Abstract
We are investigating treatments for cocaine abuse based on viral gene transfer of a cocaine hydrolase (CocH) derived from human butyrylcholinesterase, which can reduce cocaine-stimulated locomotion and cocaine-primed reinstatement of drug-seeking behavior in rats for many months. Here, in mice, we explored the possibility that anti-cocaine antibodies can complement the actions of CocH to reduce cocaine uptake in brain and block centrally-evoked locomotor stimulation. Direct injections of test proteins showed that CocH (0.3 or 1 mg/kg) was effective by itself in reducing drug levels in plasma and brain of mice given cocaine (10 mg/kg, s.c., or 20 mg/kg, i.p). Administration of cocaine antibody per se at a low dose (8 mg/kg, i.p.) exerted little effect on cocaine distribution. However, a higher dose of antibody (12 mg/kg) caused peripheral trapping (increased plasma drug levels), which led to increased cocaine metabolism by CocH, as evidenced by a 6-fold rise in plasma benzoic acid. Behavioral tests with small doses of CocH and antibody (1 and 8 mg/kg, respectively) showed that neither agent alone reduced mouse locomotor activity triggered by a very large cocaine dose (100 mg/kg, i.p.). However, dual treatment completely suppressed the locomotor stimulation. Altogether, we found cooperative and possibly synergistic actions that warrant further exploration of dual therapies for treatment of cocaine abuse.
1. INTRODUCTION
In vivo drug-interception by antibody-binding or enzymatic destruction is emerging as a potential treatment for substance abuse, through preventing addiction relapse in recovering users who re-encounter their drug of choice [1,2]. Two reasons make cocaine abuse a promising target for such approaches. First, vaccines that elicit high-affinity antibodies against this drug have been developed [3] and one such vaccine has already shown measurable efficacy in clinical trial [4]. Second, several enzymes that rapidly hydrolyze cocaine and serve as another type of cocaine interceptor have been engineered from human butyrylcholinesterase [5–8]. One such cocaine hydrolase (CocH) accelerates cocaine’s metabolism and sharply curtails its actions in mice and rats [9]. We are now investigating possible synergistic actions of CocH with anti-cocaine antibodies in reducing drug access to the central nervous system. Prior in vitro work has shown that enzymatic destruction of cocaine continues efficiently even when a large fraction of the drug is antibody-bound [10]. Thus, antibody and enzyme should cooperate to protect the brain from repeated exposures to cocaine, an action that may prove therapeutically advantageous. We recently presented evidence for this concept from rodent studies involving an anti-cocaine vaccine and CocH delivery by gene transfer [11]. The present mouse experiments were designed to test the concept further with pharmacokinetic measures of cocaine uptake into plasma, distribution into brain, and metabolic release of benzoic acid after direct administration of anti-cocaine antibody and CocH protein. We also determined the relative ability of the same agents to suppress cocaine-induced locomotor hyperactivity, a classic behavioral effect of cocaine in rodents.
2. MATERIALS AND METHODS
2.1 Drug and biological sources
Cocaine HCl was obtained from NIDA (National Institute on Drug Abuse, Bethesda MD). Purified CocH, a quadruple mutant of human butyrylcholinesterase (A199S/S287G/A328W/Y332G) first reported by Pan et al [7], was produced in the form of a C-terminal fusion with human serum albumin in clonal lines of stably transfected Chinese hamster ovary cells (D. LaFleur, Cogenesys Inc.). The enzyme was purified on DEAE Sepharose followed by ion exchange chromatography as described elsewhere [10] and was stored at −80°C until used.
2.2 Animals
Balb/c male mice obtained at 6 to 7 weeks of age from Harlan Sprague Dawley (Madison WI) were housed in plastic cages with free access to water and food (Purina Laboratory Chow, Purina Mills, Minneapolis, MN, USA) in rooms controlled for temperature (24 °C), humidity (40–50%), and light (light/dark, 12/12-h with lights on at 6:00 a.m.). The animal use protocol (A4309) was approved by the Mayo Clinic Institutional Care and Use Committees. All experiments were conducted in accordance with the Principles of Laboratory Animal Care in AAALAC-accredited laboratories.
2.3 Sample Collection
Blood samples (< 100 μl) for enzyme and antibody determination were collected at appropriate intervals, by cheek poke with a 21-gauge mouse-bleeding lancet, into tubes with separating gel for red cell removal (Fisher Scientific, Pittsburgh, PA. Bleeding was stopped with a sterile gauze pad applied with modest compression. Plasma separated by centrifugation for 10 min at 8000 g was used fresh or stored at −20 °C pending analysis for cocaine, metabolite, antibody and CocH enzyme levels. Brain samples harvested postmortem at selected intervals were homogenized in 10 volumes of 10 mM sodium phosphate, pH 7.4 with 0.1% Tween-20, and centrifuged along with blood samples.
2.4 Preparation of antibody and vaccine
Anti-cocaine antibodies were elicited as previously described [3], by a vaccine consisting of a norcocaine adduct conjugated to keyhole limpet hemocyanin (KLH). The vaccine (5.7 mg/kg, 100 μg / mouse) was injected along with 1.5 g of alum (Sigma), into the upper thigh of each hind leg (80 μl per site). At three weeks a booster immunization was given in the same dose. At four weeks, levels of specific anti-cocaine antibodies were determined with a cocaine-binding assay. For this purpose, diisopropylfluorophosphate (DFP, 10−5 M) was added to plasma aliquots and 5 min were allowed for inactivation of hydrolytic enzymes. Samples were then incubated 50 min with 3H-cocaine in near saturating concentration (5 μM). Subsequently, 50 μl aliquots were centrifuged on a Sepharose spin-column (1000 × g for 4 min), and 30 μl of the void volume fraction was mixed for scintillation counting in 4 ml “BioSafe” fluor (RPI Inc, Mt Prospect IL). Validation experiments showed that > 80% of the sample protein passed into the collection tube (including IgG with bound cocaine), while > 98% of free cocaine remained on the column. Assay signals (counts per min), proportional to IgG concentration over a wide range, were calibrated with reference to a standard of purified anti-cocaine IgG. For in vivo experiments (see below), calibrated antisera were delivered directly by i.v. injection.
2.5 Injections of cocaine, antiserum, and enzyme
Trace quantities of 3H-labeled cocaine (5 μCi per mouse) were injected i.p. or s.c. (10 or 20 mg/kg, with sample collection at 5, 15, or 45 min) or i.v. (0.1 mg/kg with sample collection at 2 min. Protein pretreatments were a) CocH (0.3. 1, 2 or 4 mg/kg), and b) cocaine antiserum (“AB”, equivalent to 8 or 12 mg/kg IgG). Initially AB and CocH were both given i.v. After finding that i.p. and i.v. injections gave similar plasma levels of cocaine hydrolase activity and specific anti-cocaine IgG from 1 hour onward, we switched to i.p. protein administration followed 1 or 2 hours later by cocaine.
2.6 Assay of cocaine hydrolase activity and cocaine drug levels
Cocaine hydrolase activity in 50-μl aliquots of plasma or brain supernatant was assayed by incubating 30 min with 3H-cocaine (50 nCi, 18 μM) and partitioning liberated 3H-benzoic acid into toluene-fluor for scintillation counting [12]. A related procedure determined levels of 3H-cocaine and benzoic acid [13], with DFP added to halt enzymatic breakdown. A validation experiment confirmed that the assay signal was not affected by the presence or absence of anti-cocaine IgG in test samples. Native mouse BChE activity was assayed in DFP-free samples by a radiometric method [14] using 1 mM 3H-acetylcholine as substrate and 10−5 M BW284c51 as an acetylcholinesterase inhibitor.
2.7 Locomotor Apparatus and Procedure
Cocaine-induced locomotion in mice was assessed in sound-insulated rectangular activity chambers from Med Associates Inc, St Albans, VT USA (27-cm W x 27-cm L x 20-cm D) with continually running fans and infrared lasers and sensors. Beam breaks (2-min bins over 60 min) were converted automatically to distance travelled (cm) and recorded on a computer with Med-PC software Version 4.0. Untreated mice and mice immunized 5 weeks earlier with KLH-norcocaine vaccine were habituated by the following procedure. Before entering the locomotor chamber (time zero) each animal received i.p. saline (6.7 ml /kg). A second saline injection was given at 1 hour, and locomotor activity was recorded for another hour. After three days of habituation, acute pretreatments were administered in a series of similarly timed i.p. injections: 1) at time zero, saline or enzyme (CocH, 1 mg/kg) and / or anti-cocaine antiserum (AB, 12 mg/kg); and 2); at 60 min, cocaine hydrochloride (40 or 100 mg/kg).
2.8 Statistical Analysis
Travel distance served as the primary dependent measure of locomotor activity in the present study. Analysis of variance (ANOVA) compared locomotor activity among groups during the cocaine challenge. Post hoc analyses used Fisher’s least significant difference procedure following a significant interaction between factors. Similar statistical analysis was applied to pharmacokinetic data. The criterion for statistical significance was p<0.05.
3. RESULTS
3.1 Effect of antibody and enzyme on plasma and brain cocaine
Three separate experiments were performed to determine how effectively anti-cocaine antibody and CocH enzyme reduce brain uptake of cocaine by sequestration or destruction in the blood (respectively), and whether their actions would be additive or even synergistic. First, mice (n = 7 per group) received i.v. CocH (1 mg/kg) and/or AB (8 mg/kg) followed 5 min later by 3H-cocaine (0.1 mg/kg, i.v.). Plasma and brain samples were collected after a further 2-min delay. The results (data not shown) revealed that CocH by itself reduced plasma cocaine levels by 95% and brain levels by 51% (p < 0.01 for both effects). The AB treatment (yielding 0.6 ± 0.1 μM of cocaine-specific IgG in plasma) caused a 330% increase in plasma cocaine (p < 0.01) but no effect on brain levels. The combined treatment, AB + CocH, reduced brain cocaine levels to 41% of control but this effect was not significantly different from that of CocH alone.
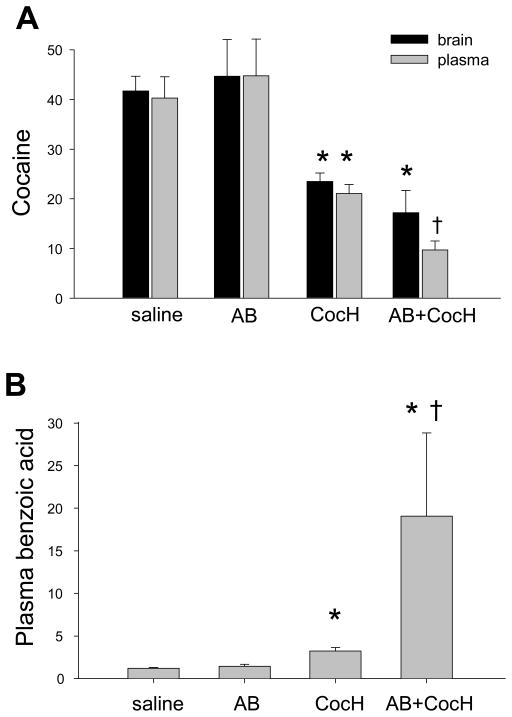
To better balance antibody and enzyme effects, mice in a second experiment were pretreated (i.v. as before) with the same 8-mg/kg dose of AB but a smaller dose of CocH (0.3 mg/kg), separately or together. Five min later 3H-cocaine (20 mg/kg i.p) was injected. After another 15 minutes, blood and brain samples were collected for analysis. To reduce variability, cocaine levels were expressed as a percentage of total measured radiolabel. By this metric, enzyme alone cut plasma and brain cocaine levels by about 50% (p < 0.01) and elevated plasma benzoic acid almost 3-fold (Fig. 1). Again AB on its own had weaker effects. It did not significantly reduce brain uptake of cocaine or change cocaine levels in the blood), but when given along with CocH, AB greatly enhanced the enzyme-induced rise in plasma benzoic acid (p < 0.02), suggesting increased cocaine destruction in the periphery.
Fig 1.
Mice were given i.p. cocaine, 20 mg/kg, 5 min after i.v. treatment with saline (n = 7), AB (8 mg/kg, n = 6), CocH (0.3 mg/kg, n = 6), or CocH + AB (n= 7). Tail vein blood samples were drawn 15 min later. Statistical significance: * (p < 0.01 vs saline and AB-treated groups; †(p < 0.01 vs saline, AB-only, and CocH-only group).
In the third experiment, mice were given 50% more AB (12 mg/kg, i.p. instead of i.v., 2 hr beforehand) and 50% less cocaine (10 mg/kg, s.c.). Under these conditions antibody on its own did exert a clear effect. In particular, plasma cocaine levels 5 min after 3H-cocaine injection were nearly 3-fold higher in AB-treated mice than in saline-pretreated mice (p < 0.005), and at 45 min they were still twice the control levels (Table 1). This outcome implies a degree of peripheral drug trapping even though cocaine uptake was not significantly reduced in brain samples taken at the same time (data not shown). Nonetheless, it was clear that antibody binding of cocaine did not hinder enzyme-mediated hydrolysis in the blood stream but may have actually enhanced it. Thus, in the dual-treated mice, plasma cocaine dropped to approximately the same levels seen in the mice that received CocH without AB. Since the mice that received AB alone showed cocaine levels about twice those in saline controls, the impact of CocH in dual-treated animals must have been about twice as great as in the absence of AB. For example, at 45 min, the cocaine level in saline-treated mice was 1.6 μM, while in mice given 4 mg/kg CocH it was 0.19 μM, a net difference of 1.4 μM. On the other hand, mice given AB alone showed a cocaine level of 3.1 μM, while mice given AB+4 mg/kg CocH showed a level of 0.17, for a net difference of 2.9 μM. Such effects were consistent across CocH dose levels and sampling times and they were statistically significant (p < 0.05). In our view, they indicate that more cocaine was destroyed in the periphery as a result of antibody binding.
Table 1.
Shown are plasma levels, in micromolar units, of cocaine (A) and benzoic acid (B) observed 5 and 45 min after 3H-cocaine injection (10 mg/kg, i.p.) in mice pretreated with saline, CocH (2 or 4 mg/kg, i.p.), or antibody (AB, 12 mg/kg, s.c.), singly or in combination.
| A Cocaine (μM) | ||||||
|---|---|---|---|---|---|---|
| Saline | CocH 2 mg | CocH 4 mg | AB | AB+ CocH 2 mg | AB + CocH 4 mg | |
| 5-min | ||||||
| n | 5 | 4 | 4 | 5 | 4 | 4 |
| Mean ± SEM | 0.40 ± 0.05 | 0.04 ± 0.00 | 0.02 ± 0.00 | 1.00 ± 0.15 | 0.40 ± 0.04 | 0.06 ± 0.03 |
| 45-min | ||||||
| n | 3 | 3 | 2 | 3 | 3 | 2 |
| Mean ± SEM | 1.60 ± 0.22 | 1.20 ± 0.11 | 0.19 | 3.10 ± 0.79 | 1.20 ± 0.33 | 0.17 |
| B Benzoic acid (μM) | ||||||
|---|---|---|---|---|---|---|
| Saline | CocH 2 mg | CocH 4 mg | AB | AB+ CocH 2 mg | AB + CocH 4 mg | |
| 5-min | ||||||
| n | 5 | 4 | 4 | 5 | 4 | 4 |
| Mean ± SEM | 0.21 ± 0.03 | 6.00 ± 0.53 | 6.20 ± 0.67 | 0.34 ± 0.11 | 2.70 ± 0.47 | 6.00 ± 0.77 |
| 45-min | ||||||
| n | 3 | 3 | 2 | 3 | 3 | 2 |
| Mean ± SEM | 3.70 ± 0.82 | 12.6 ± 1.6 | 14.5 | 3.00 ± 0.29 | 16.0 ± 1.90 | 14.0 |
3.2 Reduced locomotor activation in presence of antibody and enzyme
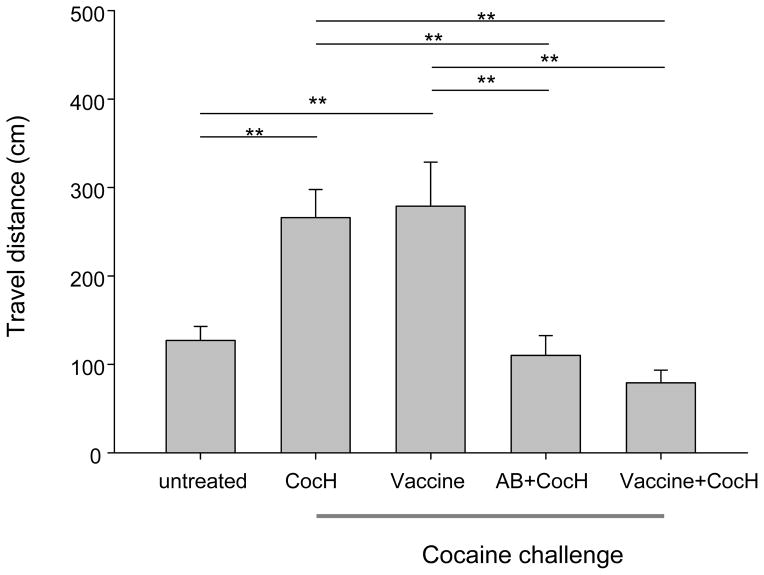
Further investigation of additive or synergistic effects of enzyme and antibody utilized a model of cocaine-induced hyper-locomotion. Cocaine was administered to mice that had received AB (12 mg/kg, i.p) or CocH (1 mg/kg, i.p.) one hour before, or had been vaccinated with KLH-norcocaine (tests were run 4 to 6 weeks after initial immunization--see Methods). Two different cocaine challenges were given. The first was a strong but sub-toxic dose (40 mg/kg, i.p.), which in unprotected animals caused sustained hyper-locomotion for the entire 60 min of observation. In this case, CocH at 1 mg/kg completely prevented the drug-induced locomotion, while neither AB nor vaccine in isolation had a significant effect (data not shown). A second challenge delivered a frankly toxic dose of cocaine, 100 mg/kg i.p (Fig. 2). Unprotected mice and mice pretreated only with antibody became incapable of locomotion, owing to extreme motor weakness and a fixed pattern of head-bobbing and other stereotypic behaviors (repetitive movements without ambulation). Mice pretreated only with CocH or only with vaccine did not show such stereotypy. Instead they exhibited dramatic cocaine-induced hyper-locomotion, just like the unprotected animals given 40 mg/kg. In other words, their response reflected stimulation rather than toxicity. In contrast, dual-treated mice (given either vaccine or AB in addition to CocH) showed neither stereotypy nor excess locomotion. Rather, they continued their moderate exploratory activity from before cocaine exposure. This outcome can also be viewed as a synergistic action of the antibody-based and enzyme-based treatment modalities combining to prevent any observable, centrally-mediated effect of the stimulant drug.
Fig 2.
Cocaine-induced locomotor activity. One group of mice were given only saline (“untreated”, n =12). The other groups were challenged with cocaine 100 mg/kg i.p., 1 hr after the following i.p. pretreatments (all 1 hr beforehand, except vaccine): enzyme (CocH, 1 mg/kg, n = 10), antibody (AB, 8 mg/kg, n = 8, data not shown), vaccine (Vac, n = 11), antibody plus enzyme (AB + CocH, n = 12), and vaccine plus enzyme (Vac + CocH, n = 6). Statistical significance: **(p < 0.01 vs CocH-only treatment).
4. DISCUSSION AND CONCLUSIONS
Antidrug antibodies (including monoclonal antibodies) and vaccines have been developed against many substances of abuse, including nicotine [15], cocaine [16,17], methamphetamine [18], heroin [19,20], and other substances [21]. They pose a relatively small risk to the recipient, and they act to sequester drugs in the peripheral circulation, thereby preventing access to brain reward centers. Signs of altered drug pharmacokinetics in previous studies include increased area under the concentration-versus-time curve in plasma, with corresponding decreases in distribution of drug to the brain [22,23]. The scope of the present work did not permit full-scale analysis of such phenomena, but the biochemical results are in line with previous work with drug-specific antibodies in demonstrating a degree of drug-trapping in the circulation and signs of enhanced drug metabolism in the periphery.
To the best of our knowledge only two other studies, both from our laboratories, have tested dual treatments involving a drug-metabolizing enzyme as well as anti-drug antibodies. The outcomes of those studies also supported complementary or synergistic actions of the combined treatment modalities. The first study [11] focused on locomotor behavior in rats treated singly or in combination with CocH delivered by viral gene transfer and/or AB elicited by vaccination. Although antibody levels after vaccination (~10 μM) were much higher than those achieved in the present work after direct injection (~ 1 μM), the effect on cocaine-induced locomotion was still modest. In contrast, vector-generated CocH (also about 10-times more abundant than in the present study) again reduced that effect on its own after the first exposure to cocaine. Of great interest, dual treatment (vaccine plus vector) was stronger in two respects. First, it completely suppressed initial responses to cocaine and, second, it impaired the locomotor sensitization that typically accompanies repeated cocaine exposure [24].
The most compelling evidence for synergy between antibody and enzyme-based treatments, however, emerged from a second set of experiments focused on severe liver damage in mice exposed to a near fatal cocaine dose, of 120 mg/kg [25]. In that case, whereas single administration of AB and CocH caused only minor reduction in cocaine-induced hepatic necrosis, dual treatment abolished all signs of toxicity. We regard these outcomes as strong support for further development of a complementary therapy for cocaine abuse based on the generation of effective antibodies and drug-metabolizing enzyme in vivo.
Research Highlights.
Small amounts of cocaine hydrolase reduce cocaine levels in mouse plasma and brain
Anti-cocaine antibody can sequester cocaine in plasma
Antibody plus hydrolase enhance cocaine metabolism and reduce brain drug levels
Acknowledgments
This research was supported by NIDA grants DP1 DA031340, R01 DA023979, and R01 DA023979 S1 (to SB) and by R01 DA030338 (FMO), and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brimijoin S. Interception of cocaine by enzyme or antibody delivered with viral gene transfer: a novel strategy for preventing relapse in recovering drug users. CNS Neurol Disord Drug Targets. 2011;10(8):880–891. doi: 10.2174/187152711799219398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brimijoin S, Gao Y. Cocaine hydrolase gene therapy for cocaine abuse. Future Med Chem. 2012;4(2):151–162. doi: 10.4155/fmc.11.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR. Vaccines for cocaine abuse. Hum Vaccin. 2009;5(4) doi: 10.4161/hv.5.4.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun H, Pang YP, Lockridge O, Brimijoin S. Re-engineering butyrylcholinesterase as a cocaine hydrolase. Mol Pharmacol. 2002;62(2):220–224. doi: 10.1124/mol.62.2.220. [DOI] [PubMed] [Google Scholar]
- 6.Pan Y, Gao D, Yang W, et al. Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci U S A. 2005;102(46):16656–16661. doi: 10.1073/pnas.0507332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Y, Gao D, Zhan CG. Modeling the catalysis of anti-cocaine catalytic antibody: competing reaction pathways and free energy barriers. J Am Chem Soc. 2008;130(15):5140–5149. doi: 10.1021/ja077972s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng F, Yang W, Ko MC, et al. Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc. 2008;130(36):12148–12155. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brimijoin S, Gao Y, Anker JJ, et al. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology. 2008;33(11):2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem Biol Interact. 2008;175(1–3):83–87. doi: 10.1016/j.cbi.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll ME, Zlebnik NE, Anker JJ, et al. Combined Cocaine Hydrolase Gene Transfer and Anti-Cocaine Vaccine Synergistically Block Cocaine-Induced Locomotion. PLoS One. 2012 doi: 10.1371/journal.pone.0043536. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brimijoin S, Shen M, Sun H. Radiometric solvent-partitioning assay for screening cocaine hydrolases and measuring cocaine levels in milligram tissue samples. Analytical Biochemistry. 2002;309:200–205. doi: 10.1016/s0003-2697(02)00238-5. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Expt Ther. 2004;310:1046–1052. doi: 10.1124/jpet.104.068122. [DOI] [PubMed] [Google Scholar]
- 14.Johnson CD, Russell RL. A rapid simple radiometric assay for cholinesterase, suitable for multiple determinations. Anal Biochem. 1975;64:229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- 15.Pentel PR, Malin DH, Ennifar S, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol Biochem Behav. 2000;65(1):191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 16.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci U S A. 2001;98(4):1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantak KM, Collins SL, Bond J, Fox BS. Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology (Berl) 2001;153(3):334–340. doi: 10.1007/s002130000555. [DOI] [PubMed] [Google Scholar]
- 18.McMillan DE, Hardwick WC, Li M, et al. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309(3):1248–1255. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- 19.Killian A, Bonese K, Rothberg RM, Wainer BH, Schuster CR. Effects of passive immunization against morphine on heroin self-administration. Pharmacol Biochem Behav. 1978;9(3):347–352. doi: 10.1016/0091-3057(78)90295-2. [DOI] [PubMed] [Google Scholar]
- 20.Stowe GN, Vendruscolo LF, Edwards S, et al. A vaccine strategy that induces protective immunity against heroin. J Med Chem. 2011;54(14):5195–5204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012;91(1):60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurenzana EM, Byrnes-Blake KA, Milesi-Halle A, Gentry WB, Williams DK, Owens SM. Use of anti-(+)-methamphetamine monoclonal antibody to significantly alter (+)-methamphetamine and (+)-amphetamine disposition in rats. Drug Metab Dispos. 2003;31(11):1320–1326. doi: 10.1124/dmd.31.11.1320. [DOI] [PubMed] [Google Scholar]
- 23.Keyler DE, Dufek MB, Calvin AD, et al. Reduced nicotine distribution from mother to fetal brain in rats vaccinated against nicotine: time course and influence of nicotine dosing regimen. Biochem Pharmacol. 2005;69(9):1385–1395. doi: 10.1016/j.bcp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Reith ME, Benuck M, Lajtha A. Cocaine disposition in the brain after continuous or intermittent treatment and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243(1):281–287. [PubMed] [Google Scholar]
- 25.Gao Y, Geng L, Orson F, et al. Effects of anti-cocaine vaccine and viral gene transfer of cocaine hydrolase in mice on cocaine toxicity including motor strength and liver damage. Chem Biol. 2012 doi: 10.1016/j.cbi.2012.08.006. pending. [DOI] [PMC free article] [PubMed] [Google Scholar]