Abstract
Objectives
Assessment of a pancreaticoduodenectomy specimen by pathologists requires specialized knowledge of anatomy. Standardized assessment, description and documentation of the retroperitoneal margin are crucial for the accurate interpretation of studies evaluating adjuvant therapy for pancreatic cancer patients.
Methods
Twenty-five patients who underwent a pancreaticoduodenectomy for pancreatic adenocarcinomas had their pathological specimens examined prospectively, using an anatomical-based mapping approach. All margins, including the bile duct, pancreatic neck, superior mesenteric artery, superior mesenteric vein and posterior surface of the uncinate process, were microscopically examined in their entirety. The assessment of an R1 margin in terms of distance was assessed in two ways: first defining it as a tumour at the margin or secondary as tumour within 1 mm (1 mm rule).
Results
If the existing College of American Pathologists recommendations were applied (assessing only the bile duct, pancreatic neck and superior mesenteric artery margins), a R1 status would be achieved in only 9 of 25 patients. Extending the examination by assessment and reporting of the entire retroperitoneal resection margin, including the Superior Mesenteric Vein margin and the Posterior surface of the uncinate process margin, increased the number of patients with a R1 resection to 14 out of 25. Applying the 1-mm rule further increased the number of patient with a R1 resection to 20 of 25 patients.
Conclusions
The above findings illustrate that different approaches to the assessment and reporting of the retroperitoneal margin can change the results and adversely affect the final statistics used in pancreatic cancer studies and clinical trials.
Introduction
A pancreaticoduodenectomy with adjuvant chemotherapy remains the only hope of a cure for localized cancers of the pancreatic head and uncinate process (UP).1,2 Pathological assessment of the pancreaticoduodenectomy (PD) specimen requires specialized knowledge of anatomy. Standardization of surgical and pathological techniques is crucial to the interpretation of the studies needed to plan adjuvant therapy for pancreatic cancer.3 However, consensus guidelines for the handling and reporting of a PD specimen are distinctly lacking.4–9 A major explanation lies in the inconsistent assessment, description and documentation of the posterior, retroperitoneal tissue/circumferential surfaces.3,9
To highlight the lack of standardization, Katz et al.3 reviewed 79 pathology reports from patients included in the multicentre ACOSOG Z5031 trial and found documentation of the ‘uncinate process margin’, ‘retroperitoneal margin’, ‘superior mesenteric artery (SMA) margin’, ‘radial margin’, ‘deep margin’ and ‘posterior margin’; all referring to margins in the retroperitoneum. Even the American Joint Committee on Cancer (AJCC)10 and the College of American Pathologists (CAP) guidelines11 appear to disagree on the designation and relative importance of tissue in this region.3 CAP11 emphasized the oncological significance of the non-peritonealized surface of the uncinate margin and posterior retroperitoneal surface of the pancreas. While the definition of the former is relatively clear, the definition of the latter lacks detailed guidance3,7,9 and as a result, in day-to-day practice, there is inconsistency and ambiguity in the evaluation and reporting of the pancreatic margin.6,9,10 Recently, the AJCC Cancer Staging Manual10 recommended using the term superior mesenteric artery (SMA) margin (SMAM) instead of the retroperitoneal or uncinate margin.
The approach to analyse the PD margins is another challenge (Table 1). The oldest and the most common approach in North America4 requires assessment of the bile duct resection (transection) margin (BDM), distal pancreatic (pancreatic neck) resection (transection) margin (PNM) and the SMAM. However, there is no consensus about pathological examination of the rest (excluding SMAM) of the posterior (retroperitoneal) surface of the PD. Staley et al.,4 Luttges et al.,5 as well as CAP11 recommend sampling of the tumour at its closest point to the posterior surface, emphasizing the importance of the assessment of mainly SMAM (CAP uses the term uncinate margin). Verbecke et al.7,12 offered a through discussion of the concept of the circumferential margin of the PD, which is divided into three components: the anterior, posterior and medial (facing superior mesenteric vessels).0008,0012 In 2009, Verbeke and Menon redefined resection margin status in pancreatic carcinoma13 by dividing the circumferential resection margin into the anterior surface, SMV surface, SMA surface and posterior surface, which was accepted by the 2010 edition of The Royal College of Pathologists of United Kingdom.14
Table 1.
Comparison of margins reported in pancreaticoduodenectomy specimen
| Margin | CAP11 | RCPUK,14 Verbeke and Menon13 | Leeds Protocol/Verbeke et al0007,0012 | Anatomical-based mapping9/author's approach |
|---|---|---|---|---|
| SMAM/uncinate (retroperitoneal) margin | + | + | − | + |
| SMVM | − | + | − | + |
| Mediala | − | − | + | − |
| Posterior | − | + | + | + |
| PNM | + | + | + | + |
| BDM | + | + | + | + |
| Anterior | − | + | + | +/−c |
| Otherb | + | − | − | − |
+ margin reported.
− margin not reported.
‘Medial circumferential resection margin, the part of the surface of the pancreatic head that faces the superior mesenteric vessels’.12
The following description is available in CAP Cancer protocols. Pancreas (exocrine), 2012:11 ‘deep retroperitoneal posterior surface of the pancreas … recommend inking the posterior surface of the pancreas and submission of sections through the tumour at its closest approach to this surface’.
In the author's opinion, the anterior margin present in a pylorus-preserving pancreaticoduodenectomy and absent in a standard Whipple procedure.
BDM, Bile duct margin; CAP, College of American Pathologists; PNM, Pancreatic neck margin; RCPUK, The Royal College of Pathologists of United Kingdom; SMAM, Superior mesenteric artery margin; SMVM, Superior mesenteric vein margin.
Similar, an anatomical-based approach was described by Khalifa et al. in 2009.9 The entire posterior retroperitoneal surface of the head of the pancreas was divided (mapping) into the uncinate margin (facing to SMA), the groove or vascular bed (facing to SMV) and the posterior surface of the UP.
The goals of this study were to (i) present the results of the prospective evaluation of the pancreatic margins, based on anatomic landmarks and (ii) demonstrate how different approaches to reporting of pancreatic margins can affect R1 status, synoptic reporting and statistical data.
Material and methods
From July 2006 to March 2011, 62 consecutive patients underwent PD at the Department of Surgery, Eastern Health, St. John's, Newfoundland, Canada. In all 62 patients, mapping of the entire retroperitoneal margin (RPM) (see Figs 1,2) was performed prospectively according to an anatomical-based mapping protocol described below. Of these 62 patients, 28 had primary pancreatic adenocarcinoma with a macroscopically free margin (R0 or R1 resection). An important part was the submission of the entire RPM in total. However, in 3 of the 28 patients, RPM was not examined in total microscopically, and, therefore these patients were excluded from the study. Twenty-five patients were included in this study.
Figure 1.
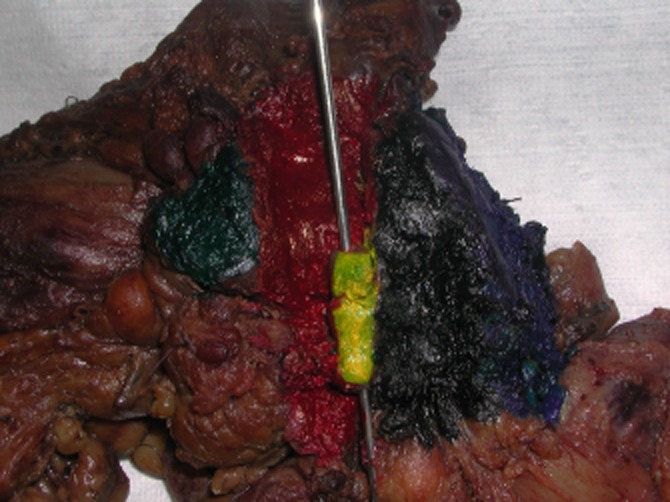
Pancreaticoduodenectomy (standard) specimen. Posterior view (mapping using different colours/dyes). Green, distal pancreatic resection margin; Red, superior mesenteric vein (vascular groove) dissection margin; Yellow, superior mesenteric vein (SMV) adhered to the posterior lateral aspect of the SMV dissection margin (the probe inserted in the lumen of the SMV); Black, SMA resection margin; Blue, posterior surface of the uncinate process dissection margin
Figure 2.
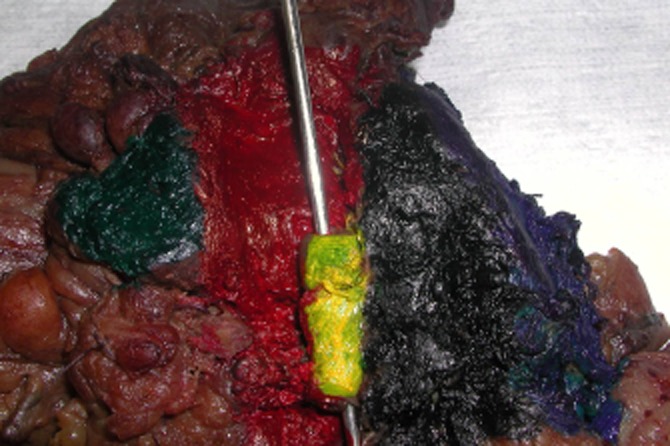
Pancreaticoduodenectomy (standard) specimen (the same case as previous Fig. 1, view from the closer distance). Posterior view (mapping using different colours/dyes). Green, distal pancreatic resection margin; Red, superior mesenteric vein (vascular groove) dissection margin; Yellow, superior mesenteric vein (SMV) adhered to the posterior lateral aspect of the SMV dissection margin (the probe inserted in the lumen of the SMV); Black, SMA resection margin; Blue, posterior surface of the uncinate process dissection margin
Standardized, anatomical-based mapping protocol for examining PD specimens
The specimen was assessed fresh by the same experienced hepato-pancreato-biliary pathologist (VM) and when necessary, it was reviewed with the surgeon. The retroperitoneal surface of the specimen was divided into the following components (margins): the SMAM, SMVM and posterior surface of the uncinate process margin (PUPM) and, if an extended PD was performed, the medial aspect (posterior surface along the neck of the pancreas). Each margin was inked with a different colour (mapping) including the corresponding superior and inferior aspect for each margin (Figs 1,2). SMAM was carefully evaluated before inking and a comment in the gross description was made if there was exposed pancreatic tissue (incomplete skeletonization of the SMA) at the margin. This could result from cutting through the tip of the UP facing the SMA and, as a result, pancreatic tissue adjacent to the SMA may be left behind in the patient. When non-exposed pancreatic tissue was found, this implied that it was more likely that complete skeletonization of the SMA took place. The stomach, duodenum and common bile duct were open and assessed in situ. After fixation in formalin for at least 24 h, gastric, duodenal, pancreatic neck margin (PNM) and bile duct margin (BDM) were submitted en face. BDM and PNM submitted in total. The entire RPM, divided into the SMAM, SMVM and PUPM, was shaved (0.5 cm thick rim of tissue), kept oriented and submitted in total for microscopic examination, serially sectioned, perpendicular to RPM, into maximum 3–5 mm thick slices, from the superior to inferior, with clear identification of orientation and identification of the superior, medial and inferior aspect. Contrary to all existing/previous protocols, the entire RPM, including the SMAM, SMVM and PUPM, was submitted in total for microscopic examination.
The ampulla of the Vater was submitted in total for microscopic examination, en block with underlying pancreas, sectioned longitudinally along the long axis of the CBD. This approach showing the relationship between CBD, main pancreatic duct, pancreas and duodenal wall in an aim to help differentiate carcinoma originating from ampulla, distal CBD and periampullary pancreatic carcinoma.
Table 1 shows the difference in margins reporting by different jurisdictions and author's approach.
Distance of the tumour from the closest margin was measured microscopically. A microscopically measured 1-mm tumour-free zone was used as the cutoff. A presence of the tumour at the margin (0 mm rule) as is recommended by CAP or at 1 mm or less (1 mm rule) was documented for the purpose of this study.
Results
Of the 25 patients (12 women and 13 men, median age 65.5 years; range 46–76), 24 patients underwent a standard PD (in 1 cases standard PD was transformed in total pancreatectomy) and 1 patient underwent a pylorus-preserving PD. There were 4 patients with vascular resection. Frozen sections from the BDM and pancreatic neck RM were performed upon macroscopic suspicion of tumour involvement in 15 and 18 cases, respectively. None of the patients died within 30 days of surgery.
The results of R1 status by definition are shown in Table 2.
Table 2.
Margin involvement
| Margin | Based on CAP approach | Based on author's approach | |
|---|---|---|---|
| (0 mm rule) | (1 mm rule) | ||
| SMAM | 9a | 9a | 14 |
| SMVM | - | 9 | 18 |
| PUPM | - | 1 | 5 |
| PNM | 1 | 1 | 1 |
| BDM | 1 | 1 | 1 |
| Total R1 | 9b | 14b | 20b |
In one patient, the SMAM was involved by metastatic carcinoma in a lymph node and this patient was not included as it was considered as locoregional metastasis.
Owing to multifocality of resection margin involvement within the same specimen in some patients, the total number of involved margin exceeds the total number of patients with an R1 margin.
BDM, Bile duct margin; CAP, College of American Pathologists; PNM, Pancreatic neck margin; PUPM, Posterior surface of the uncinate process margin; SMAM, Superior mesenteric artery margin; SMVM, Superior mesenteric vein margin.
The most frequently involved margins were SMAM and SMVM. Based on 1-mm rule SMVM was involved in 18 out of 20 patients with R1 resection and SMAM in 14 out of 20 patients with an R1 margin. More details are shown in Table 3.
Table 3.
The number of patients with retroperitoneal margin involvement by definition
| Based on definition by CAP (0 mm rule) | Based on 1 mm rule | |
|---|---|---|
| SMAM only | 5 | 2 |
| SMVM only | 5 | 5 |
| SMAM and SMVM | 3 | 8 |
| TOTAL (R1) cases | 13 | 15 |
| SMAM ± SMVM & PUPM | 1 | 5 |
| PUPM only | - | - |
| Total (R1) | 14 | 20 |
CAP, College of American Pathologists; PUPM, Posterior surface of the uncinate process margin; SMAM, Superior mesenteric artery margin; SMVM, Superior mesenteric vein margin.
The number of margins involved based on definition is shown in Table 4.
Table 4.
The number of margins involved by definition of retroperitoneal margin involvement
| Number of involved RP margins | Based on definition by CAP (0 mm rule) | Based on 1 mm rule |
|---|---|---|
| 1 | 10 | 7 |
| 2 | 3 | 9 |
| 3 | 1 | 4 |
| Total (R1) | 14 | 20 |
CAP, College of American Pathologists.
Discussion
PNM and/or BDM are usually assessed intra-operatively by frozen sections and it is unusual to have positive margins on permanent sections unless a frozen section examination was not done. The main focus of this study was a RPM which is the entire posterior, deep surface of the pancreas. Assessment and reporting of the RPM remain controversial and performed differently.3 CAP emphasize the importance of SMAM and recommends reporting involvement of the rest of deep retroperitoneal posterior surface of the head of the pancreas without attempting to map and report separately involvement of SMVM and PUPM.11
The aim of this study was to investigate how assessment of the RPM using an anatomical-based mapping approach of sampling9,15,16 can affect final reporting and hence the number of patients with an R1 resection. To achieve this, the entire RPM, divided into the SMAM, SMVM and PUPM, was assessed microscopically in total, which is currently followed in only few centres.17–19 The existing literature is predominantly based on the assessment of SMAM only20 or with ‘submission of section through the tumour at its closest point to deep retroperitoneal posterior surface’,11 which is often called the ‘peripancreatic margin’.0021,0022 Following this trend and applying the 0-mm rule, the number of patients with a R1 resection in the current series would have been 9 out of 25, these figures correlates with the existing literature (16%-36%) based on a large series where only assessment of the SMAM20 or peripancreatic tissue has been performed.0021,0022 However, extending the examination by assessment and reporting of the entire RPM while still adopting the 0-mm rule, would increase the number of patients with R1 resection to 14 out of 25 patients.
There is lack of consensus for the definition of margin involvement. It ranges from 0 mm definition in North America11 to 1 mm in Europe0012,0023 and Japan.24 Applying the 1-mm rule, the number of patients with R1 resection in the current series increased from 14 to 20 patients. The result is very similar to the R1 rate reported by Verbeke et al.7 and Esposito et al.8 and correlates with the high (67% to 86%) local recurrence rate.7,8,25 Recent discussions related to the work of Hernandez et al.,22 who showed that survival after PD is not improved by extending resections to achieve negative margins, indicated that controversies still exist and a 1.5-mm clearance might be needed to obtain a long-term survival benefit.26
It is a common belief that SMAM is the most commonly involved margin,0020,0025 but often it is based on the protocols examining only the BDM, PNM and SMAM.20 In the current series, the most frequently involved margins were SMVM, SMAM and less often the PUPM. Interestingly, the SMAM was involved as often as the SMVM (9 patients), but based on the 1-mm rule, the SMVM was involved in 18 out of 20 patients with an R1 resection and SMAM in 14 out of 20 patients with an R1 resection. The SMAM, SMVM or both were involved in 13 out of 14 patients with an R1 resection and based on the 1-mm rule in 15 out of the 20 patients with an R1 resection. The PUPM was involved less often and it was never a single source of R1 status. It is related to the nature of the anatomy, surgery and criteria for the selection of patients. Very often owing to the close anatomical relationship of the retroperitoneal surface to the SMA and SMV it is impossible or difficult to reach a negative margin and many surgeons are still hesitant to proceed with en block resection, especially if it was not planned in advance. The situation is different along the posterior surface of the uncinate process, which is separated by fat from the underlying structures such as the inferior vena cava and it is much easier to obtain a clear margin.
Two or more margins were involved only in 4 out of 14 patients with a R1 resection. However, using the 1-mm rule would increase the number patients with two or more positive margins to 13 out of 20 patients. This finding indicates that careful examination of the entire RPM is important to accurately identify patients with an R1 resection. Clearly this has important implications for clinical trials as it could alter the R1 status dramatically.
Coexisting fibrosis might be a misleading feature during gross assessment and selection of sections. At the same time, invasion of the peripancreatic adipose tissue by single malignant glands cannot be grossly identifiable (Fig. 3) therefore highlighting the importance of complete assessment of the RPM.
Figure 3.
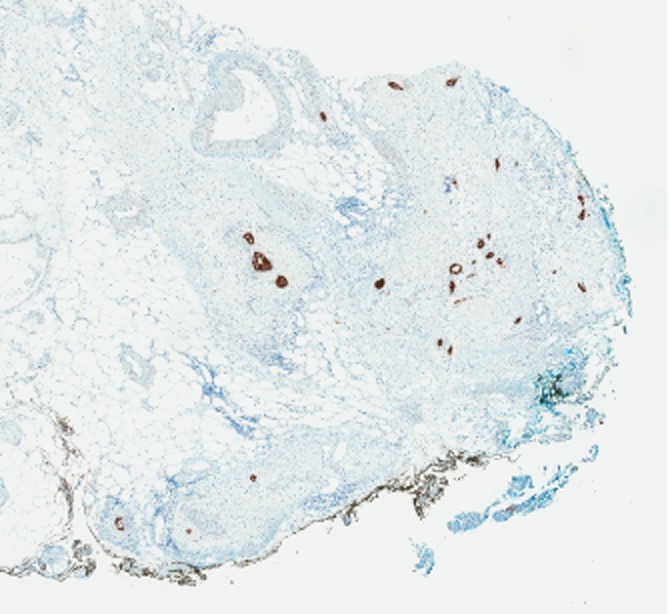
Invasion of the peripancreatic adipose tissue by single malignant glands (black coloured structures highlighted by keratin stain AE1/AE3) along the SMA margin, marked by black ink, sectioned perpendicular to the resection margin (see standardized protocol). AE1/AE3 keratin stain, original magnification 2×
The described approach can reasonably be adopted in specialized centres that developed enough expertise and confidence in gross assessment. As it is now widely accepted that the SMAM can be identified, selection, recognition and inking of other retroperitoneal zones (surfaces) should not be too difficult. It is the author's view that it is much easier to recognize the SMAM in patients with incomplete skeletonization of the SMA than in the patients with complete skeletonization. Surgeons can also be encouraged to mark the SMAM with a suture during surgery. In the author's experience, borderline resectable patients where this particular issue is important, the SMAM is easier to identify. The critical assessment of this margin, particularly in the intra-operative setting, has already been described in detail.27
In conclusion, this study illustrates that different approaches to the assessment and reporting of the RPM can completely change the frequency of R1 status within a series and affect the final understanding in related clinical trials. Involvement of the SMVM and SMAM should be included in standard protocols. It is important for quality assurance and for providing reliable feedback.
Acknowledgments
No financial support was received.
Conflicts of interest
The authors have no potential conflicts of interest.
References
- 1.Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointestinal Surg. 2006;10:1338–1345. doi: 10.1016/j.gassur.2006.09.008. et al; discussion 13451346. [DOI] [PubMed] [Google Scholar]
- 2.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz MH, Merchant NB, Brower S, Branda M, Posner MC, William Traverso L. Standardization of surgical and pathologic variables is needed in multicenter trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Ann Surg Oncol. 2011;18:337–344. doi: 10.1245/s10434-010-1282-y. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staley CA, Cleary KR, Abbruzzese JL, Lee JE, Ames FC, Fenoglio CJ. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–380. doi: 10.1097/00006676-199605000-00009. et al. [DOI] [PubMed] [Google Scholar]
- 5.Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas. A proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999;16:291–296. doi: 10.1159/000018738. [DOI] [PubMed] [Google Scholar]
- 6.Chatelain D, Flejou JF. Pancreatectomy for adenocarcinoma: prognostic factors, recommendations for pathological reports. Ann Pathol. 2002;22:422–431. [PubMed] [Google Scholar]
- 7.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 8.Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H. Most Pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. et al. [DOI] [PubMed] [Google Scholar]
- 9.Khalifa MA, Maksymov V, Rowsell C. Retroperitoneal margin of the pancreaticoduodenectomy specimen: anatomic mapping for the surgical pathologist. Virchows Arch. 2009;454:125–131. doi: 10.1007/s00428-008-0711-9. [DOI] [PubMed] [Google Scholar]
- 10.American Joint Committee on Cancer. Exocrine pancreas. In: Edge SB, Byrd DR, Compton CC, editors. 7th edn. Chicago: Springer; 2009. pp. 241–249. . In: et al., eds. AJCC Cancer Staging Manual, pp. [Google Scholar]
- 11.College of American Pathologists Cancer Protocols. 2012. Pancreas (exocrine). Available at http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/PancreasExo_12protocol_3200.doc (last accessed 9 September 2012).
- 12.Verbeke CS. Resection margin and R1 rates in pancreatic cancer-are we there yet? Histopathology. 2008;52:787–796. doi: 10.1111/j.1365-2559.2007.02935.x. [DOI] [PubMed] [Google Scholar]
- 13.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB. 2009;11:282–289. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Royal College of Pathologists of United Kingdom. 2010. Standards and datasets for reporting cancers. Data set for the histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct. May . Available at http://www.rcpath.org/NR/rdonlyres/954273A2-3F01-4B97-B0F6-C136231DF65F/0/datasethistopathologicalreportingcarcinomasmay10.pdf (last accessed 9 September 2012).
- 15.Maksymov V, Khalifa MA, Rowsell CH. Grossing and evaluation of the retroperitoneal resection margin in the pancreaticoduodenectomy specimen: consensus needed. p. 165. 8th Congress of the European Hepato Pancreato Billiary Association, June 1820, 2009, Athens, Greece, p.
- 16.Maksymov V, Khalifa MA, Rowsell CH. Grossing, evaluation and reporting of the retroperitoneal resection margin in the pancreaticoduodenectomy specimen. European Journal of Surgical Oncology. 2010;36:895. [Google Scholar]
- 17.Willett CG, Lewandrowski K, Warshaw AL, Efird J, Compton CC. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg. 1993;217:144–148. doi: 10.1097/00000658-199302000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagakawa T, Konishi I, Ueno K, Ohta T, Akiyama T, Kanno M. The results and problems of extensive radical surgery for carcinoma of the head of the pancreas. Jpn J Surg. 1991;21:262–267. doi: 10.1007/BF02470944. et al. [DOI] [PubMed] [Google Scholar]
- 19.Nagakawa T, Sanada H, Inagaki M, Sugama J, Ueno K, Konishi I. Long-term survivors after resection of carcinoma of the head of the pancreas: significance of histologically curative resection. J Hepatobiliary Pancreat Surg. 2004;11:402–408. doi: 10.1007/s00534-004-0917-4. et al. [DOI] [PubMed] [Google Scholar]
- 20.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O. Resectable adenocarcinomas in the pancreatic head: the retroperitoneal resection margin is an independent prognostic factor. BMC Cancer. 2008;8:5. doi: 10.1186/1471-2407-8-5. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez J, Mullinax J, Clark W, Toomey P, Villadolid D, Morton C. Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann Surg. 2009;250:76–80. doi: 10.1097/SLA.0b013e3181ad655e. et al. [DOI] [PubMed] [Google Scholar]
- 23.Esposito I, Born D. Pathological Reporting and staging following pancreatic cancer resection. In: Neoptolemos JP, Urritia R, Abbruzzese JL, Buchler MW, editors. New York: Springer; 2010. pp. 1010–1034. . In: , eds. Pancreatic Cancer, pp. [Google Scholar]
- 24.Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219–230. doi: 10.1097/00006676-200404000-00002. et al. [DOI] [PubMed] [Google Scholar]
- 25.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang DK, Johns AL, Merrett ND, Gill AJ, Colvin EK, Scarlett CJ. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;7:2855–2862. doi: 10.1200/JCO.2008.20.5104. et al. [DOI] [PubMed] [Google Scholar]
- 27.Khalifa MA, Maksymov V, Rowsell CH, Hanna S. A novel approach to the intraoperative assessment of the uncinate margin of the pancreaticoduodenectomy specimen. HPB. 2007;9:146–149. doi: 10.1080/13651820701278273. [DOI] [PMC free article] [PubMed] [Google Scholar]


