Abstract
Introduction:
Regional therapy with trans-arterial chemoembolization (TACE) is a common treatment for unresectable hepatocellular carcinoma (HCC). Outcomes were examined in patients with the best radiological response (BR) after the initial TACE.
Methods:
This was a retrospective cohort study of patients who underwent TACE as the initial treatment for HCC between the years 2000 and 2010. BR was defined as complete disappearance of the tumour or no enhancement with contrast on the first cross-sectional imaging study after the initial TACE.
Results:
Seventy-eight out of 104 total consecutive patients were identified with the potential for a BR to TACE therapy for unresectable HCC, and 24 met the criteria for BR. Patients with BR had a median survival of 12.8 months (2.2–54.9) compared with 18.9 months(1.3–56.7) for the entire cohort (P= 0.313). The median time to progression was 10.6 months (1.2–24.3) in the BR group and 3.2 months (0.7–49.2) in the patients without a BR (P= 0.003).
Discussion:
BR to initial TACE for unresectable HCC is associated with comparable survival to those without BR in spite of a longer time to cancer progression. It may be reasonable to consider further therapy such as repeat TACE or biological/systemic therapy in patients with HCC even when the radiological response to the initial TACE is favourable.
Introduction
Liver cancer has a devastating global impact being the third leading cause of cancer-related death worldwide. Hepatocelluar carcinoma (HCC) compromises 70–85% of liver cancer diagnoses resulting in well over 500 000 deaths worldwide each year.1,2 While this disease is most prevalent in the African and Asian continents, its incidence is on the rise in the United States with liver cancers as a whole being the fastest growing group of cancers over the past decade.1,3 In spite of the prevalence of this disease treatment options are limited resulting in a 1-year survival rate of less than 50% for all stages worldwide.3,4
Potentially curative treatment with surgical resection or transplantation is limited to a small subset of HCC patients.3,5 Many patients are not candidates for these procedures because of advanced cirrhosis precluding safe resection or tumours outside transplant criteria.5 While other options such as local ablative therapy are available these are limited by the extent of cirrhosis and the size and location of tumour(s).6 Therefore a commonly used treatment for HCC in the presence of cirrhosis is trans-arterial chemoemboization (TACE).7 TACE has been shown to be safe and improve survival in patients with unresectable HCC compared with no therapy or best supportive care.8–11 As expected outcomes are best in patients with compensated or Child's A cirrhosis, TACE has also been used by some in properly selected patients with more advanced cirrhosis.12 Favourable outcomes from TACE are associated with Child's Class A cirrhosis13 lack of ascites,13 age younger than 60 years,14 alkaline phosphatase less than 120 U/l,14 albumin greater than 35 g/l,14 smaller tumours,15 solitary tumours,15 good baseline performance status,16 low billirubin level,7,16 and multiple TACE procedures.15,16
A best or complete radiological response to TACE has been defined as a lack of contrast enhancement on imaging after the procedure.7,15–18 Such a best response to TACE has been associated with improved survival in patients with recurrent HCC after a hepatectomy15 and in patients with class A and B cirrhosis.7,16 Of note, some of these previous studies allowed for multiple serial TACE procedures to be done in order to achieve a best response after the final TACE.7,15,16 One study by Imai suggested that a favourable response after subsequent TACE is associated with the response seen at previous TACE procedures (not specifically the initial TACE), but the present study did not examine survival outcomes based upon response.17 In this study, outcomes for individuals having a best response (BR) after initial TACE for unresectable HCC are compared with the individuals who do not have a BR.
Methods
After Institutional Review Board approval, the medical records of 104 patients with unresectable HCC treated with initial TACE therapy between 2000 and 2010 were reviewed. All patients had the diagnosis of HCC made by characteristic radiographical findings on pre-procedure liver magnetic resonance imaging (MRI) or computed tomography (CT); ultrasound or a CT-guided biopsy was done if tumour features were suggestive, but not diagnostic for HCC. Patients with extra-hepatic disease or major vascular invasion were excluded. Patients without post-TACE imaging were also excluded.
TACE therapy was generally offered to patients with unresectable HCC, Child's class A or B cirrhosis, an Eastern Cooperative Oncology Group performance status of 0–2, no encephalopathy and no ascites detectable on physical examination. Very rarely, patients with Child's C cirrhosis were offered TACE. TACE therapy was triple drug TACE (cisplatin 50 mg, doxorubicin 30 mg, and mitomycin C 20 mg with Visipaque at 320 mg/ml) using a 10-ml Lipiodol carrier and most of the procedures targeted the right or left lobar distribution. After 2009, LC Beads (coated with 100 mg of doxorubicin) (Biocompatibles, Farnham, Surrey, UK) were used for all TACE procedures in any patient with HCC. Some patients received bland embolization. Most patients were discharged the day after TACE. A physical examination and laboratory studies were conducted within a few days after discharge from the initial hospitalization in the outpatient setting. Liver protocol CT or MRI was done around 1 to 2 months after TACE, and if no further therapy was needed, surveillance imaging and labs were repeated every 3 months for the first year then every 6 months for at least another 2 years.
A radiological BR to TACE therapy was determined using the first imaging study available at least 2 weeks after the initial TACE therapy. Both abdominal contrast-enhanced MRI and multiphase CT were allowed. BR was defined as no abnormal enhancement or density consistent with a viable tumour present at the previous target site(s) for TACE or complete disappearance of tumour(s) after the initial TACE without any new tumours within the liver or abdomen concerning early progression. For the patients that received Lipiodol, areas of enhancement that had washout or obvious tumour lesions without Lipiodol contrast were considered to have a residual tumour. If a patient had additional TACE therapy less than 2 months after the intial TACE, they were considered to not have a BR.
Clinical tumour characteristics and outcomes were compared between those with BR after the initial TACE for unresectable disease to those without BR using Student's t-test for parametric, continuous data, Mann–Whitney U-test for non-parametric, continuous data, and Fisher’ exact test for all nominal data. From data obtained through the chart review, the Child–Pugh score, model of end-stage liver disease (MELD) score, and TACE survival score14 were calculated for each patient. Overall survival was defined as the time from initial TACE therapy to date of death or censored at last follow-up. Time to progression was defined as the time from the initial TACE to the first sign of progression after TACE. Kaplan–Meier curves were used to compare groups and significance was determined by the log-rank test. Factors were considered to be associated with overall survival when a P-value of less than 0.05 was obtained by univariate analysis. All statistics were performed using Statistical Package for Social Sciences (SPSS) Version 19 (SPSS Inc., Chicago, IL, USA).
Results
There were 104 patients who underwent TACE for initial treatment of unresectable HCC between 2000 and 2010. A total of 26 (25%) patients were excluded from the analysis. Seven had extra-hepatic disease, five had major vascular invasion and two had a combination of these findings on pre-procedure imaging. Twelve patients did not have post-TACE imaging owing to the following reasons: six died in less than 2 months after the TACE procedure, one was assumed to need additional TACE procedures without imaging because of the size of the original tumour, one had a secondary malignancy discovered that prioritized their therapy, one had a brain metastasis discovered after the procedure that altered the plan of therapy, one patient had a liver transplant and two had reasons that were unknown. Of the remaining patients, 24 (30.8%) met the criteria for BR whereas 54 (79.2%) did not have BR (Fig. 1). A small number of patients in the study (n= 11) underwent TACE for recurrent HCC after previous potentially curative ablation or surgical resection; none of these recurrences were local. There were no significant differences in patient demographics between patients in the BR group and the remaining portion of the TACE cohort who did not have a BR. A difference was observed in the synthetic liver function between the groups, but not any other lab values. All patients had liver cirrhosis and the degree of liver impairment was not significantly different between the two groups (Table 1).
Figure 1.
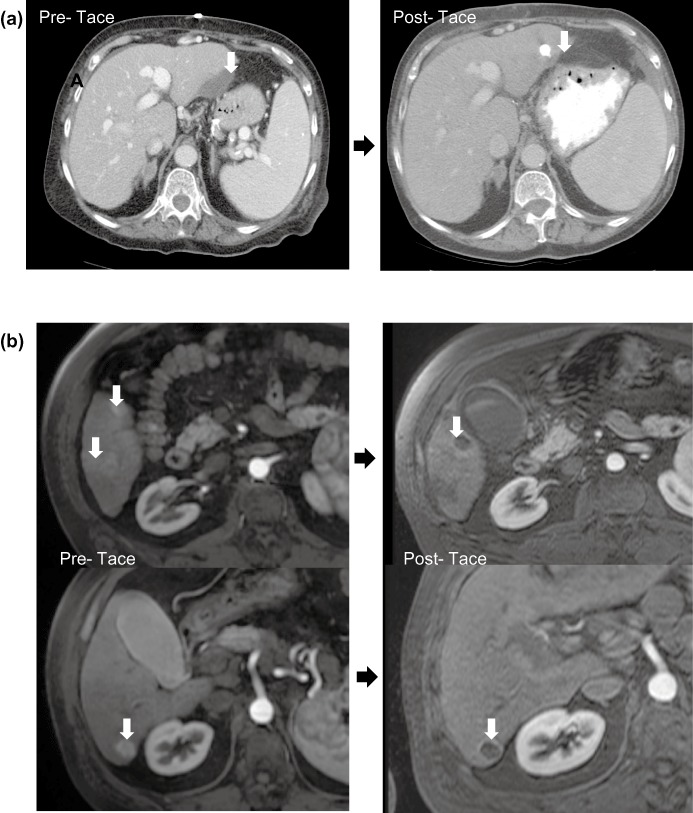
(a) An example of a best response: this is a computed tomography (CT) image from a 76-year-old woman who presented with haemoperitoneum from a bleeding left lateral segment hepatocellular carcinoma (HCC) (arrow, left CT). Multiple comorbidities and portal hypertension made surgical resection not feasible therefore she was treated with trans-arterial chemoembolization (TACE). A CT scan performed 1 month after the initial TACE revealed no evidence of a residual viable tumour (arrow right CT). Several more serial CT scans showed no evidence of a mass until she developed local recurrence/progression 2 years after the initial TACE. (b) An example of not a best response: magnetic resonance imaging (MRI) images are shown from a patient with cirrhosis and three arterial-enhancing tumours in the right liver (arrows, left MRI) treated with TACE. MRI images obtained approximately 1 month after TACE revealed a tumour response with overall loss of enhancement but some residual peripheral enhancement around two of the masses (arrows, right MRI)
Table 1.
Patient demographics and baseline liver function
| TACE cohort (n= 78) | No BR (n= 54) | BR (n= 24) | P-value | |
|---|---|---|---|---|
| Age, years [median (range)] | 60 (31–83) | 59 (31–81) | 59 (48–83) | 0.380 |
| Gender [n, (% male)] | 58 (74.4) | 42 | 16 | 0.400 |
| HBV [n (%)] | 9 (11.5) | 8 | 1 | 0.261 |
| HCV [n (%)] | 33 (42.3) | 22 | 11 | 0.805 |
| Total bilirubin [median (range)] | 1.1 (0.4–4.4) | 1.0 (0.5–3.8) | 1.2 (0.40–4.40) | 0.173 |
| Albumin [median (range)] | 3.1 (1.5–4.9) | 3.1 (1.7–4.9) | 2.8 (1.5–3.7) | 0.014 |
| INR [median (range)] | 1.2 (0.9–2.0) | 1.2 (0.9–2.0) | 1.2 (1.1–1.7) | 0.042 |
| Creatinine [median (range)] | 0.82 (0.46–4.94) | 0.86 (0.52–4.94) | 0.79 (0.46–1.66) | 0.309 |
| Ascites [n (%)] | 18 (23.1) | 13 | 5 | 1.000 |
| C-P score [median (range)] | 6 (5–11) | 6 (5–11) | 7 (5–10) | 0.071 |
| Class A (n, %) | 42 (54.8) | 31 | 11 | 0.461 |
| Class B (n, %) | 34 (43.6) | 23 | 11 | 0.803 |
| Class C (n, %) | 2 (2.6) | 0 | 2 | 0.092 |
| MELD score [median (range)] | 9.5 (6.4–23.8) | 9.1 (6.4–23.8) | 9.7 (7.9–17.0) | 0.059 |
| TACE prognotic survival score [mean] | 1.55 | 1.44 | 1.79 | 0.129 |
C-P, Child-Pugh; HBV, Hepatitis-B Virus; HCV, Hepatitis-C Virus; INR, International Normalized Ratio; TACE, trans-arterial chemoembolization. Bold values indicated significant P-values in the comparison of BR to non-BR groups.
Baseline tumour characteristics were similar between the two groups. (Table 2). There was no statistical difference in the type or specifity of the TACE therapy used between those with BR and those without, and alpha-feto protein (AFP) levels before and after TACE were not different (Table 2). The pre-TACE imaging was performed at this institution for 63 (80.8%) of the patients and all patients had post-TACE imaging performed at this hospital. Imaging was generally obtained at a median of 52 days (15–810) after the initial TACE. The median length of hospital stay was 2 days (1–15) in the entire cohort and 2 days in both the non-BR (1–6 days) and the BR groups (1–15 days) (P= 0.160). Only one 60-day mortality occurred in the entire cohort and that was in the non-BR group. There were three patients who experienced major complications in the BR group compared with 15 in the non-BR group (P= 0.160). Common complications included infection, hepatic insufficiency and gastrointestinal bleeding. There were three with hepatic failure in the non-BR group and two with hepatic failure in the BR group (P= 0.641).
Table 2.
Tumour characteristics and trans-arterial chemoembolization (TACE) treatment among the cohort
| TACE cohort (n= 78) | No BR (n= 54) | BR (n= 24) | P-value | |
|---|---|---|---|---|
| Pre-procedure AFP level [median, (range)] | 47.0 (1.3–524 000) | 47.0 (1.3–524 000) | 44.5 (2.0–4787) | 0.830 |
| Post-procedure AFP level [median, (range)] | 24.6 (1.4–58 623) | 37.6 (1.8–58 623) | 23.1 (1.4–2668) | 0.922 |
| Diameter of largest tumour (cm) [median (range)] | 4.4 (0.7–12.1) | 4.2 (0.7–12.1) | 4.8 (2.1–12.1) | 0.700 |
| More than one tumour [n (%)] | 48 (61.5) | 34 | 14 | 0.802 |
| Location of tumour (s) | ||||
| Right lobe [n (%)] | 36 (46.2) | 22 | 14 | 0.219 |
| Left lobe [n (%)] | 7 (9.0) | 4 | 3 | 0.670 |
| Bilobar [n (%)] | 35 (44.8) | 28 | 7 | 0.085 |
| TACE procedure details | ||||
| Selective TACE (smaller than hepatic arteries) [n (%)] | 9 (11.5) | 6 | 3 | 1.000 |
| Chemotherapy TACE [n (%)] | 37 (47.5) | 23 | 14 | 0.227 |
| TACE with chemo-coated beads [n (%)] | 24 (30.7) | 17 | 7 | 1.000 |
| Bland TACE (embolization) [n (%)] | 17 (21.8) | 14 | 3 | 0.242 |
AFP, Alpha-feto protein.
Progression of disease occurred in 69 of the 78 (82.2%) patients within the cohort. In the BR group, 17 out of 24 had progression of disease compared with 52 of the 54 within the non-BR group. The median time to progression (TTP) after the initial TACE was 4.6 months (0.7–54.9) in the entire cohort, and the BR group experienced a median TTP of 10.6 months (1.2–54.9) compared with 3.2 months (0.7–49.2) in the non-BR group (P= 0.003, Fig. 2). The most common site of progression was an intrahepatic tumour in both groups (n= 12 and n= 41, respectively), and the most common therapy given for progression after the initial TACE was another TACE (n= 8 and n= 18, respectively). Two (2.6%) patients in the entire cohort underwent liver transplantation after the initial TACE.
Figure 2.
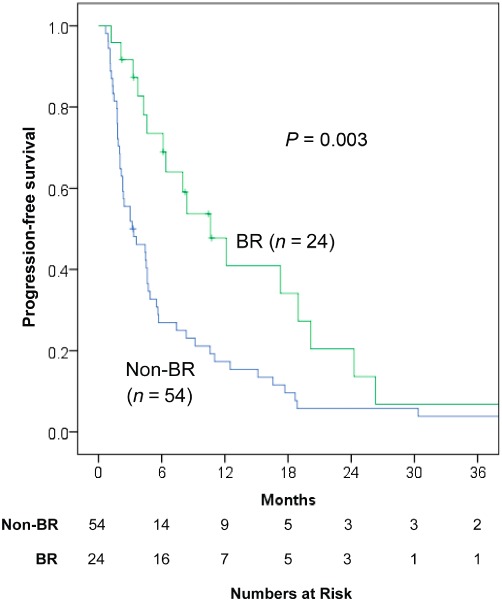
Time to progression of hepatocellular carcinoma (HCC) after the initial trans-arterial chemoembolization (TACE) comparing patients with a best response (BR) and those without response BR
The median overall survival (OS) was 16.0 months (1.3–56.7) after the initial TACE. In the group with BR after TACE, the median OS was 12.8 months (2.2–54.9) compared with 18.9 months (1.3–56.7) in the remaining patients without BR (P= 0.313, Fig. 3). In the patients with compensated cirrhosis (Child's A), the median OS after TACE in the BR group was not different compared with the without BR group (P= 0.675, Fig. 4). Other factors associated with overall survival in the entire cohort included Child's class and MELD score (Table 3).
Figure 3.

Overall survival compared between patients with a best response (BR) and without after an initial trans-arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (HCC)
Figure 4.
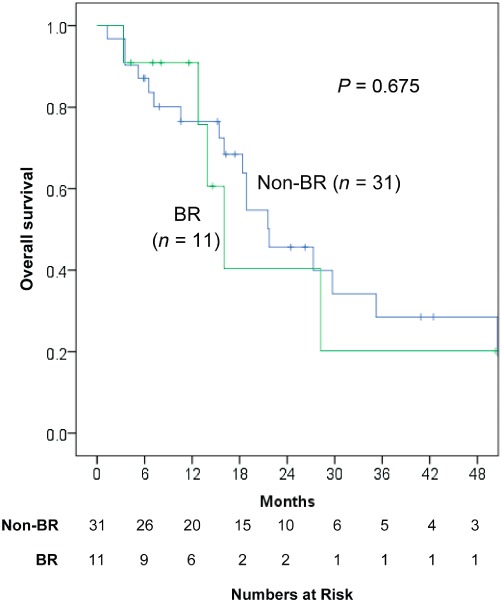
Overall survival compared between patients with Child's A cirrhosis and with or without a best response to an initial trans-arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (HCC)
Table 3.
Factors associated with overall survival among the entire cohort
| Factor | Univariate |
|---|---|
| Demographics | |
| Age | 0.147 |
| Gender | 0.289 |
| Liver function | |
| Child's class | 0.024 |
| MELD score | 0.031 |
| HCC tumour burden | |
| AFP | 0.755 |
| Milan criteria | 0.167 |
| More than 1 tumour | 0.132 |
| Largest diameter | 0.758 |
| TACE therapy | |
| Specific TACE | |
| Bland TACE | 0.244 |
| Chemotherapy with embolization | 0.940 |
| Bead Therapy | 0.155 |
AFP, alpha-feto Protein; MELD, model for end-stage liver disease.
Discussion
After the initial TACE for HCC, a best or complete radiological response is not a rare occurrence and can be seen in the range of 14–70% of TACE procedures for HCC.7,15–17 The TACE best response rate of 31% in this study falls within this range, and notably represents a response after the initial TACE whereas some other studies with higher rates allowed for multiple TACE procedures to achieve BR.15 These studies have also demonstrated that a complete response after TACE is associated with improved survival. While BR in this study's patients was associated with improved progression-free survival, outcomes were otherwise similar between the BR and non-BR groups.
The underlying liver disease (cirrhosis) seems to influence outcomes more than tumour stage or radiological response to therapy in this study. Thirty-six patients (46.2%) in this study had Child's B or C compared with rates of 13–23% in other (mostly prospective) studies which excluded patients with more advanced cirrhosis.7–9,15–17 The overall survival reported in this study reflects this inclusion of more patients with baseline poorer liver function. In other studies similar to this one with higher rates of advanced cirrhosis (34–63%) and survival rates range from 6–21 months.19–21 In Dhanasekaran et al. ‘s work on prognostic factors in doxorubicin-bead TACE therapy in HCC, they found 7 out of 10 reported prognostic factors were associated with liver function rather than tumour stage.20
The present study has several limitations. As stated, outcomes were examined based only on radiological response to the initial TACE. Others have found a complete or best response to correlate with outcomes allowing for more than one TACE procedure, and such a treatment strategy when feasible may result in better outcomes overall. As stated above, almost half of the patients in this study had more advanced cirrhosis. This likely explains why only 26 out of 69 patients in the cohort with disease progression went on to receive additional TACE therapy. It may be that combination therapies or a use of selective TACE over multiple sessions when feasible may improve outcomes in patients with unresectable HCC compared with a single lobar TACE followed by observation. The use of selective TACE has increased at this institution since 2009. Finally, the first post-TACE imaging study was used at a median of 52 days to determine BR or no BR, but this may not be the ideal timing in terms of measuring this response outcome.
In summary, the best response to the initial TACE was not associated with a survival advantage in patients with unresectable HCC in spite of an improved time to progression. It may be reasonable to offer other therapies such as systemic/biological therapy even in patients who have a favourable radiological response to the initial TACE. A radiological response is inadequate as a sole variable for determining prognosis in patients with HCC who undergo regional therapy. Therefore, better molecular information and biomarkers are needed to determine outcomes and personalize therapies.
Conflicts of interest
None declared.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Marsh JW, Geller DA, Finkelstein SD, Donaldson JB, Dvorchik I. Role of liver transplantation for hepatobiliary malignant disorders. Lancet Oncol. 2004;5:480–488. doi: 10.1016/S1470-2045(04)01527-X. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capussotti L, Ferrero A, Vigano L, Polastri R, Tabone M. Liver resection for HCC with cirrhosis: surgical perspectives out of EASL/AASLD guidelines. Eur J Surg Oncol. 2009;35:11–15. doi: 10.1016/j.ejso.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivo M, Valenza F, Buccellato A, Scala L, Virdone R, Sciarrino E. Transcatheter arterial chemoembolisation for hepatocellular carcinoma in cirrhosis: survival rate and prognostic factors. Dig Liver Dis. 2010;42:515–519. doi: 10.1016/j.dld.2009.09.012. et al. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. et al. [DOI] [PubMed] [Google Scholar]
- 9.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. et al. [DOI] [PubMed] [Google Scholar]
- 10.Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173–1178. doi: 10.1016/j.ejrad.2011.03.046. et al. [DOI] [PubMed] [Google Scholar]
- 11.Camma C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. et al. [DOI] [PubMed] [Google Scholar]
- 12.Nouso K, Ito Y, Kuwaki K, Kobayashi Y, Nakamura S, Ohashi Y. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br J Cancer. 2008;98:1161–1165. doi: 10.1038/sj.bjc.6604282. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsin IF, Hsu CY, Huang HC, Huang YH, Lin HC, Lee RC. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol. 2011;45:556–562. doi: 10.1097/MCG.0b013e318210ff17. et al. [DOI] [PubMed] [Google Scholar]
- 14.Wigmore SJ, Redhead DN, Thomson BN, Parks RW, Garden OJ. Predicting survival in patients with liver cancer considered for transarterial chemoembolization. Eur J Surg Oncol. 2004;30:41–45. doi: 10.1016/j.ejso.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Shim JH, Kim KM, Lee YJ, Ko GY, Yoon HK, Sung KB. Complete necrosis after transarterial chemoembolization could predict prolonged survival in patients with recurrent intrahepatic hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2010;17:869–877. doi: 10.1245/s10434-009-0788-7. et al. [DOI] [PubMed] [Google Scholar]
- 16.Cabibbo G, Genco C, Di Marco V, Barbara M, Enea M, Parisi P. Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Aliment Pharmacol Ther. 2011;34:196–204. doi: 10.1111/j.1365-2036.2011.04694.x. et al. [DOI] [PubMed] [Google Scholar]
- 17.Imai N, Ikeda K, Seko Y, Kawamura Y, Sezaki H, Hosaka T. Previous chemoembolization response after transcatheter arterial chemoembolization (TACE) can predict the anti-tumor effect of subsequent TACE with miriplatin in patients with recurrent hepatocellular carcinoma. Oncology. 2011;80:188–194. doi: 10.1159/000328749. et al. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Choi MS, Kang JY, Choi DI, Park CK, Gwak GY. Prediction of complete necrosis of hepatocellular carcinoma treated with transarterial chemoembolization prior to liver transplantation. Gut Liver. 2009;3:285–291. doi: 10.5009/gnl.2009.3.4.285. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862–1868. doi: 10.1038/sj.bjc.6602590. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Prognostic factors for survival in patients with unresectable hepatocellular carcinoma undergoing chemoembolization with doxorubicin drug-eluting beads: a preliminary study. HPB. 2010;12:174–180. doi: 10.1111/j.1477-2574.2009.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin B, Wang D, Lewandowski RJ, Ryu RK, Sato KT, Larson AC. Quantitative 4D transcatheter intraarterial perfusion MRI for standardizing angiographic chemoembolization endpoints. AJR Am J Roentgenol. 2011;197:1237–1243. doi: 10.2214/AJR.10.5821. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]


