Abstract
Background:
Resection of liver metastases from neuroendocrine cancer (NEC) prolongs survival and provides durable symptom relief. Not all hepatic lesions are amenable to resection, particularly when there is multifocal involvement. In this study, it was hypothesized that ablation of concomitant non-resectable NEC liver metastases is safe and salvages patients who would not have been selected for cytoreductive surgery.
Methods:
Patients who underwent adjuvant ablation of NEC liver metastases between 1995 and 2008 were reviewed. NEC was classified by patient and tumour characteristics. Regression and Kaplan–Meier models were used to compare variables and generate survival curves.
Results:
Ninety-four patients underwent hepatic resection and intra-operative ablation of metastatic NEC. The median number of lesions ablated was 3, and median size was 1.4 cm. One abscess occurred at an ablation site. Local recurrence was detected in four patients (3.8%). Overall survival was 80% and 59% at 5 and 10 years. Age, gender, tumour type, grade, primary site and need for repeat ablation had no significant association with survival. The Ki67 proliferative index was a significant predictor of decreased survival. Symptom-free survival was 34% at 3 years and 16% at 5 years, independent of the tumour grade.
Conclusion:
Concurrent ablation of NEC metastases to the liver not amenable to resection is safe and increases the candidacy of patients for cytoreductive surgery. Ablation performed intra-operatively and repeated post-operatively as needed provides significant symptom control regardless of the tumour grade.
Introduction
Neuroendocrine cancers (NEC) are slow-growing epithelial neoplasms with predominant neuroendocrine differentiation. In spite of their indolent course, more than half of the patients develop hepatic metastases.1,2 Because of their low proliferative rate, cytotoxic chemotherapeutic agents are not effective against NEC.3 Similarly, somatostatin analogues that are used for symptom control do not alter overall survival.4 Hence, surgical resection of the primary tumour and hepatic metastases remain the mainstay of treatment. In patients with hepatic metastases from NEC, cytoreductive surgery improves symptom control, slows disease progression and prolongs survival.5,6 Although the 5-year survival of patients with unresectable liver metastases is about 30%,7 surgical resection of these metastases prolongs 5-year survival to 60–80%.1,6
In spite of the apparent benefits of surgical resection, only about 10% of patients with metastatic NEC to the liver are candidates for cytoreductive surgery8 owing to the extent or the location of the liver metastases. Radiofrequency ablation (RFA) is an additional method of liver-directed therapy. RFA of metastatic NEC to the liver has been reported to provide 48% 5-year survival in patients with unresectable disease.9 To date, most reports of RFA for metastatic NEC to the liver include patients that are deemed unresectable9 or patients who underwent RFA after recurrence after resection.10 In this study, it was hypothesized that resection with adjunctive intra-operative RFA would increase the number of candidates for cytoreductive surgery and provide more complete cytoreduction. As a result, this combined treatment strategy has not been incorporated into our practice.
Methods
This study was approved by the Mayo Clinic Institutional Review Board. Ninety-eight consecutive patients who underwent combined resection of metastatic NEC to the liver and intra-operative RFA between January 1995 and December 2008 were identified and their medical records reviewed. Four patients who were lost to follow-up were excluded. All other patients were followed routinely with physical examination, radiological imaging (either computerized tomography, magnetic resonance imaging or an indium-111 octreotide scan), and laboratory evaluation including serum hormone (in islet cell tumours) and 5-hydroxyindoleacetic acid (5-HIAA) (in carcinoid tumours) levels. In post-operative imaging, ablation sites were routinely assessed for evidence of incomplete ablation.
The tumour burden in the liver was determined by pre-operative imaging and confirmed routinely by intra-operative ultrasonography. Two RFA systems were used over the time period including those with a multitined electrode (StarBurst® Radiofrequency Ablation System; Angio Dynamics®, Latham, NY, USA) and those with an internally cooled electrode (Cool-TipTM; Covidien, Boulder, CO, USA). For lesions larger than 4 cm, the probe was deployed multiple times to ensure complete destruction of the tumour. The histopathology of resected metastases was re-reviewed by an expert hepato-pathologist, who was unaware of the clinical outcomes. The slides were stained with routine haematoxylin and eosin and scored for tumour grade, number of mitoses per high powered fields, angioinvasion and inflammation. Immunohistochemistry for Ki67 was done on stored specimens of 90 patients, and the Ki67 index was determined by an automated quantification method using the Automated Cellular Imaging System III (Dako, Carpinteria, CA, USA).
Descriptive statistics are reported as number (percentage) or either mean (± SD) or median (range) as appropriate. Post-operative survival and symptom control were estimated using Kaplan–Meier survival. Cox proportional hazards regression was used to assess an univariate association of patient factors with both overall survival and symptom control. The multivariate analysis of overall survival was assessed using the a priori chosen variables of primary disease (islet cell vs. carcinoid) and Ki67 index (≤3.5 vs. >3.5). Results of these models are reported as a hazard ratio (HR) and a 95% confidence interval (CI). Categorical variables were compared using Fisher's exact test. The alpha-level was set at 0.05 for statistical significance.
Results
Ninety-four patients with metastatic NEC who underwent combined liver resection and ablation were reviewed (Table 1). There were 41 men (43.6%) and 53 women (56.4%). The mean age was 53.7 years (± 1.6). Forty-eight patients (51.1%) had carcinoid cancers and 46 (48.9%) had islet cell cancers. The most common site of the origin of primary cancer was the pancreas (n= 44), followed by the small bowel (n= 40), colon/rectum/appendix (n= 4), bronchus (n= 2), ovary (n= 1) and kidney (n= 1). The site of the primary cancer was not found in two patients. Fifty-nine patients (62.8%) had symptomatic endocrinopathies associated with their tumour; 19 were on pre-operative somatostatin therapy as a result of severe symptoms. Most NEC were intermediate to high grade based on mitoses/high-power fields (HPF) and the Ki67 index (Table 1). The median Ki67 index was 3.5%.
Table 1.
Patient demographics and tumour characteristics
| Age (years), mean ± SD | 53.7 ± 11.6 |
| Gender (M : F) | 41 : 53 |
| Primary disease, n (%) | |
| Carcinoid | 48 (51.1) |
| Islet cell | 46 (48.9) |
| Non-functional | 30 (31.9) |
| Gastrinoma | 8 (8.5) |
| Insulinoma | 4 (4.3) |
| Glucagonoma | 3 (3.2) |
| VIPoma | 1 (1.1) |
| Location of the primary disease, n (%) | |
| Pancreas | 44 (46.8) |
| Small bowel | 40 (42.6) |
| Colon/rectum/appendix | 4 (4.3) |
| Bronchus | 2 (2.1) |
| Ovary | 1 (1.1) |
| Kidney | 1 (1.1) |
| Unknown | 2 (2.1) |
| Pre-operative symptoms, n (%) | 59 (62.8) |
| Pre-operative somatostatin, n (%) | 19 (20.2) |
| Tumour histology | |
| Mitoses, per 10 HPF (%) | |
| <2 | 71 (75.5) |
| ≥2 | 23 (24.5) |
| Ki67 index, n (%) | |
| ≤2 | 33 (35.1) |
| 3–20 | 53 (56.4) |
| >20 | 4 (4.3) |
HPF, high-power fields.
The majority of patients underwent hepatic segmentectomy or subsegmentectomy (n= 70, 74.5%), whereas a hepatic lobectomy was done in 18 patients (19.1%) (Table 2). Six patients (6.4%) underwent a bisegmentectomy. Intra-operative ultrasonography was used routinely to confirm the metastases detected by pre-operative imaging. A median of three metastases were ablated intra-operatively (range: 1–14 per patient). The median size of the ablated metastases was 1.4 cm (range: 0.3–8.0 cm). All patients had near-total gross resection and ablation at the end of the operation with minimal measurable residual disease. Quantification of residual metastatic disease within the liver was precluded because accurate measurement intra-operatively was not possible by imaging. There was no peri-operative mortality (Table 3). One patient who had previously underwent a pancreaticoduodenectomy for the primary NEC developed an abscess at an ablation site 2 weeks post-operatively that resolved after percutaneous drainage.
Table 2.
Operative procures and characteristics of ablated lesions
| Surgical resection, n (%) | |
| Hepatic lobectomy | 18 (19.1) |
| Bisegmentectomy | 6 (6.4) |
| Segmentectomy/subsegmentectomy | 70 (74.5) |
| Patients requiring additional post-operative RFA, n (%) | 21 (22.3) |
| Number of lesions ablated, median (range) | 3 (1–14) |
| Size of lesions ablated, cm, median (range) | 1.4 (0.3–8) |
RFA, radiofrequency ablation.
Table 3.
Outcomes
| Follow-up (months), mean ± SD | 58 ± 36.9 |
| Local complications | |
| Abscess at the ablation site, n (%) | 1 (1.1) |
| Recurrence at the ablation site, n | 4 |
| Tumour recurrence, n | 81 |
| Time to recurrence (months), median | 19 |
| Symptom-free survival (months), median | 24 |
Overall, in 57 out of 59 (96.6%) patients with pre-operative symptoms, symptom relief was achieved after the operation. By 5 years, the cumulative probability of symptom recurrence was 84%, with a median time to recurrence of 2 years. Similarly, progression by imaging was seen in 86% of patients. Four patients (4.2%) had tumour recurrence at the site of ablation. Twenty-one (22%) patients underwent additional post-operative ablation for metastatic progression at a median interval of 19 months post-operatively (Table 3). The overall 5- and 10-year survivals were 80% and 59%, respectively (Fig. 1a).
Figure 1.
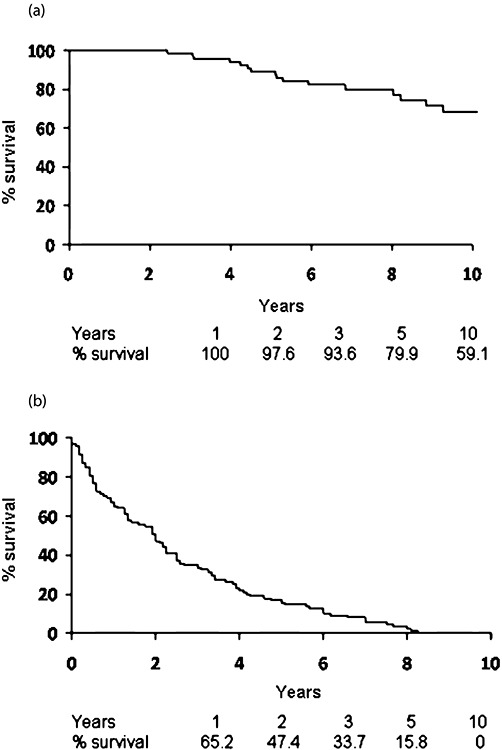
Overall (a), and symptom-free survival (b) of patients after the combined resection and ablation. Actuarial survival estimates are shown under each graph
Univariate regression models showed that age, gender, type and site of the primary cancer were not significantly associated with survival (Table 4). Similarly, repeat RFA of metastatic NEC during the follow-up was not associated with survival. Of the histopathological characteristics of the metastases which were assessed, the Ki67 index (≥3.5 relative to <3.5) was the most significant predictor for decreased survival in both univariate (HR = 4.28, CI 1.51–12.14, P= 0.006) and multivariate (HR = 5.01, CI 1.65–15.18, P= 0.004) analyses. The Ki67 index (≥3.5 relative to <3.5) had a borderline, although non-significant association with symptom-free survival (HR = 1.53, CI 0.98–2.38, P= 0.06). Preoperative somatostatin analogue use correlated with decreased symptom-free duration post-operatively (HR = 1.73, CI 1.04–2.89, P= 0.03) (Table 5).
Table 4.
Risk factors associated with decreased survival
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95% CI) | P | |
| Gender (M vs. F) | 0.79 (0.32–1.95) | 0.61 | ||
| Age, per 1 year | 1.00 (0.95–1.04) | 0.86 | ||
| Primary disease (islet cell vs. carcinoid) | 1.21 (0.51–2.89) | 0.66 | 0.67 (0.26–1.71) | 0.40 |
| Functional vs. non-functional (islet cell tumour) | 0.95 (0.28–3.27) | 0.94 | ||
| Need for repeated RFA | 1.31 (0.49–3.50) | 0.59 | ||
| Pre-operative octreotide | 0.53 (0.12–2.28) | 0.39 | ||
| Tumour histology | ||||
| Grade (Grade 2 vs. Grade 1) | 2.30 (0.91–5.84) | 0.08 | ||
| Mitoses, per 1 unit | 1.02 (0.99–1.05) | 0.15 | ||
| Angioinvasion (Yes vs. No) | 2.06 (0.81–5.19) | 0.18 | ||
| Ki67 index (≥3.5 vs. <3.5) | 4.28 (1.51–12.14) | 0.006 | 5.01 (1.65–15.18) | 0.004 |
| Inflammation (Yes vs. No) | 1.57 (0.46–5.38) | 0.47 | ||
RFA, radiofrequency ablation.
Table 5.
Risk factors associated with decreased symptom-free survival
| Univariate | ||
|---|---|---|
| HR (95%CI) | P | |
| Gender (M vs. F) | 1.04 (0.68–1.58) | 0.87 |
| Age, per 1 year | 0.98 (0.97–1.00) | 0.13 |
| Primary disease (islet cell vs. carcinoid) | 1.38 (0.91–2.09) | 0.13 |
| Functional vs. non-functional (islet cell tumour) | 1.19 (0.69–2.06) | 0.52 |
| Need for repeated RFA | 1.33 (0.83–2.13) | 0.24 |
| Preoperative octreotide | 1.73 (1.04–2.89) | 0.03 |
| Tumour histology | ||
| Grade (Grade 2 vs. Grade 1) | 1.02 (0.63–1.65) | 0.94 |
| Mitoses, per 1 unit | 1.01 (0.99–1.02) | 0.62 |
| Angioinvasion (Yes vs. No) | 1.17 (0.76–1.79) | 0.48 |
| Ki67 index (≥ 3.5 vs. < 3.5) | 1.53 (0.98–2.38) | 0.06 |
| Inflammation (Yes vs. No) | 1.52 (0.73–3.19) | 0.26 |
RFA, radiofrequency ablation.
Discussion
The management of patients with metastatic NEC to the liver has evolved over the past two decades. In the absence of effective systemic therapy, liver-directed cytoreductive therapy is widely accepted as the standard of care. Whether complete or near-total operative cytoreduction is performed, resection provides the most prolonged survival and durable control of symptomatic endocrinopathies.11 In 2003, we reported our single-centre experience, in which resection of metastatic NEC to the liver in 170 patients resulted in overall survival of 61% and 35% at 5- and 10-years, respectively.6 In a recent multicentre international study of 339 patients who underwent liver-directed therapy of metastatic NEC to the liver, overall 5- and 10-year survival were 74% and 51%, respectively.5 Because resection alone of hepatic metastases can be offered only to a subset of patients, ablation, either alone or in conjunction with resection, has recently been introduced successfully as a treatment option for patients with metastatic NEC to the liver. The rationale of combining these two modalities is that more complete cytoreduction can be achieved with adjunctive ablation in patients with extensive hepatic tumour burden or those with deep-seated metastases in whom resection would risk an adequate functional remnant. Hence resection and RFA could increase the number of candidates for cytoreductive surgery and decrease the overall risk of insufficient remnant volume. We have been using ablation in combination with resection since 1995, and the current series is the largest to our knowledge.
The present results demonstrate that the adjunctive ablation is safe and well tolerated. Although not common, thermal injury to bile ducts with subsequent stricturing12 or adjacent organs13 during percutaneous ablation of liver lesions have been reported. Intra-operative ablation with the aid of real-time ultrasonographic examination and protection of adjacent organs help prevent both these potential complications. In the present cohort, we have not had any complications secondary to thermal injury. One patient (1.1%) had a local abscess at the site of ablation detected by imaging 2 weeks post-operatively. This patient had a previous pancreatoduodenectomy with a choledochoenterostomy. The abscess resolved after percutaneous drainage with no long-term sequela. In four patients (4.3%), we found local recurrence during follow-up, presumably as a result of incomplete ablation. This incidence is lower than the 11% local recurrence reported by Mazzaglia and colleagues9 in a 10-year experience of laparoscopic RFA of metastatic NEC to the liver.
The overall 5-year and 10-year survival of 80% and 59%, respectively, herein are greater than several previous reports by others5,14 and us.6 However, these findings are inadequate to support improved survival because of the retrospective design of this study and the probability of accounted selection factors. Although it is possible that more complete cytoreduction through the addition of RFA accounts for these findings, quantification of such was not possible owing to the limits of resolution of current intra- and post-operative imaging. While a comparison of survival among different patient groups and eras is difficult, it is noteworthy that most patients herein had intermediate to high grade NEC which was unexpected given the overall survival. The proliferative rate of NEC, as assessed by the mitotic rate and Ki67 index, has been accepted by both the World Health Organization (WHO) and the European Neuroendocrine Tumor Society (ENETS) grading systems as the main determinant of tumour aggressiveness of NEC overall.15 We found that the high Ki67 index was the most significant predictor of decreased survival in both univariate and multivariate analyses, confirming earlier reports in patients that underwent resection of primary16 or metastatic NEC,10 or liver transplantation for metastatic NEC.17 Other histopathological characteristics of NEC, such as mitoses, inflammation and angioinvasion, had no significant association with survival. The grade of the tumour conferred a 2.3 times higher risk of decreased survival; however this did not reach statistical significance, possibly because of the relatively small sample size. A high Ki67 index was also associated with the probability of the need for repeated RFA as more patients with a high index required repeated RFA (Fisher's exact test, P= 0.023).
Malignant endocrinopathies are common in patients with metastatic NEC of the liver. In this study, it was demonstrated that combined resection and RFA adequate symptom control was achieved in 96.6% of our patients, similar to previously published reports.5,6,18 Symptom recurrence with disease progression was almost uniform in our patients because complete resection was seldom feasible. The median symptom-free post-operative period was 24 months (Fig. 1b). Not surprisingly, somatostatin analogue therapy pre-operatively predicted a shorter symptom-free period after cytoreduction. Importantly, tumour grade and the Ki67 index had no significant predictive impact on the symptom-free survival. Consequently, we believe that these results provide support for liver-directed therapy in all eligible candidates, regardless of the tumour biology.
Prior to incorporation of RFA to our surgical practice, only patients in whom an expected near complete resectability based on preoperative imaging were offered surgical treatment.19 We have since relaxed that selection criteria and have shifted towards more aggressive cytoreduction in patients with NEC. Because of our inability to fully cull all patients with metastatic NEC who had a surgical consultation, comparison of practice differences between eras is not feasible retrospectively. Thus, we lack objective data to determine the ratio of patients who underwent operative cytoreduction after surgical consultation. However, we are convinced the present cohort includes patients who would not have been offered resection prior to the incorporation of RFA to our practice. The present study has several limitations inherent to retrospective analyses. The patients represent a carefully selected cohort among the many with metastatic NEC. In addition, the extent of metastatic involvement of the liver, which may be used for selection of different treatment modalities,14,20 was not evaluated. In patients in whom near complete resection is feasible, we continue to offer resection alone, even if selective portal venous embolization is required to increase future liver remnant volume.
In summary, our results show that the combined liver-directed therapy of metastatic NEC to the liver can be done safely. Adjunctive ablation of otherwise non-resectable metastatic NEC to the liver complements cytoreductive resection and provides effective but transient symptom control. Incorporation of RFA as an essential component of operative cytoreduction will expand selection of patients for operation and potentially increase the durability of response.
Conflicts of interest
None declared.
References
- 1.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. et al. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13 715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Bajetta E, Ferrari L, Procopio G, Catena L, Ferrario E, Martinetti A. Efficacy of a chemotherapy combination for the treatment of metastatic neuroendocrine tumours. Ann Oncol. 2002;13:614–621. doi: 10.1093/annonc/mdf064. et al. [DOI] [PubMed] [Google Scholar]
- 4.Rinke A, Muller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. et al. [DOI] [PubMed] [Google Scholar]
- 5.Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–3136. doi: 10.1245/s10434-010-1154-5. et al. [DOI] [PubMed] [Google Scholar]
- 6.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 7.Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5:1502–1522. doi: 10.1200/JCO.1987.5.10.1502. [DOI] [PubMed] [Google Scholar]
- 8.Gamblin TC, Christians K, Pappas SG. Radiofrequency ablation of neuroendocrine hepatic metastasis. Surg Oncol Clin N Am. 2011;20:273–279. doi: 10.1016/j.soc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson J, Stalberg P, Nilsson A, Krause J, Lundberg C, Skogseid B. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg. 2008;32:930–938. doi: 10.1007/s00268-008-9510-3. et al. [DOI] [PubMed] [Google Scholar]
- 11.Maithel SK, Fong Y. Hepatic ablation for neuroendocrine tumor metastases. J Surg Oncol. 2009;100:635–638. doi: 10.1002/jso.21196. [DOI] [PubMed] [Google Scholar]
- 12.Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 14.Frilling A, Li J, Malamutmann E, Schmid KW, Bockisch A, Broelsch CE. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg. 2009;96:175–184. doi: 10.1002/bjs.6468. [DOI] [PubMed] [Google Scholar]
- 15.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 16.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. et al. [DOI] [PubMed] [Google Scholar]
- 17.Rosenau J, Bahr MJ, von Wasielewski R, Mengel M, Schmidt HH, Nashan B. Ki67, E-cadherin, and p53 as prognostic indicators of long-term outcome after liver transplantation for metastatic neuroendocrine tumors. Transplantation. 2002;73:386–394. doi: 10.1097/00007890-200202150-00012. et al. [DOI] [PubMed] [Google Scholar]
- 18.Atwell TD, Charboneau JW, Que FG, Rubin J, Lewis BD, Nagorney DM. Treatment of neuroendocrine cancer metastatic to the liver: the role of ablative techniques. Cardiovasc Intervent Radiol. 2005;28:409–421. doi: 10.1007/s00270-004-4082-6. et al. [DOI] [PubMed] [Google Scholar]
- 19.Que FG, Nagorney DM, Batts KP, Linz LJ, Kvols LK. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36–42. doi: 10.1016/s0002-9610(99)80107-x. [DOI] [PubMed] [Google Scholar]
- 20.Mayo SC, de Jong MC, Bloomston M, Pulitano C, Clary BM, Reddy SK. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18:3657–3665. doi: 10.1245/s10434-011-1832-y. [DOI] [PubMed] [Google Scholar]


