Abstract
Rationale
Impulsive behavior is categorically differentiated between impulsive action, the inability to withhold from acting out a response, and impulsive choice, the greater preference for an immediate and smaller reward over a delayed but more advantageous reward. While the effects of N-methyl-D-aspartic acid (NMDA) receptor antagonists on impulsive action have been extensively characterized, there are very few and conflicting reports on the effects of this class of drugs on impulsive choice.
Objectives
Using a modified adjusting delay task, we investigated the effects of uncompetitive and competitive blockade of NMDA receptors on impulsive choice.
Methods
Male Wistar rats were trained in a modified adjusting delay task, which involved repeated choice between a low reinforcing solution delivered immediately and a highly reinforcing solution delivered after a variable delay. Rats were then administered either the NMDA receptor uncompetitive antagonists ketamine or memantine, or the competitive antagonists D-AP-5 or CGS 19755.
Results
Ketamine treatment dose-dependently increased impulsive choice, and this effect was selective for low-impulsive but not high-impulsive rats. Similarly, memantine treatment dose-dependently increased impulsive choice with a preferential effect for low-impulsive rats. While D-AP-5 treatment did not affect impulsive choice, CGS 19755 increased impulsivity, however, at the same doses at which it caused a marked response inhibition.
Conclusions
NMDA receptor uncompetitive, but not competitive, antagonists significantly increased impulsive choice, preferentially in low-impulsive rats. These findings demonstrate that the effects of NMDA receptor blockade on impulsive choice are not generalizable and depend on the specific mechanism of action of the antagonist used.
Keywords: Impulsivity, Delay discounting, Ketamine, Memantine, D-AP-5 or AP-5 or D-AP5 or AP5, CGS 19755, Glutamate, NMDA receptor antagonist, Decision making, Impulsive choice
Introduction
Impulsivity is a personality construct defined as the tendency for rapid, unplanned reactions to stimuli with diminished regard to the negative consequences of these reactions (Chamberlain and Sahakian 2007; Potenza 2007). Impairment of impulsive behavior is thought to play a role in the psychopathology of many psychiatric disorders such as schizophrenia, major depression, addictive disorders, post-traumatic stress disorder, and attention deficit hyperactivity disorder (Heresco-Levy and Javitt 1998; Moeller et al. 2001).
As a multifaceted personality trait, impulsivity is comprised of a number of dimensions typically classified into two main categories: impulsive action and impulsive choice (Evenden 1999). Impulsive action can be defined as the inability to withhold from acting out a response, while impulsive choice is marked by a greater preference for an immediate and smaller reward over a delayed but more advantageous reward (Mar and Robbins 2007; Winstanley et al. 2006).
The N-methyl-D-aspartic acid (NMDA) receptor system is involved in numerous physiological and pathological processes in the central nervous system (synaptic plasticity, learning and memory, attention, excitotoxicity, and neuro-degeneration) (Heresco-Levy and Javitt 1998); it has received increased attention regarding its potential role in the control of impulsive behavior. Blockade of NMDA receptors has been shown to precipitate impulsivity in humans and animals (Breier et al. 1997; Floresco et al. 2008; Higgins et al. 2003; Malhotra et al. 1997), raising the hypothesis that a decreased glutamate/NMDA function may contribute to diminished inhibition of behavior in psychiatric disorders (Adams et al. 2004; Amitai and Markou 2010; Pattij and Vanderschuren 2008; Turic et al. 2004). NMDA receptors are heteromeric ligand-gated ion channels which show a complex pharmacological profile and require the simultaneous binding of glutamate and glycine for activation (Johnson and Kotermanski 2006). Given the pharmacological complexity of this ion channel receptor, numerous classes of antagonists have been described and differentiated based on their inhibitory mechanism of action: a first class of competitive antagonists which bind to the glutamate binding site; a second class of competitive antagonists which bind to the glycine binding site; uncompetitive antagonists which require that the receptor pore be open to allow binding to internal sites, different from those of the agonists; non-competitive antagonists which bind to sites different from those of the agonists to all the receptor states; finally, negative allosteric modulators which bind to other modulatory sites of the receptor (Monaghan et al. 2012; Ogden and Traynelis 2011; Traynelis et al. 2010).
The effects of NMDA receptor blockade on impulsive action have been extensively characterized (Carli et al. 2006; Fletcher et al. 2011; Murphy et al. 2005; Pozzi et al. 2011). However, to the best of our knowledge, very few studies have investigated the effects of antagonism of NMDA receptors on impulsive choice (Floresco et al. 2008; Oberlin et al. 2010). Indeed, only the uncompetitive class of NMDA receptor antagonists has been tested in impulsive choice, and the reported findings have been conflicting (Floresco et al. 2008; Oberlin et al. 2010). In addition, the effects of competitive NMDA receptor antagonists on intolerance to delay of reinforcement have not yet been investigated.
Therefore, the first aim of this study was to investigate the effects of two uncompetitive NMDA receptor antagonists, ketamine and memantine, on impulsive choice by using a modified adjusting delay task. The second aim was to investigate the effects of two competitive antagonists, DAP-5 and CGS 19755, on the same behavioral task.
Materials and methods
Subjects
Male Wistar rats, weighing 180–230 g and 41–47 days old at arrival (Charles River, Wilmington, MA), were double-housed in wire-topped, plastic cages (27×48×20 cm) on a 12-h reverse light cycle (lights off at 10:00 am) in an AAALAC-approved humidity-controlled (60 %) and temperature-controlled (22 °C) vivarium. Rats had access to corn-based chow (Harlan Teklad LM-485 Diet 7012 (65 % [kilocalories] carbohydrate, 13 % fat, 21 % protein, metabolizable energy 341 cal/100 g; Harlan, Indianapolis, IN) and water ad libitum at all times unless otherwise specified. Procedures adhered to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH publication number 85–23, revised 1996), the Principles of Laboratory Animal Care, and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee.
Drugs
Ketamine hydrochloride ((±)-2-(2-chlorophenyl)-2-(methyl-amino)cyclohexanone hydrochloride) was purchased from Sigma-Aldrich (St. Louis, MO); memantine hydrochloride (1,3-dimethyl-5-aminoadamantane hydrochloride) was purchased from Acros Organics (Geel, Belgium); D-AP-5 ((R)(−)-2-amino-5-phosphonopentanoic acid) and CGS 19755 (cis-4-phosphonomethyl-2-piperidine carboxylic acid) were obtained from the NIMH’s Chemical Synthesis and Drug Supply Program. All drugs were freshly dissolved in isotonic saline and administered systemically using a volume of 1 mL/kg, except CGS 19755 which was administered using a volume of 2 mL/kg. D-AP5 for intracerebroventricular administration was dissolved in sterile isotonic saline and administered in a volume of 5 μL/rat. All doses were calculated as weight of salt. Ketamine and memantine block the NMDA receptor in an uncompetitive manner (Ki=~0.5 μM Ki =~1 μM, respectively) (Ebert et al. 1997; Parsons et al. 1999; Traynelis et al. 2010). D-AP-5 and CGS 19755 block the NMDA receptor in a competitive manner (Ki=~1.93 μM and Ki =~0.34 μM, respectively) (Benveniste and Mayer 1991; Traynelis et al. 2010). Angiotensin II was generously provided by Dr. Jean Rivier (The Salk Institute, La Jolla, CA, USA).
Apparatus
The test chambers (Med Associates, Inc., St. Albans, VT) had grid floors and were located in sound-attenuating, ventilated environmental cubicles (Cottone et al. 2009, 2007b). Two syringe pumps dispensed the solutions into two stainless steel drinking cups mounted 2 cm above the grid floor in the middle of an aluminum panel (Sabino et al. 2006). Two retractable levers were located 3.2 cm to either side of the drinking cups, and two 28-V stimulus cue lights were located above each lever. Liquid delivery and operant responses were recorded automatically by a microcomputer with a 10-ms resolution.
Modified adjusting delay task procedure
The modified adjusting delay task procedure was performed as previously described (Blasio et al. 2011). The procedure was adopted from Dr. Perry and colleagues (Perry et al. 2011, 2005) and modified to eliminate typical energy–homeostasis limitations induced by food restriction or by the use of reinforcers with dissimilar caloric content. In the present modified adjusting delay task, subjects were fed and watered ad libitum, and the reinforcers were isocaloric as we described previously (Blasio et al. 2011). Subjects were allowed two overnight fixed ratio 1 sessions (Sabino et al. 2011), with each of the two levers yielding 0.1 mL of 1.5 % glucose solution. The rats were then moved to the next training phase. Beginning 3–4 h into the dark cycle, the rats were transferred to the testing room daily and individually placed in the test chambers. The procedure consisted of a 30-min pre-session and a 15-block session, the latter ending either after 2 h or after completion of the 15 blocks (whichever occurred first). Therefore, the procedure lasted no longer than 2.5 h. During the 30-min pre-session, levers were in the retracted position, and the stimulus lights were off. This pre-session allowed subjects to acclimate to the chambers, as well as to signal the imminent availability of the reinforcers. During the training phase, each of the 15 blocks consisted of four forced-choice trials. Forced-choice trials were signaled by the extension of the response-appropriate lever and the illumination of the stimulus light above it. In forced-choice trials, levers retracted immediately following a lever press response. A single response on one lever yielded 0.1 mL of the glucose solution delivered immediately while a response on the other lever yielded 0.1 mL of the supersaccharin (1.5 % glucose + 0.4 % saccharin) solution delivered after a constant delay of 6 s. Supersaccharin solution serves as a potent reinforcer and makes it unnecessary to water restrict animals to induce a very high liquid intake (Blasio et al. 2011; Roberto et al. 2010; Sabino et al. 2009; Valenstein et al. 1967). Levers retracted at the end of each four-trial block. Each rat was then moved to the modified adjusting delay task as soon as it learned to complete at least ten out of the 15 trials. In the modified adjusting delay task, the first and second trials of each block were forced-choice while the third and fourth were free-choice trials. In the free-choice trials, rats were given the opportunity to choose between the two levers. The free-choice trials were signaled by illumination of the stimulus lights above both levers. The initial delay to the delivery of the more preferred reinforcer was 6 s and was adjusted only after the third and fourth trials in each block, depending on which lever was chosen by the subject. A response on the immediately reinforcing lever resulted in a 1-s decrease in the delay of the supersaccharin solution’s delivery while a response on the delayed reinforcement lever resulted in a 1-s increase in the delay of the super-saccharin solution’s delivery. The lower and upper limits of the delay were set to 0 and 36 s, respectively. Following each lever press, an inter-trial interval (ITI) was imposed so that each trial would last 36 s (i.e., Delay+ITI=36 s). During the ITI, the stimulus lights were turned off, and responses on the levers had no programmed consequences. During the delay, the stimulus lights above each lever were turned off. The delivery of the glucose solution was kept constant. A mean adjusted delay (MAD) was calculated at the end of each session by averaging all of the adjusting delays on the free-choice trials, and this procedure was repeated until the MAD stabilized (varying by less than 3 s across 5 days with no consistent increasing or decreasing trends). The MAD ranged between 0.5 s (in the event that the immediately reinforcing lever was always chosen during the 30 free-choice trials) and 21.5 s (in the event that the adjusting delay lever was always chosen). The MAD values were used as a quantitative measure of impulsive choice, with lower MADs indicating higher levels of impulsive choice. The MADs were transformed into impulsivity scores (ISs) using the following mathematical expressions:
Therefore, IS is a dimensionless variable in a linear inverse relationship with MAD. IS is a more intuitive variable relative to MAD and ranges between 0 and 1, representing the lowest and highest degree of impulsivity, respectively (Blasio et al. 2011).
Intracranial surgery and microinfusion procedures
Following training in the modified adjusting delay task and performance stabilization, rats were implanted intracerebro-ventricular (i.c.v.) guide cannulas. Intracranial surgeries were performed as previously described (Cottone et al. 2007a; Sabino et al. 2007). Briefly, for cannulation, anaesthetized (isoflurane, 2–3 % in oxygen) subjects were secured in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) and a stainless steel, 24-gauge guide cannula (Plastics One, Inc., Roanoke, VA, USA) was lowered above the third ventricle. Four stainless steel jeweler’s screws were fastened to the rat’s skull around the cannula. Dental restorative filled resin (Henry Schein, Inc., Melville, NY, USA) and acrylic cement were applied, forming a pedestal which firmly anchored the cannula (Iemolo et al. 2012). The cannula was placed 1 mm posterior to the bregma, ±1.5 mm lateral to midline, terminating 2.3 mm below the outer surface of the skull with the interaural bar set at flat skull (dorsal/ventral: bregma = lamda). Coordinates were based on the atlas of Paxinos and Watson (Paxinos and Watson 1986). A 31-gauge stainless steel dummy stylet (Plastics One, Inc., Roanoke, VA, USA) maintained patency. After surgery, the rats were returned to their cages for a 1-week recovery period, during which they were handled daily.
Drug treatments
The uncompetitive antagonists ketamine (0, 2.5, 5, 10, 20 mg/kg, intraperitoneal (i.p.); n=17) and memantine (0, 1.25, 2.5, 5 and 10 mg/kg, i.p.; n=12), and the competitive antagonists D-AP-5 (0, 2.5, 5, 10, 20 mg/kg, subcutaneous (s.c.); n=12 for the systemic experiment) and CGS 19755 (0, 2.5, 5, 10, 20 mg/kg, s.c.; n=11) were administered 30 min prior to the beginning of the session’s first block (immediately prior to the pre-session).
For i.c.v. microinfusions of D-AP-5 (0, 7.5, 15, 30 μg, i.c.v.; n=9), the dummy stylet was removed from the guide cannula and was replaced with a 31-gauge stainless steel injector projecting 2.5 mm beyond the tip of the guide cannula which was connected via PE 20 tubing to a Hamilton microsyringe driven by a microinfusion pump (Kd Scientifics/Biological Instruments, Holliston, MA, USA). D-AP-5 was administered 30 min prior to the beginning of the session’s first block (immediately prior to the pre-session). Microinjections were performed in 5-μL volume delivered over 1 min; injectors were left in place for an additional minute to minimize backflow. Immediately after, each rat was moved to the test chamber. Cannula placement was functionally verified at the beginning and the conclusion of all testing as a positive dipsogenic response (>5 mL of water intake within 30 min) to i.c.v. angiotensin II (25 ng/5 μL).
All of the treatments were given in full Latin square designs with 1–3 intervening treatment-free test days, in which the MAD returned to baseline. Rats were given 3 days of acclimation to daily sham injections, prior to the beginning of drug treatments.
Statistical analysis
The effects of ketamine, memantine, D-AP-5, and CGS 19755 on MAD, IS, latency, and total responses were analyzed using one-way repeated measures analyses of variance (ANOVAs) with dose as a within-subject factor. Latency to respond was not a normally distributed variable, so the values were ln-transformed for statistical analysis. Pairwise dose effects were interpreted using Fisher’s least significant difference (LSD) tests. To determine subpopulations of low-impulsive and high-impulsive rats, a median split analysis of MADs was performed. For this purpose, in each of the drug treatment studies, subjects were ranked based on their MAD under vehicle conditions, and the median was calculated. All of the subjects with a MAD ≤ median were included in the high-impulsive group while the rest of them were included in the low-impulsive group. The effects of ketamine, memantine, D-AP-5, and CGS 19755 on MAD, IS, latency, and total responses in low-impulsive and high-impulsive rats were analyzed using two-way split-plot ANOVAs with dose as a within-subject factor and impulsivity as a between-subjects factor. The statistical/graphic software packages used were SigmaPlot 11.0 (Systat Software, Inc., Chicago, IL) and Statistica 7.0 (Statsoft, Inc., Tulsa, OK).
Results
Effects of the uncompetitive NMDA receptor antagonist ketamine on impulsive choice in a modified adjusting delay task
Ketamine significantly and dose-dependently affected MAD and IS (dose: F(4,64)=6.24, p<0.0005; Fig. 1a, b). Post hoc analysis revealed that rats treated with the 10- and 20-mg/kg doses showed significantly lower MAD and higher IS compared to those treated with vehicle. The effect on impulsive choice exerted by ketamine was not secondary to motor impairment or to an overall behavioral disruption, since the drug treatment did not affect the latency to respond (dose: F(4,64)=0.60, not significant (n.s.); Fig. 1c) or the number of completed trials (dose: F(4,64)=0.42, n.s.; Table 1). Interestingly, median split subgroup analysis (vehicle (veh)/low-impulsive vs. veh/high-impulsive, p= 0.0006) showed that ketamine treatment selectively affected impulsive choice in low-impulsive rats by increasing the choice for the immediate reinforcer without affecting MAD in high-impulsive rats (dose: F(4,60)=7.38, p< 0.001; Dose×Impulsivity: F(4,60)=2.98, p<0.05; Fig. 2). Ketamine treatment significantly increased impulsive choice in low-impulsive rats, when injected at the 10- and 20-mg/kg doses by ~27 % and ~51 %, respectively, compared to vehicle condition.
Fig. 1.
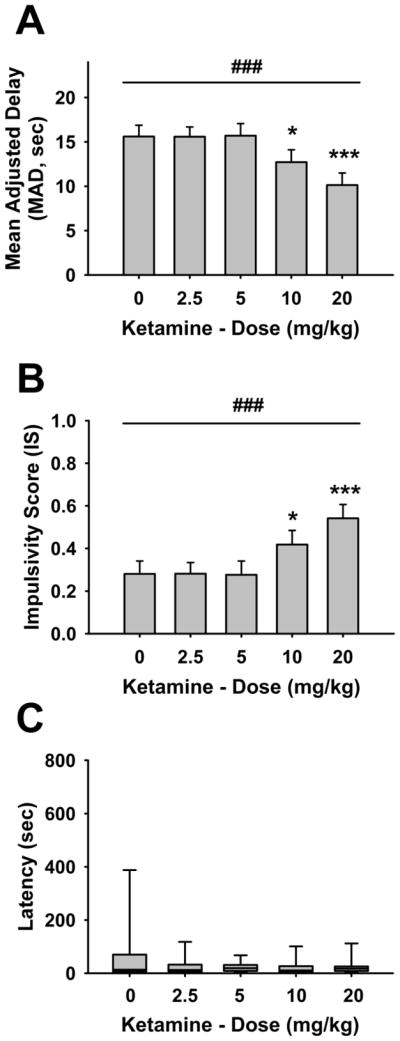
Effects of pretreatment with the uncompetitive NMDA receptor antagonist ketamine (n=17, the entire group of rats before being split into low-impulsive and high-impulsive) (i.p., 30 min prior to the beginning of the first block of the session) on a mean adjusted delay (MAD) and b impulsivity score (IS), in male Wistar rats. Panels (a) and (b) show M ± SEM. Panel (c) shows the box and whisker plots of latency where the boundaries indicate the 25th and 75th percentiles, the line within the box marks the median, and the whiskers (error bars) indicate the 90th and 10th percentiles. Symbols denote significant differences from vehicle: *p<0.05; ***p<0.001 (Fisher’s LSD test); significant main effect of dose ###p<0.001
Table 1.
Number of completed trials following treatments with uncompetitive and competitive NMDA receptor antagonists
| Dose
|
|||||||
|---|---|---|---|---|---|---|---|
| Vehicle | 1.25 mg/kg | 2.5 mg/kg | 5 mg/kg | 10 mg/kg | 20 mg/kg | ||
| ANOVA | |||||||
| Drug | |||||||
| Ketamine | n.s. | 26.6±1.7 | – | 27.2±1.3 | 28.2±0.8 | 27.8±1.3 | 28.5±0.8 |
| Memantine | n.s. | 24.8±1.9 | 25.5±1.9 | 28.5±1.1 | 27.7±1.6 | 27.7±1.6 | – |
| CGS 19755 | –* | 24.5±2.3 | – | 24.2±2.4 | 24.5±2.2 | 22.5±2.7 | 13.8±3.3** |
Data are expressed as M ± SEM. Symbols denote significant differences from vehicle. Effects of pretreatment with ketamine, memantine, and CGS 19755 on the number of completed trials in male Wistar rats, n.s. not significant,
p<0.05;
p<0.01, significant main effect of dose and Fisher’s LSD test, respectively
Fig. 2.
Effects of pretreatment with the uncompetitive NMDA receptor antagonist ketamine (n=8–9/group) (i.p., 30 min prior to the beginning of the first block of the session) on mean adjusted delay (MAD), in low-impulsive and high-impulsive male Wistar rats. Panels show M ± SEM. Symbols denote significant differences from vehicle: *p<0.05; ***p< 0.001 (Fisher’s LSD test); significant main effect of dose ###p<0.001
Effects of the uncompetitive NMDA receptor antagonist memantine on impulsive choice in a modified adjusting delay task
The uncompetitive antagonist memantine significantly and dose-dependently affected MAD and IS (dose: F(4,44)=16.85 p<0.0001; Fig. 3a, b). Post hoc analysis revealed that rats treated with the 5- and 10-mg/kg doses showed significantly lower MAD and higher IS compared to the vehicle treated rats. No significant effect of drug treatment was observed on latency (dose: F(4,44)=1.76, n.s.; Fig. 3c) or on number of completed trials (dose: F(4,44)=1.08, n.s.; Table 1). Conversely to what was observed with the ketamine treatment, median split subgroup analysis (veh/low-impulsive vs. veh/high-impulsive, p=0.00008) showed that the effect of memantine treatment on impulsive choice did not differ between low-impulsive and high-impulsive rats (dose: F(4,40)=17.82, p<0.001; Dose×Impulsivity: F(4,40)= 1.63, n.s.; Fig. 4). Indeed, memantine injected at 10 mg/kg dramatically decreased MAD by ~71 % and ~38 % in low-impulsive and high-impulsive rats, respectively. However, at the 5-mg/kg dose, memantine reduced MAD by ~33 % in low-impulsive rats but not in high-impulsive rats, suggesting that perhaps there is an increased potency of the drug in rats with the tendency to choose the large, delayed reinforcer.
Fig. 3.

Effects of pretreatment with the uncompetitive NMDA receptor antagonist memantine (n=12, the entire group of rats before being split into low-impulsive and high-impulsive) (i.p., 30 min prior to the beginning of the first block of the session) on a mean adjusted delay (MAD) and b impulsivity score (IS), in male Wistar rats. Panels (a) and (b) show M ± SEM. Panel (c) shows the box and whisker plots of latency where the boundaries indicate the 25th and 75th percentiles, the line within the box marks the median, and the whiskers (error bars) indicate the 90th and 10th percentiles. Symbols denote significant differences from vehicle: **p<0.01; ***p<0.001 (Fisher’s LSD test); significant main effect of dose ###p<0.001
Fig. 4.
Effects of pretreatment with the uncompetitive NMDA receptor antagonist memantine (n=6/group) (i.p., 30 min prior to the beginning of the first block of the session) on mean adjusted delay (MAD), in low-impulsive and high-impulsive male Wistar rats. Panels show M ± SEM. Symbols denote significant differences from vehicle: **p<0.01; ***p<0.001 (Fisher’s LSD test); significant main effect of dose #p<0.05; ###p<0.001
Effects of the competitive NMDA receptor antagonist D-AP-5 on impulsive choice in a modified adjusting delay task
Following systemic treatment with the competitive NMDA antagonist D-AP-5, no significant effect was observed in either MAD or IS (dose: F(4,44)=1.42, n.s.; Table 2). No significant effect of D-AP-5 treatment was observed on latency (dose: F(4,44)=0.75, n.s.; Table 2) or on number of completed trials (dose: F(4,44)=1.10, n.s.; Table 2). DAP-5 treatment did not affect impulsive choice (dose: F (4,40)=1.31, n.s.; Dose×Impulsivity: F(4,40)=0.20, n.s.; not shown), latency (dose: F(4,40)=1.31, n.s.; Dose× Impulsivity: F(4,40)=0.05, n.s.; not shown), or number of completed trials (dose: F(4,40) =1.07, n.s.; Dose × Impulsivity: F(4,40)=0.72, n.s.; not shown) in low-impulsive and high-impulsive rats (veh/low-impulsive vs. veh/high-impulsive, p=0.0008).
Table 2.
Effects of subcutaneous and intracerebroventricular treatment with the competitive NMDA receptor antagonist AP-5 on the modified adjusting delay task
| Route | Dose | Parameter MAD | IS | Latency | Number of completed trials |
|---|---|---|---|---|---|
| Subcutaneous | |||||
| ANOVA | n.s. | n.s. | n.s. | n.s. | |
| Vehicle | 14.4±1.4 | 0.3±0.1 | 63.7 [8.6, 15.3, 119.4, 156.4] | 23.2±1.8 | |
| 2.5 mg/kg | 13.8±1.3 | 0.4±0.1 | 75.2 [5.1, 12.5, 129.4, 506.4] | 21.8±2.1 | |
| 5 mg/kg | 13.9±1.3 | 0.4±0.1 | 86.1 [8.7, 19.6, 144.8, 267.5] | 21.8±1.6 | |
| 10 mg/kg | 14.4±1.6 | 0.3±0.1 | 46.2 [7.0, 23.4, 185.2, 419.3] | 22.0±2.4 | |
| 20 mg/kg | 15.7±1.3 | 0.3±0.1 | 16.8 [7.1, 9.7, 104.0, 269.0] | 24.5±2.0 | |
| Intracerebroventricular | ANOVA | n.s. | n.s. | n.s. | n.s. |
| Vehicle | 10.3±1.5 | 0.5±0.1 | 113.7 [6.1, 24.8, 194.7, 278.4] | 19.6±2.4 | |
| 7.5 μg | 8.0±1.2 | 0.6±0.1 | 75.2 [3.5, 5.4, 233.5, 365.2] | 22.1±3.3 | |
| 15 μg | 9.3±1.3 | 0.6±0.1 | 530.0 [5.3, 22.5, 284.9, 3,515.6] | 22.5±3.8 | |
| 30 μg | 7.3±1.2 | 0.7±0.1 | 165.4 [3.6, 13.7, 412.3, 786.8] | 22.5±2.9 | |
Data for MAD, IS, and number of completed trials are expressed as M ± SEM. Data for latency are expressed as median [10th, 25th, 75th, 90th percentiles]. Effects of pretreatment with the competitive NMDA receptor antagonist D-AP-5 (s.c., n=12 and i.c.v., n=9, 30 min prior to the beginning of the first block of the session) on mean adjusted delay, impulsivity score, latency, and number of completed trials in male Wistar rats
When injected intracerebroventricularly, D-AP-5 did not significantly alter any of the modified adjusting delay task variables (MAD or IS, dose: F(3,24)=1.25, p=0.31; latency, dose: F(3,24)=0.79, p=0.51; number of completed trials, dose: F(3,24)=0.34, p=0.79; Table 2). Under vehicle condition, the MAD of the i.c.v. experiment was lower compared to the MAD of the systemic experiment, possibly due to the more stressful nature of the central injection procedure.
Effects of the competitive NMDA receptor antagonist CGS 19755 on impulsive choice in a modified adjusting delay task
The competitive NMDA antagonist CGS 19755 significantly decreased MAD and increased IS (dose: F(4,40)=2.82, p<0.05; Fig. 5a, b). Only the highest dose injected (20 mg/kg) increased the choice for the small, immediate reinforcer, when compared to the vehicle condition, as revealed by post hoc analysis. However, CGS 19755 treatment increased latency (dose: F(4,40)=2.88, p<0.05; Fig. 5c) and decreased the number of completed trials (dose: F(4,40)=3.69, p<0.05; Table 1) at the same dose which significantly increased impulsive choice, suggesting an effect on locomotor activity and an overall decrease of subjects’ performance. Median split analysis (veh/low-impulsive vs. veh/high-impulsive, p=0.0002) revealed that CGS 19755 overall decreased MAD in both low-impulsive and high-impulsive rats (dose: F(4,36)=2.80, p<0.05; Dose×Impulsivity: F(4,36)=0.48, n.s.; not shown); furthermore, it confirmed that the effect on impulsive choice was accompanied by an increased latency (dose: F(4,36)=2.65, p<0.05; Dose×Impulsivity: F(4,36)=0.17, n.s.; not shown) and a decreased number of completed trials (Dose: F(4,36)= 3.27, p<0.05; Dose×Impulsivity: F(4,36)=0.17, n.s.; not shown).
Fig. 5.

Effects of pretreatment with the competitive NMDA receptor antagonist CGS 19755 (n=11) (i.p., 30 min prior to the beginning of the first block of the session) on a mean adjusted delay (MAD) and b impulsivity score (IS), in male Wistar rats. Panels (a) and (b) show M ± SEM. Panel (c) shows the box and whisker plots of latency where the boundaries indicate the 25th and 75th percentiles, the line within the box marks the median, and the whiskers (error bars) indicate the 90th and 10th percentiles. Symbols denote significant differences from vehicle: *p<0.05; **p<0.01 (Fisher’s LSD test); significant main effect of dose #p<0.05
Discussion
The major findings of the present study can be summarized as follows: (1) the uncompetitive NMDA receptor antagonists ketamine and memantine increased the choice for a small, immediate reinforcer over a large, delayed reinforcer in a modified adjusting delay task; (2) ketamine selectively precipitated impulsive choice in low-impulsive rats but not in high-impulsive rats; (3) memantine preferentially precipitated impulsive choice in low-impulsive rats; (4) the competitive NMDA receptor antagonists D-AP-5 and CGS 19755 did not affect impulsive choice.
In this study, two uncompetitive NMDA receptor antagonists—ketamine and memantine—precipitated impulsive behavior by increasing the choice for a small, immediate reinforcer over a large, delayed reinforcer. The observed effects were not secondary to motor impairment or a generalized behavioral deficit, as both drugs did not affect the number of completed trials or the latency to respond. In addition, the alternative explanation that the increased preference for the small, immediate reinforcer could be due to intrinsic energy homeostatic or satiety effects of ketamine and memantine (Foltin et al. 2008; Springer and Baker 2007) can be excluded, since the reinforcers used in this modified adjusting delay task were isocaloric (Blasio et al. 2011). To the best of our knowledge, so far only two studies have evaluated the effects of uncompetitive antagonists of the NMDA receptor on impulsive choice, with conflicting results (Floresco et al. 2008; Oberlin et al. 2010). The first study in question, by Floresco and colleagues (Floresco et al. 2008), demonstrated the ability of ketamine to increase responding for a small, immediate reinforcer when injected at a 5-mg/kg dose. Our results confirm this previous observation and extend it by demonstrating that the precipitating effect on impulsive choice is a common effect exerted by uncompetitive NMDA antagonists. The second study by Oberlin and colleagues (Oberlin et al. 2010) found that memantine injected in mice at the doses of 1 and 5 mg/kg did not affect impulsive choice in a delay discounting task. In contrast to this previously published study, we have shown here that memantine increased impulsive choice when injected at the doses of 5 and 10 mg/kg. An important factor which could explain this discrepancy is the difference in species used (high alcohol preferring mice vs. Wistar rats). Mice have a higher metabolism in comparison to rats; therefore, the highest dose of memantine tested in the Oberlin study (5 mg/kg) likely resulted in a lower drug bioavailability compared to that of rats in the lowest effective dose in the present study (5 mg/kg).
The findings of this study are in agreement with the effects observed following ketamine and memantine on impulsive action. Indeed, both drugs have been shown to exacerbate premature responding in the five-choice serial reaction time task (5-CSRTT) (Nemeth et al. 2010; Oliver et al. 2009; Smith et al. 2011). The same effects have been demonstrated for other uncompetitive NMDA receptor antagonists such as dizocilpine (MK-801) (Ardayfio et al. 2008; Grottick and Higgins 2000; Paine and Carlezon 2009; Terry et al. 2012) and phencyclidine (PCP) (Smith et al. 2011; Thomson et al. 2011). It is likely that NMDA receptors exert an overall fundamental modulatory function, which seems to be important in all the different dimensions of trait impulsivity.
Interestingly, we have shown here that both ketamine and memantine treatments preferentially increased the intolerance to delay of reinforcement in low-impulsive subjects. Indeed, ketamine increased impulsive responding selectively in low-impulsive subjects, while memantine showed a decreased efficacy in increasing impulsive choice in high-impulsive subjects. A possible explanation for the observed effects can be found in the particular mechanism of action displayed by this class of channel blockers. Since uncompetitive antagonists such as ketamine and memantine can only reach their binding site when the NMDA channel is open, they show a tendency for higher affinity inhibition at higher agonist concentrations (Johnson and Kotermanski 2006). Therefore, the preferential efficacy of these drugs for low-impulsive subjects suggests that higher intolerance to delay of reinforcement may be associated with decreased glutamate/NMDA function. In support of this hypothesis, decreased glutamate/NMDA activity has been proposed to be an important factor driving impulsive behavior, and it has also been associated with disorders characterized by heightened impulsive behavior such as attention deficit/hyperactivity disorder (Adams et al. 2004; Carlsson 2001; Turic et al. 2004), as well as schizophrenia (Amitai and Markou 2010; Robbins and Murphy 2006).
The difference between the effects of ketamine and memantine on impulsive choice in low-impulsive and high-impulsive subjects is an interesting point of discussion. Ketamine and memantine share many different pharmacological properties: following the activation of the NMDA receptors, both drugs bind the internal portion of the pore and can remain trapped inside the channel following its closure; in addition, at higher agonist concentrations the two drugs inhibit with faster kinetics, and both also show similar specificity for the NMDA receptor subtypes (Johnson and Kotermanski 2006; Traynelis et al. 2010). However, a relevant point of difference between these two drugs’ action mechanisms, which may be useful in explaining the findings of this study, is that memantine has been proposed to bind the receptor not only inside the channel, as ketamine does, but also superficially near the extracellular end of the pore in GluN1/GluN2A. Binding to this additional shallow site can lead to the opening of the receptor even in the absence of an agonist, exposure of the deep binding site, and thus resulting in important consequences on the inhibitory action of memantine (Blanpied et al. 1997; Johnson and Kotermanski 2006; Sobolevsky et al. 1998; Traynelis et al. 2010). Therefore, even in a condition of decreased glutamate/NMDA activity, we hypothesized that for the high-impulsive subjects, memantine would still be able to exert a blocking action of the channel, therefore, exacerbating impulsive choice.
We have also shown that the two competitive NMDA receptor antagonists D-AP-5 and CGS 19755 exerted no effect on impulsive choice. Systemic D-AP-5 treatment did not significantly affect any of the variables measured in the modified adjusting delay task. In addition, given the low blood brain barrier penetrability of D-AP-5 and to rule out that the doses used for the systemic administration were not sufficiently high, the drug was also administered intracere-broventricularly at a dose as high as 30 μg/rat. This dose has been demonstrated to be effective on a number of different measures in several studies (Heale and Harley 1990; Koek et al. 1987; Leung and Shen 1999; Paoli et al. 1990; Takashima et al. 1990). Confirming the results obtained with the systemic treatment, centrally-administered D-AP-5 did not alter any of the variables of the modified adjusting delay task. An alternative interpretation of the lack of effect in impulsive choice following i.c.v. administration of AP-5 is that the drug injected through this route of administration does not effectively reach the target areas. Moreover, CGS 19755 treatment increased the choice for the small, immediate reinforcer when injected at 20 mg/kg, a sufficiently high dose, which has been validated to be effective in several studies (Bennett et al. 1989; Berz et al. 1992; Bespalov and Zvartau 1997). However, the same dose of CGS 19755 was also responsible for the disruption of overall behavioral performance, demonstrated by the increase of the latency to respond and the number of completed trials. Therefore, CGS 19755 treatment suppressed instrumental responding, and none of the doses tested was able to selectively influence impulsive choice without a marked response inhibition. However, the possibility that the effects observed in the latency and in the number of completed trials may be dissociable from the effect observed in impulsive choice following CGS 19755 treatment cannot be ruled out; therefore, in such a case, the drug would increase impulsive choice. As such, although the two competitive antagonists tested in this study did not affect impulsive choice, our data may not necessarily be representative of the entire class, and it would be helpful to assess the effects of other competitive NMDA receptor antagonists in future studies for the purpose of generalizing the concept.
While a broad range of literature has described the effects of competitive NMDA receptor antagonists on impulsive action, it is to our understanding that this is the first study investigating the effects of competitive NMDA receptor antagonists on impulsive choice. Indeed, microinfusion of the competitive NMDA receptor antagonist 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) into fronto-cortical brain regions has been shown to reduce accuracy, as well as to increase omission errors and perseverative responding in rats performing the 5-CSRTT (Baviera et al. 2008; Mirjana et al. 2004; Murphy et al. 2005; Murphy et al. 2012). However, when CPP was administered systemically, the drug was able to dose-dependently weaken the attentional effect of nicotine, but it lacked any intrinsic effect in the 5-CSRTT, likely suggesting that an increased local drug concentration is required to observe an intrinsic effect on impulsive responding in a reaction time paradigm (Quarta et al. 2007). Moreover, Smith and colleagues have recently shown that in all tested doses, the competitive NMDA receptor antagonist SDZ 220,581 suppressed instrumental responding in the 5-CSRTT, but it was not capable of selectively influencing task accuracy without the accompaniment of a marked response inhibition (Smith et al. 2011). Therefore, the results obtained in the present study with the two competitive NMDA receptor antagonists on impulsive choice are not in conflict with existing findings of the same class antagonists on impulsive action.
In summary, we here demonstrate that the effects of NMDA receptor blockade on impulsive choice are not generalizable and depend on the specific mechanism of action of the antagonist used. Specifically, of the two classes of NMDA receptor antagonists studied here, uncompetitive but not competitive antagonists were able to increase impulsive responding for the small, immediate reinforcer. Moreover, ketamine selectively increased impulsive behavior in low-impulsive but not in high-impulsive subjects. Memantine, on the other hand, increased impulsive choice in both groups, but it was still more efficacious in low-impulsive subjects, an effect that might be explained by the peculiar pharmacological property of the ability to bind to a shallow site of the NMDA ion channel even in the absence of an agonist. The preferential effect of NMDA receptor antagonists in low-impulsive subjects observed in this study is hypothesized to depend on a preexisting decrease in glutamatergic activity of subjects intolerant to delay of reinforcement.
Acknowledgments
We thank Tamara Zeric, Fanny Jiang, Shawn Hsu, and Stephen St. Cyr for technical assistance, as well as Angelo Blasio for helpful discussions. We thank the NIMH’s Chemical Synthesis and Drug Supply Program for providing D-AP-5 and CGS 19755.
Funding This publication was made possible by grant numbers DA023680, DA030425, MH091945, MH093650A1 and AA016731 from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), by the Peter Paul Career Development Professorship (P.C.), and by Boston University’s Undergraduate Research Opportunities Program (UROP) (A.R.N., J.K.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Pietro Cottone, Email: cottone@bu.edu, Department of Pharmacology, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA. Department of Psychiatry, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA.
Attilio Iemolo, Department of Pharmacology, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA. Department of Psychiatry, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA.
Aditi R. Narayan, Department of Pharmacology, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA. Department of Psychiatry, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA
Jina Kwak, Department of Pharmacology, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA. Department of Psychiatry, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA.
Duncan Momaney, Department of Pharmacology, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA. Department of Psychiatry, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA.
Valentina Sabino, Email: vsabino@bu.edu, Department of Pharmacology, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA. Department of Psychiatry, Laboratory of Addictive Disorders, Boston University School of Medicine, 72 E Concord St., R-618, Boston, MA 02118, USA.
References
- Adams J, Crosbie J, Wigg K, Ickowicz A, Pathare T, Roberts W, Malone M, Schachar R, Tannock R, Kennedy JL, Barr CL. Glutamate receptor, ionotropic, N-methyl D-aspartate 2A (GRIN2A) gene as a positional candidate for attention-deficit/hyperactivity disorder in the 16p13 region. Mol Psychiatr. 2004;9:494–499. doi: 10.1038/sj.mp.4001455. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 2010;68:5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardayfio PA, Benvenga MJ, Chaney SF, Love PL, Catlow J, Swanson SP, Marek GJ. The 5-hydroxytryptamine2A receptor antagonist R-(+)-alpha-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and enhancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. J Pharmacol Exp Ther. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- Baviera M, Invernizzi RW, Carli M. Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology (Berl) 2008;196:269–280. doi: 10.1007/s00213-007-0959-9. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Bernard PS, Amrick CL, Wilson DE, Liebman JM, Hutchison AJ. Behavioral pharmacological profile of CGS 19755, a competitive antagonist at N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1989;250:454–460. [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. Structure-activity analysis of binding kinetics for NMDA receptor competitive antagonists: the influence of conformational restriction. Br J Pharmacol. 1991;104:207–221. doi: 10.1111/j.1476-5381.1991.tb12409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berz S, Battig K, Welzl H. Effects of CGS 19755 and dizocilpine (MK 801) on delayed time discrimination performance. Behav Brain Res. 1992;51:185–192. doi: 10.1016/s0166-4328(05)80212-8. [DOI] [PubMed] [Google Scholar]
- Bespalov A, Zvartau E. NMDA receptor antagonists prevent conditioned activation of intracranial self-stimulation in rats. Eur J Pharmacol. 1997;326:109–112. doi: 10.1016/s0014-2999(97)85403-9. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol. 1997;77:309–323. doi: 10.1152/jn.1997.77.1.309. [DOI] [PubMed] [Google Scholar]
- Blasio A, Narayan AR, Kaminski BJ, Steardo L, Sabino V, Cottone P. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: effects of 5-HT(2A/C) and 5-HT (1A) receptor agonists. Psychopharmacology (Berl) 2011;219:377–386. doi: 10.1007/s00213-011-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Carlsson ML. On the role of prefrontal cortex glutamate for the antithetical phenomenology of obsessive compulsive disorder and attention deficit hyperactivity disorder. Progr Neuro Psychopharmacol Biol Psychiatr. 2001;25:5–26. doi: 10.1016/s0278-5846(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatr. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol. 2007a;583:487–504. doi: 10.1113/jphysiol.2007.138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology. 2007b;32:1069–1081. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333:99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology. 2011;61:468–477. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Danysz W, Bisaga A. A novel procedure for assessing the effects of drugs on satiation in baboons: effects of memantine and dexfenfluramine. Psychopharmacology (Berl) 2008;199:583–592. doi: 10.1007/s00213-008-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- Heale V, Harley C. MK-801 and AP5 impair acquisition, but not retention, of the Morris milk maze. Pharmacol Biochem Behav. 1990;36:145–149. doi: 10.1016/0091-3057(90)90140-d. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC. The role of N-methyl-D-aspartate (NMDA) receptor-mediated neurotransmission in the pathophysiology and therapeutics of psychiatric syndromes. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 1998;8:141–152. doi: 10.1016/s0924-977x(97)00050-3. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive-type’ behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, Sabino V, Cottone P. Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol. 2012;23(5–6):593–602. doi: 10.1097/FBP.0b013e328357697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Koek W, Woods JH, Ornstein P. A simple and rapid method for assessing similarities among directly observable behavioral effects of drugs: PCP-like effects of 2-amino-5-phosphonovalerate in rats. Psychopharmacology (Berl) 1987;91:297–304. doi: 10.1007/BF00518181. [DOI] [PubMed] [Google Scholar]
- Leung LS, Shen B. N-methyl-D-aspartate receptor antagonists are less effective in blocking long-term potentiation at apical than basal dendrites in hippocampal CA1 of awake rats. Hippocampus. 1999;9:617–630. doi: 10.1002/(SICI)1098-1063(1999)9:6<617::AID-HIPO2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Mar AC, Robbins TW. Delay discounting and impulsive choice in the rat. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience/editorial board. Unit 8. Chapter 8. 2007. p. 22. [DOI] [PubMed] [Google Scholar]
- Mirjana C, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Irvine MW, Costa BM, Fang G, Jane DE. Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neuro-chemistry international. 2012 doi: 10.1016/j.neuint.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, Dalley JW, Robbins TW. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl) 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Paine TA, Rittiner JE, Beguin C, Carroll FI, Roth BL, Cohen BM, Carlezon WA., Jr Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl) 2010;210:263–274. doi: 10.1007/s00213-010-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharmacologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcohol Clin Exp Res. 2010;34:1363–1375. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends Pharmacol Sci. 2011;32:726–733. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology (Berl) 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56:788–797. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli F, Spignoli G, Pepeu G. Oxiracetam and D-pyroglutamic acid antagonize a disruption of passive avoidance behaviour induced by the N-methyl-D-aspartate receptor antagonist 2-amino-5-phosphonovalerate. Psychopharmacology (Berl) 1990;100:130–131. doi: 10.1007/BF02245803. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic; Orlando: 1986. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65:124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. To do or not to do? The complexities of addiction, motivation, self-control, and impulsivity. Am J Psychiatry. 2007;164:4–6. doi: 10.1176/ajp.2007.164.1.4. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Baviera M, Sacchetti G, Calcagno E, Balducci C, Invernizzi RW, Carli M. Attention deficit induced by blockade of N-methyl D-aspartate receptors in the prefrontal cortex is associated with enhanced glutamate release and cAMP response element binding protein phosphorylation: role of metabotropic glutamate receptors 2/3. Neuroscience. 2011;176:336–348. doi: 10.1016/j.neuroscience.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Morris HV, Patel S, Genn RF, Stolerman IP. Different effects of ionotropic and metabotropic glutamate receptor antagonists on attention and the attentional properties of nicotine. Neuropharmacology. 2007;53:421–430. doi: 10.1016/j.neuropharm.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27:141–148. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Steardo L, Schmidhammer H, Zorrilla EP. 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2007;192:537–546. doi: 10.1007/s00213-007-0746-7. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009;34:1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology (Berl) 2011;217:255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Koshelev SG, Khodorov BI. Interaction of memantine and amantadine with agonist-unbound NMDA-receptor channels in acutely isolated rat hippocampal neurons. J Physiol. 1998;512(Pt 1):47–60. doi: 10.1111/j.1469-7793.1998.047bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer DA, Baker KC. Effect of ketamine anesthesia on daily food intake in Macaca mulatta and Cercopithecus aethiops. Am J Primatol. 2007;69:1080–1092. doi: 10.1002/ajp.20421. [DOI] [PubMed] [Google Scholar]
- Takashima A, Maeda Y, Itoh S. Effect of caerulein on decreased latency of passive avoidance response in rats treated with NMDA receptor antagonists. Peptides. 1990;11:1263–1267. doi: 10.1016/0196-9781(90)90161-w. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol. 2012;83:941–951. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DM, McVie A, Morris BJ, Pratt JA. Dissociation of acute and chronic intermittent phencyclidine-induced performance deficits in the 5-choice serial reaction time task: influence of clozapine. Psychopharmacology (Berl) 2011;213:681–695. doi: 10.1007/s00213-010-2020-7. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turic D, Langley K, Mills S, Stephens M, Lawson D, Govan C, Williams N, Van Den Bree M, Craddock N, Kent L, Owen M, O’Donovan M, Thapar A. Follow-up of genetic linkage findings on chromosome 16p13: evidence of association of N-methyl-D aspartate glutamate receptor 2A gene polymorphism with ADHD. Mol Psychiatr. 2004;9:169–173. doi: 10.1038/sj.mp.4001387. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]




