Abstract
The heat-labile enterotoxins (HLTs) of Escherichia coli and Vibrio cholerae are classified into two major types on the basis of genetic, biochemical, and immunological properties. Type I and Type II HLT have been intensively studied for their exceptionally strong adjuvant activities. Despite general structural similarities, these molecules, in intact or derivative (non-toxic) forms, display notable differences in their mode of immunomodulatory action. The molecular basis of these differences has remained largely uncharacterized until recently. This review focuses on the Type II HLTs and their immunomodulatory properties which depend largely on interactions with unique gangliosides and Toll-like receptors that are not utilized by the Type I HLTs.
1. Introduction
The heat-labile enterotoxins (HLT) of E. coli and Vibrio cholerae belong to a family of structurally-related proteins that is divided into two major groups based on genetic, biochemical, and immunological characteristics. The Type I subfamily consists of cholera toxin (CT) from Vibrio cholerae, the LT enterotoxin (referred herein as LT-I) of E. coli, and antigenically-related enterotoxins from other enteric bacteria (Connell and Holmes, 1995). LT-IIa, LT-IIb, and LT-IIc comprise the Type II subfamily of HLT (Holmes et al., 1995; Nawar et al., 2011; Nawar et al., 2010b). Type I and Type II heat-labile enterotoxins are oligomeric ‘AB5’ proteins, i.e., composed of a single A polypeptide which is non-covalently bound to a pentameric array of B polypeptides (Gill et al., 1981b; Mekalanos et al., 1979). The A subunit possesses strong ADP-ribosyltransferase activity and consists of two subcomponents: the N-terminal A1, which represents the actual catalytic moiety, formed by proteolytic cleavage and reduction of an intrachain disulfide bond in the A polypeptide, and the C-terminal A2 tail that is inserted noncovalently into the central pore of the ring-shaped B pentamer (Gill et al., 1981a; van den Akker et al., 1996). The B pentameric subunit is nontoxic by itself but, upon high-affinity binding to receptors on the plasma membrane, delivers the A subunit intracellularly. Intoxication by an HLT constitutively activates the Gsα regulatory subunit of adenylate cyclase which, in most mammalian cells, substantially increases intracellular concentration of cAMP, a secondary messenger within the cell. In gut epithelial cells, cAMP elevation causes massive secretion of electrolytes and water into the gut lumen, which is clinically manifested as diarrhea in humans and animals.
The amino acid sequences of the A polypeptides of the Type I and Type II enterotoxins are highly homologous, which reflects the conservation in structures required for retention of enzymatic activity. In contrast, the amino acid sequences of the B polypeptides of the type I and type II HLTs are highly divergent (Connell, 2007; Mekalanos et al., 1979; Pickett et al., 1986). It is this divergence in sequences of the B polypeptides that underlies the differences observed in the binding activities of the members of both subfamilies for their respective cellular receptors. This in turn determines, in great part, their distinct immunomodulatory activities (see section 3).
2. Interactions of Type II enterotoxins with gangliosides
Receptors for the Type I and Type II heat-labile enterotoxin subfamilies are one or more members of the family of gangliosides (Table 1), a structurally complex group of sialic acid-decorated glycolipids that are located in the plasma membrane of the cells of most higher eukaryotes (Sonnino et al., 1986). Structurally, gangliosides are oligoglycosylceramides that contain one or more N-acetylneuraminic acid (NeuAc) or N-glycolylneuraminic acid (NeuGc) forms of sialic acid that are conjugated by glycosidic linkages to the ceramide core. While the ceramide core is commonly embedded in the plasma membrane, the sugar groups are exposed on the surface of the cells (Levery, 2005). It is proposed that gangliosides are structural components of the plasma membrane. Yet, it is also clear that gangliosides have roles in regulating various cellular metabolisms and responses. Platelet-derived growth factor, epidermal growth factor, and insulin each bind to gangliosides (Hakomori and Igarashi, 1995; Zeller and Marchase, 1992). It is likely that binding of ligands to gangliosides, either directly or indirectly, transduces signals across the plasma membranes that drive specific cellular responses (Fishman, 1986; Hakomori, 1993; Hakomori and Igarashi, 1995; Hannun and Linardic, 1993; Nagai and Iwamori, 1984).
Table 1.
Ganglioside-binding specificities of HLTs1
| Enterotoxin | Gangliosides bound: | References |
|---|---|---|
| Cholera toxin |
Strong: GM1 Weaker: asialo GM1, GM2, GM3, GD1a, GD1b, GT1b |
Kuziemko et al., 1996; Fukuta at al., 1988 |
| LT-I |
Strong: GM1 Weaker: GM2, GD1b |
Mudrak and Kuehn, 2010; Fukuta et al., 1988 |
| LT-IIa |
Strong: GD1b Weaker: GD1a, GM1, GT1B, GQ1B, GD2, GM2, GM3 |
Fukuta et al., 1988 |
| LT-IIb |
Strong: GD1a Weaker: GT1b, GM1b, GD1α, GM2, GM3 |
Fukuta et al., 1988; Berenson et al., 2010 |
| LT-IIc |
Strong: GD1a, GM1, GM2, GM3, GD1a Weaker: GQ1b |
Nawar et al., 2010b |
The binding activities of the different HLT for gangliosides were evaluated using different methods, e.g., ganglioside-dependent ELISA, surface plasmon resonance, TLC immunoblotting, etc. Thus, it is difficult to compare the binding data between HLTs that were obtained using these disparate assays or to determine for any of the HLTs which of the gangliosides have the potential to participate as receptors for modulating toxicity and/or immune stimulation.
Notably, the types of gangliosides that are expressed are heterogeneous, differ between species and different cell types within a single species may express different forms of gangliosides (Levery, 2005). For example, gangliosides GM3 and GD1a are highly expressed on the surface of resting T cells (Levery, 2005; Nohara et al., 1997; Nohara et al., 1992) and gangliosides GD1a, GM1b, and GM1a are found on the plasma membranes of mouse macrophages (Yohe et al., 1997). While cows, sheep, mice, and other higher mammals express gangliosides containing both NeuAc and NeuGlc, due to a frameshift mutation in the Cmah gene which encodes CMP-Neu5Ac hydroxylase, the enzyme that converts NeuAc to NeuGc, humans express only NeuAc gangliosides (Brinkman-Van der Linden et al., 2000; Chou et al., 1998). The ramifications of this restricted expression of gangliosides in humans has only recently determined to have importance in enterotoxin biology (Nawar et al., 2010a).
In vitro binding assays using commercially-available gangliosides was employed to define the ganglioside-binding activities of the type I and type II HLTs. These data demonstrated that each member of the two subfamilies of enterotoxins has a distinctive pattern of binding to one or more gangliosides (Table 1). CT and LT-I bind avidly to ganglioside GM1 (Critchley et al., 1981; Fukuta et al., 1988; Orlandi et al., 1994; Yamada et al., 1983); LT-I also has affinity for one or more glycoproteins identified on the surface of rabbit intestinal cells (Holmgren et al., 1982) or on human CaCo-2 cells (Orlandi et al., 1994). LT-IIb binds avidly to GD1a, GT1b, GM2, and GM3 (Berenson et al., 2010); LT-IIa binds to GD1b, GD1a, and GM1 (Fukuta et al., 1988). LT-IIc has significant binding affinity for GM1, GM2, GM3, and GD1a (Nawar et al., 2010a; Nawar et al., 2011) (Table 1). Immunoblotting experiments using total gangliosides from murine macrophages and thin-layer chromatography revealed that the number of ganglioside receptors recognized by LT-IIb was more extensive than indicated by experiments using commercial gangliosides (Nawar et al., 2010a). In addition to GD1a, GT1b, GM2, and GM3, these high-resolution experiments revealed that LT-IIb binds to gangliosides GM1b and GD1α. Binding of LT-IIb was independent of whether these gangliosides were decorated with NeuAc or NeuGlc. The diversity in binding of the various type I and type II HLTs to different gangliosides likely dictates the types of cells, and thus, the types of animals for which each of the enterotoxins have the capacity to intoxicate (Connell, 2007; Orlandi et al., 1994). As will be evident later in this review, the differences in binding to different gangliosides also mediate the distinctive immunomodulatory properties that are exhibited by each HLT.
To identify the domain(s) that were essential for binding to ganglioside GM1, mutants of CT and LT-I were engineered in which alternative amino acids were substituted at various positions in the B polypeptides of the enterotoxins. Substitution of the glycine at amino acid position 33 for aspartic acid (G33D) in the B polypeptides of CT and LT-I was found to abrogate binding of the holotoxins to ganglioside GM1. Concurrently, the G to D substitution highly decreased the enterotoxins’ ability to intoxicate cells (Jobling and Holmes, 1991, 2002). Similar mutants with altered affinities for binding to their ganglioside receptors were also engineered for LT-IIa and LT-IIb (Connell and Holmes, 1992; Connell and Holmes, 1995). Mutants of LT-IIa with threonine to isoleucine substitutions at amino acid positions 13, 14, and 34 (T13I, T14I, and T34I) in the B polypeptide had no detectable binding to ganglioside GD1b, the receptor for which the wild-type (wt) enterotoxin exhibited the highest binding affinity and a much reduced binding to GD1a. Binding to GM1, a ganglioside that is bound at lower affinity by wt LT-IIa, was not bound by either LT-IIa(T13I) and LT-IIa(T34I); in contrast, LT-IIa(T14I) and wt LT-IIa bound GM1 with similar avidity. LT-IIa(T13I), LT-IIa(T14I), and LT-IIa(T34I) all lacked toxicity in a cell bioassay. Amino acid positions 13 and 14 in the B polypeptides of LT-IIb also are required for binding to GD1a, the ganglioside for which that enterotoxin binds with highest avidity. Neither LT-IIb(T13I) nor LT-IIb(T14I) were capable of intoxicating cells. In comparison, a T to I substitution at amino acid position 34 in LT-IIb [LT-IIb(T34I)] had no effects on binding to GD1a or on toxicity.
In contrast to the binding affinities of LT-IIb, which binds equally well to NeuAc gangliosides and to NeuGlc gangliosides, LT-IIb(T13I) was found to have diminished binding to NeuAc gangliosides and preferentially bound to NeuGlc gangliosides. Thus, this difference in binding affinities between NeuAc gangliosides and NeuGlc gangliosides combined with the observation that LT-IIb(T13I) was non-toxic, suggested that high affinity binding to NeuAc ganglioside receptors by LT-IIb was required for toxicity.
These functional results correlated well with the structural data obtained from protein crystallization studies of CT, LT-I, and LT-IIb in that amino acid positions 13, 14, 33, and 34 are likely in or near the carbohydrate binding pockets of the B pentamers of each of the holotoxins (Sixma et al., 1993; Sixma et al., 1991; van den Akker et al., 1996; Zhang et al., 1995).
3. HLTs and the mucosal immune system
Mucosal surfaces are confronted continuously with antigenic substances in the environment. The vast majority of those substances are harmless. In most cases, the mucosal immune system is unresponsive to these Ags or responds only in a muted manner (Kuper et al., 1992). In the gastrointestinal tract, lymphoid tissue comprising the gut-associated lymphoid tissue (GALT) is concentrated in cellular aggregates known as Peyer’s Patches (PP) (Kiyono and Fukuyama, 2004). After traversing the epithelial layer of the PP through specialized cells known as M cells, Ags are processed and presented by APC to naive B and T cells, which along with Ag and APC, migrate out of the PP into the draining mesenteric lymph nodes for further rounds of activation and proliferation. In the nasal/oral mucosae, a similar arrangement of cells and tissues mediates mucosal immune responses. The nasal/oral-associated lymphoid tissue (NALT) in humans is located in the tonsils and adenoids and within tissue designated as Waldeyer’s ring (Goeringer and Vidic, 1987; Kuper et al., 1992). In the mouse, the functional equivalent of Waldeyer’s ring is located in the nasal cavity. The murine NALT, including overlying epithelia, M-like cells, T cells, B cells, dendritic cells, macrophages, and other immunocompetent cells (Asanuma et al., 1997; Heritage et al., 1997; Wu et al., 1997) responds to intranasally applied HLTs by initiating cascades of molecular and cellular events which induce enhanced mucosal immune responses to foreign Ags.
As alluded to above, most purified microbial proteins do not generally stimulate immune responses. In contrast, Type I and Type II HLTs are potent immunogens when administered by mucosal routes (Connell, 2007; Elson and Dertzbaugh, 2005; Hajishengallis et al., 2005a). In fact, mucosal administration of most protein immunogens often induces a state of immunologic unresponsiveness (tolerance) rather than active immunity (Czerkinsky et al., 1999) due to, at least in part, their failure to induce appropriate activation signals in APC (Akira et al., 2001; Hajishengallis et al., 2005a; Kwissa et al., 2007). On the other hand, induction of such activating signals is a major mechanism of adjuvant action. Importantly, Type I and Type II HLTs are also potent mucosal adjuvants for co-administered protein immunogens, as shown by a great number of experimental animal immunization studies (Hajishengallis et al., 2005a). In this regard, the enterotoxins stimulate the production of specific secretory IgA (S-IgA) Abs against both themselves and co-administered immunogens after stimulation of mucosal inductive sites (see below). However, due to their intrinsic toxicity, the use of intact enterotoxins as adjuvants in human vaccine formulations is definitely precluded. On the other hand, the tremendous health impact of infectious diseases, which remain leading causes of mortality and morbidity, and the paucity of adjuvants licensed for human use create an urgent need for novel, safe, and improved adjuvants (Holmgren and Czerkinsky, 2005; Kwissa et al., 2007; Mestecky et al., 2008). This is especially true for mucosal adjuvants since most infectious diseases occur, or are initiated, at mucosal surfaces. Indeed, the vast majority of pathogens colonize or invade the mucosae via oral, respiratory, or urogenital routes (Holmgren and Svennerholm, 2005; Mestecky et al., 2008). At least in principle, mucosal infectious diseases could be prevented by mucosal immunization for induction of specific S-IgA Abs (Hajishengallis and Russell, 2008; Holmgren and Czerkinsky, 2005) which can interfere with microbial adherence and colonization (Hajishengallis et al., 1992).
In view of the above discussed considerations, research on the adjuvant properties of heat-labile enterotoxins is performed under the premise that it is possible to dissociate adjuvant activities from undesirable toxic effects. This work has led to the development, at least at the experimental level, of a number of modified molecules, such as non-toxic point mutants of otherwise intact enterotoxins (holotoxins) or recombinantly expressed B pentamers (i.e., lacking the entire A subunit), which have retained useful adjuvant properties. For relevant work on the Type I HLTs, the reader is referred to excellent recent reviews (Connell, 2007; Cox et al., 2006; Langridge et al., 2010; Liang and Hajishengallis, 2010; Lycke and Bemark, 2010). This review will focus on the progress made with Type II enterotoxins and derivatives thereof, which possess distinct immunostimulatory properties when compared to the Type I molecules (Table 2), in part due to differential ganglioside utilization and, moreover, the ability to activate non-ganglioside receptors.
4. Adjuvant activities of Type II HLTS
The oral/gastrointestinal, nasopharyngeal, respiratory, and vaginal mucosae represent natural sites for entry of many human pathogens. Protection against those pathogens requires vaccines to evoke strong pathogen-specific, protective mucosal immune responses. To avoid responding to the myriad of harmless antigens (Ags) in the environment, however, endogenous immunoregulatory systems at the mucosae routinely suppress immune responses to most Ags. To overcome those barriers, mucosal adjuvants must be employed.
A plethora of studies have demonstrated that the most powerful mucosal adjuvants are members of the type I and type II subfamilies of HLTs. Using various mucosal immunization models, it has been shown that CT, LT-I, LT-IIa, LT-IIb, and LT-IIc are potent mucosal adjuvants which, when co-administered with a weak Ag in a murine intranasal immunization model alters or bypasses those endogenous immunoregulatory systems in the mucosa, the result of which is to dramatically enhance mucosal (IgA) and systemic (IgG) immune responses to that weak Ag (Connell, 2007; Connell et al., 1998; Nawar et al., 2005, 2007).
4.1 Adjuvant properties of LT-IIa and LT-IIb
Recent investigations have revealed that the mucosal and systemic adjuvant properties of LT-IIa and LT-IIb are equivalent, and in some cases, more potent than those of CT and LT-I (Arce et al., 2005; Connell, 2007; Connell et al., 1998; Nawar et al., 2005, 2007; Nawar et al., 2010b). Co-administration either LT-IIa or LT-IIb, by the intranasal (mucosal) or intradermal (systemic) route, of a weak antigen augmented both antigen-specific mucosal (IgA) and antigen-specific systemic (IgG) responses to that weak antigen (Mathias-Santos et al., 2011; Nawar et al., 2005, 2007). LT-IIa and LT-IIb, however, exhibit distinctive immunomodulatory properties in comparison to those induced (or suppressed) by CT and LT-I, to augment those responses. For example, CT and LT-I routinely induce Th2 responses to co-administered antigen. Based on patterns of cytokine expression and antibody isotype distributions, LT-IIa or LT-IIb, when employed as mucosal adjuvants, have a propensity to elicit a more balanced Th1/Th2 immune response. These data suggested that LT-IIa and LT-IIb either interact with one or more types of immunocompetent cells than are influenced by either CT or LT-I, or LT-IIa and LT-IIb interact differently with immunocompetent cells as compared to CT or LT-I. This model is supported by experimental evidence. CT binds to virtually all CD4+ T cells, CD8+ T cells, B cells, dendritic cells, and macrophages; in contrast, LT-IIa and LT-IIb, while binding to most dendritic cells and macrophages, binds only to a limited percentage of CD4+ T cells, CD8+ T cells, and B cells (Arce et al., 2005). Functionally, neither LT-IIa nor LT-IIb suppress production of IL-2, TNF-α, or IL-12 in anti-CD3 stimulated human peripheral blood monocytes to the same degree as when the cells were treated with CT (Arce et al., 2005; Martin et al., 2001). CD4+ T cells stimulated by LT-IIa and LT-IIb induced production of greater amounts of TNF-α and IL-12p40 by monocyte-derived dendritic cells than were induced by treatment of those cells with CT (Martin et al., 2001). Polarization to a Th2 response is often mediated by reduction in the CD8+ T cell population. CT and LT-IIa, but not LT-IIb, stimulate apoptosis in CD8+ T cells (Arce et al., 2005). Neither LT-IIa nor LT-IIb has a negative effect on mitogen-driven CD4+ T cell proliferation, in contrast to the effects of CT on those cells (Arce et al., 2005). These and other immunomodulatory effects stimulated by LT-IIa and LT-IIb, and their differences from the effects of CT, are summarized in Table 2 (Arce et al., 2007; Arce et al., 2005; Nawar et al., 2007; Nawar et al., 2010b).
4.2 Adjuvant properties of LT-IIc, the newest member of the type II HLT subfamily
LT-IIc is the most recent addition to the type II subfamily of HLTs (Nawar et al., 2010b) and, as such, has not been as extensively investigated in respect to immunodulatory properties. When used as intranasal adjuvant, LT-IIc is also capable of augmenting mucosal (IgA) and systemic (IgG) responses to AgI/II, a surface antigen from Streptococcus mutans (Russell et al., 1980). Cellular analysis indicates that the immunomodulatory properties of LT-IIc are similar to those of LT-IIb; splenic cells from mice administered either LT-IIc or LT-IIb as mucosal adjuvants exhibit enhanced production of IL-2, IL-6, IL-17a, IFN-γ, and TNF-α in comparison to mice that received only the immunizing antigen (Nawar et al., 2011). Production of the anti-inflammatory IL-10 is suppressed in cells from mice that received LT-IIc (or LT-IIb) as a mucosal adjuvant, a cytokine response which might be expected to be produced by an efficient adjuvant. Interesting, LT-IIc differs from LT-IIb in a capacity to stimulate production by peritoneal macrophages of the pro-inflammatory cytokines IL-1α and IL-1β (Nawar et al., 2011). While LT-IIa and LT-IIb appear to drive a more balanced Th1/Th2 immune response to a co-administered antigen, recent studies suggest that, in comparison to LT-IIa or LT-IIb, LT-IIc has a greater capacity to drive antigen-specific Th1 type immune responses (T.D.C., unpublished observations).
4.3 Gangliosides and adjuvanticity
It is likely that the unique patterns of immunostimulatory responses that are induced by CT, LT-I, LT-IIa, LT-IIb, and LT-IIc are modulated by the enterotoxins’ distinctive binding affinities for different gangliosides (Arce et al., 2005; Berenson et al., 2010; Nawar et al., 2005, 2007) that likely participate directly as signal transducers or indirectly as co-factors for immunological-associated signal transduction pathways. To investigate the correlation between immunomodulation and GM1-binding, LT-I(G33D) [and CT(G33D)] were employed in various immunization and immunomodulation models. LT-I(G33D), unlike the parental LT-I, was unable to augment the levels of IgA or IgG against a co-administered antigen in an oral immunization model [113]. Interestingly, LT-I(G33D) retained some toxic activity in a mouse Y1 adrenal cell bioassay and stimulated increased production of cAMP in CaCo-2 cells. These data indicated that the mutation in LT-I did not fully abrogate binding of the mutant enterotoxin to GM1. It is clear that binding to ganglioside GM1 is an essential feature for the adjuvant properties of CT and LT-I. But, could other gangliosides participate in requisite molecular and cellular events needed to promote adjuvanticity?
As noted above, LT-IIa and LT-IIb, when employed as adjuvants, induce immune responses that are distinctive from those induced by the use of either CT or LT-I. Since LT-IIa and LT-IIb bind to a number of gangliosides in addition to GM1, the ganglioside receptor for CT and LT-I, it is intriguing to speculate that binding of LT-IIa and LT-IIb to non-GM1 gangliosides, or the differential affinities of LT-IIa and LT-IIb for particular gangliosides including GM1, is essential for modulating those distinctive patterns of immune responses that are observed. Furthermore, it is clear that single amino acid changes in the B pentamers of the type II HLT dramatically alter toxicity (see above) without significantly reducing adjuvancity which is best illustrated by a series of ganglioside-binding mutants of LT-IIa. Members of this group of mutants exhibit stepwise reductions in binding affinities for the ganglioside receptors bound avidly by wt LT-IIa. LT-IIa(T14S), a mutant with a serine substitution for threonine at amino acid position 14 in the B polypeptide of LT-IIa, binds avidly to GD1b, GD1a, and GM1, and with lesser avidity to GM2, GM3, and GQ1b, a pattern that is identical to the binding patterns of wt LT-IIa (Nawar et al., 2007). Toxicity of LT-IIa(T14S) is equivocal to the toxicity of wt LT-IIa. Alternatively, LT-IIa(T14I) and LT-IIa(T14D), mutants substituted at amino acid position 14 with isoleucine or aspartic acid, respectively, have significantly reduced binding to those gangliosides, and exhibit little or no toxicity. Yet, all three mutants retained strong adjuvant properties for enhancing mucosal and systemic immune responses to a co-administered antigen (Nawar et al., 2007). These results suggested that weaker binding to one or more ganglioside receptors by LT-IIa was sufficient for immune augmentation, while strong binding was required for toxicity.
A similar pattern of loss of ganglioside-binding activity, toxicity, and adjuvanticity is observed for LT-IIb and its respective mutant holotoxins. Specifically, the non-toxic LT-IIb(T13I) mutant, which in comparison to the binding patterns of wt LT-IIb is observed in mucosal immunization model to have adjuvant properties nearly equivalent to those exhibited by LT-IIb (Nawar et al., 2005). As noted above, while LT-IIb(T13I) binds to many of the NeuAc gangliosides and NeuGlc gangliosides bound by wt LT-IIb, the mutant binds with much lower affinities. And, it binds preferentially to NeuGlc gangliosides (Berenson et al., 2010; Nawar et al., 2010a). Again, these data indicate that strong binding to the endogenous ganglioside receptors is required to mediate toxicity of LT-IIb, while less avid binding to gangliosides is sufficient for the enterotoxin’s adjuvant properties. This model is further strengthened by the use of a Cmah knock-out mouse, which expresses only NeuAc gangliosides for which LT-IIb(T13I) has limited avidity for binding. When co-immunized with AgI/II, Cmah-deficient mice receiving LT-IIb(T13I) as a mucosal adjuvant respond by elevating the levels of antigen-specific IgA in the saliva and vaginal fluids and antigen-specific IgG in the serum (Nawar et al., 2011).
Overall, these mutants indicate that potentially useful clinical adjuvants can be genetically engineered by altering the ganglioside-binding properties of LT-IIa, LT-IIb, and LT-IIc to eliminate toxicity while retaining adjuvanticity. Furthermore, these mutants will be critical reagents in experiment to define the roles of gangliosides in immunomodulation, a field that has not received significant attention in immunology.
5. Interactions of Type II B pentamers with Toll-like receptors
It is now firmly established that cellular activation by microbial molecules generally involves interactions with several co-operating host receptors within membrane lipid rafts, which serve as signaling platforms (Simons and Toomre, 2000; Triantafilou et al., 2002; Triantafilou and Triantafilou, 2005). Conversely, the interaction of ligands of microbial origin with a single host receptor (i.e., in isolation from co-receptors) may often represent an oversimplified model. As discussed above, gangliosides are the predominant receptors for Type I or Type II enterotoxins. However, circumstantial evidence accumulating since the 2000s has suggested that at least some of the immunomodulatory properties of the heat-labile enterotoxins could involve interactions with non-ganglioside receptors. In this regard, point mutations in the B subunits of Type I enterotoxins, specifically CT(H57A) and LT-I(H57S), rendered the molecules defective in immunomodulatory signaling and toxicity although both mutants retained high-affinity binding to GM1 (Aman et al., 2001). These data could be interpreted to mean that structural alterations in CT(H57A) and LT-I(H57S), while not preventing GM1 binding, inhibited their ability to interact with co-receptor(s) requiredfor signaling.
In an effort to identify immunostimulatory activities mediated exclusively by the non-catalytic B subunits of the enetrotoxins, it was discovered that the B subunits of the Type II, but not Type I, enterotoxins uniquely interact with Toll-like receptors (TLR). Specifically, LT-IIa-B5 and LT-IIb-B5 were shown to activate human monocytes or mouse macrophages in a TLR2-dependent way (Hajishengallis et al., 2005a). Moreover, it was shown that TLR1 was a signaling partner for TLR2 in response to cell activation by LT-IIa-B5 or LT-IIb-B5 (Hajishengallis et al., 2005a).
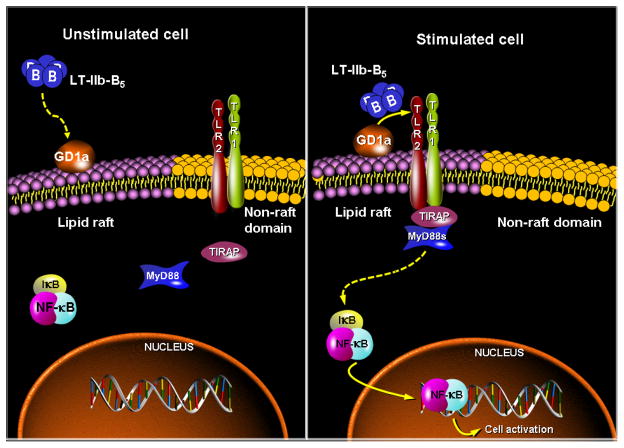
Subsequently, on the basis of combined molecular, biophysical, and cell imaging approaches, it was established that GD1a and TLR2/TLR1 cooperate in molecular proximity for recognition of LT-IIb-B5 and induction of immunostimulatory signaling (Liang et al., 2007a). These studies led to a model according to which LT-IIb-B5 initially binds the lipid raft-associated GD1a ganglioside which facilitates the interaction of LT-IIb-B5 with the TLR2/TLR1 signaling complex that is recruited ad hoc to lipid rafts. Moreover, the intracellular adaptor known as TIRAP (Toll/IL-1R domain-containing adaptorprotein) or Mal (MyD88-adaptor-like) is induced to colocalize with the LT-IIb-B5 receptor complex (GD1a/TLR2/TLR1) and is essential for NF-κB activation and induction of cytokine production by LT-IIb-B5 (Liang et al., 2007a). Additionally, the MyD88 signaling adaptor is also required for cell activation by LT-IIb-B5 (Liang et al., 2009b). These experiments were initially performed in primary cells but were confirmed by mechanistic studies in transfected cell lines, which additionally established the specificity of these interactions. Indeed, irrelevant gangliosides (GM1, GD1b) or TLRs (TLR4) could not support the immunostimulatory activity of LT-IIb-B5 (Liang et al., 2007a). These findings are summarized in a mechanistic model that is shown in Figure 1.
Figure 1. Model for ganglioside-TLR cooperation for cell activation by LT-IIb-B5.
Upon binding of LT-IIb-B5 to GD1a, GD1a facilitates the interaction of LT-IIb-B5 with the TLR2/TLR1 signaling complex, which is recruited to lipid rafts. Induction of TLR2/TLR1 signaling for NF-κB activation by LT-IIb-B5 occurs at the cell surface and requires the adaptor proteins TIRAP and MyD88, which colocalize with the LT-IIb-B5 receptor complex (GD1a/TLR2/TLR1) (Hajishengallis et al., 2005a; Liang et al., 2009b; Liang et al., 2007a).
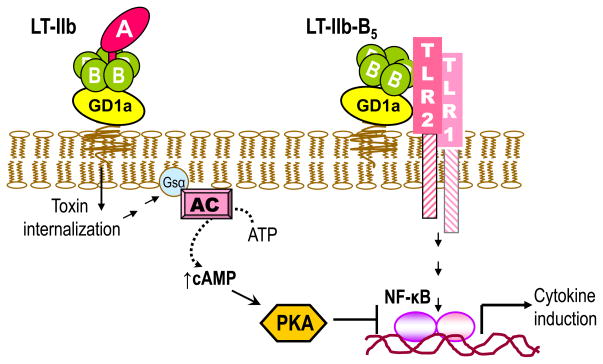
Additional evidence for the exquisite specificity of the B pentamer of LT-IIb in its interactions with TLRs, as depicted in Figure 1, was provided by findings that the LT-IIb holotoxin does not bind or activate the TLR2/TLR1 complex, although LT-IIb binds GD1a with high-affinity (Liang et al., 2007a). This was attributed to the presence of the A subunit which, in either wild-type or catalytically inactive version, appears to sterically interfere with TLR2 binding (Liang et al., 2007b). In fact, LT-IIb inhibits LT-IIb-B5-induced NF-κB activation through cAMP-dependent protein kinase A signaling (Liang et al., 2007b) (Figure 2). These data were pivotal for determining the molecular basis of the LT-IIb-B5 interaction with the TLR2/TLR1 heterodimer (see below).
Figure 2. Differential and antagonistic effects on NF-κB activation by LT-IIb holotoxin and its B pentamer.
LT-IIb-B5 activates the TLR2/TLR1 heterodimer and induces NF-κB-dependent production of proinflammatorry cytokines (Liang et al., 2009a; Liang et al., 2007b). In contrast, the holotoxin does not interact with TLR2/TLR1 due to A subunit-dependent steric hindrance (Liang et al., 2009a; Liang et al., 2007b). However, upon GD1a binding and internalization of the holotoxin, the ADP-ribosyltransferase activity of its A subunit activates the Gsα component of adenylate cyclase (AC). This leads to elevation of intracellular cAMP, activation of cAMP-dependent protein kinase A (PKA), and inhibition of NF-κB-dependent transcription of proinflammatory cytokines (e.g., TNF-α) (Liang et al., 2007b).
Although TLR2/TLR1 employs hydrophobic interactions for ligand binding (Jin et al., 2007; Nishiguchi et al., 2001; Okusawa et al., 2004), LT-IIb-B5 participates in both hydrophilic and hydrophobic interactions mediated by distinct regions of the molecule. The so-called lower region interacts hydrophilicly with the oligosaccharide moiety of GD1a, whereas the upper region of the B pentamer pore contains a large hydrophobic surface (494-Å2) which engages in hydrophobic interactions with the A2 segment of the A subunit (van den Akker et al., 1996). In the absence of the A subunit, however, the upper region hydrophobic surface becomes solvent accessible (van den Akker et al., 1996). It was, therefore, hypothesized that the solvent-accessible hydrophobic upper region of LT-IIb-B5 was critical for TLR2 binding, since the TLR2 binding interaction was abrogated when the upper region is masked by the A subunit in the LT-IIb holotoxin (Liang et al., 2007b). This region comprises a ring of hydrophobic residues, namely M69, A70, L73, and the Cβ of S74 (the Oγ atom of S74 is involved in a hydrogen bond in a way that leaves the hydrophobic Cβ atom of S74 pointing towards the interior of the pore), of each of the five B subunits. To address the hypothesis, point substitution mutations rendering these residues hydrophilic were engineered. All four point mutants constructed (M69E, A70D, L73E, S74D) were defective in binding TLR2 and inducing TLR2/TLR1-dependent cell activation, but retained full GD1a-binding capacity, thus confirming the hypothesis (Liang et al., 2009a). Moreover, the results implied that these point mutations do not cause global alterations in the B pentamer, since GD1a binding requires successful assembly of the B pentamers (van den Akker et al., 1996). Intriguingly, M69, A70, L73, and S74 are exactly shared by LT-IIa-B5, which also activates TLR2 (Hajishengallis et al., 2005b), but not by the Type I B pentamers, LT-I-B5 and CT-B5 (van den Akker et al., 1996). The fact that LT-IIc-B5 also shares these critical residues predicts that this B pentamer may also activate TLR2, although this hypothesis has not been tested yet.
To determine which segment(s) of TLR1 are required for cooperative TLR2-induced cell activation in response to LT-IIb-B5, TLR1/TLR6 chimeric receptors were constructed by reciprocally exchanging increasing segments of N-terminal leucine-rich repeat (LRR) modules between these two highly homologous receptors. The rationale was based on the finding that TLR6 does not support LT-IIb-B5-induced TLR2 activation (Hajishengallis et al., 2005b). It was found that the LRR9–12 region of TLR1 is critical for cooperative TLR2-induced cell activation by LT-IIb-B5 (Liang et al., 2009a). Moreover, specific point mutations in the TLR2/TLR1 dimer interface resulted in diminished cell activation by LT-IIb-B5 (Liang et al., 2009a). Subsequent docking analysis of the LT-IIb-B5 interaction with TLR2/TLR1 revealed that the most possible model involves interaction between the hydrophobic surface in the upper region of LT-IIb-B5 and the convex surface of the central domains of TLR2/TLR1 (Liang et al., 2009a). LT-IIb-B5 interacts primarily with the TLR2 component of the complex (the TLR1/LT-IIb-B5 interface is relatively smaller) and the putative contact surface partially overlaps with the crystallographically determined Pam3CSK4-binding site (Jin et al., 2007). This theoretical prediction was confirmed by the finding that the Pam3CSK4 lipopeptide dose-dependently inhibits TLR2 binding of LT-IIb-B5, albeit not as potently as self-competitive inhibition (Liang et al., 2009a). Therefore, the interaction of LT-IIb-B5 with TLR2/TLR1 depends on specific hydrophobic interactions. The TLR2-binding site of LT-IIb-B5 is defined by a ring of four upper region residues (M69, A70, L73, and S74) (Liang et al., 2009a), which, strikingly, are also critical for hydrophobic interactions between the B pentamer and the A2 segment of the A subunit in the fully-assembled LT-IIb holotoxin (van den Akker et al., 1996). As a result, the TLR2-binding site is blocked in the intact holotoxin.
6. TLR-dependent adjuvant activities of Type II B pentamers
The demonstration that Type II B pentamers interact with TLR2 suggested a novel mechanism whereby enterotoxin derivatives can be exploited as vaccine adjuvants, i.e., by eliciting TLR2-dependent immunostimulatory activity. Indeed, TLRs can function as adjuvant receptors by virtue of their ability to stimulate APC function and thereby bridge innate to adaptive immunity (Iwasaki and Medzhitov, 2004). In this regard, TLR agonists and synthetic analogues are currently major targets for developing vaccine adjuvants to prevent infectious diseases (Gearing, 2007).
To assess the immunostimulatory potential of LT-IIb-B5 in dendritic cells, a TLR pathway-focused real-time PCR array analysis was performed in LT-IIb-B5-stimulated bone marrow-derived dendritic cells (BMDC). Significantly upregulated genes included those for various cytokines (Il10, Il1a, Il12a, Il1b, Tnf, and Il6), TLRs or co-receptors (Tlr1, Tlr2, Cd14), or co-stimulatory molecules (Cd80) (Liang et al., 2009b). However, the upregulation in the expression of these genes was abrogated in LT-IIb-B5–stimulated MyD88-deficient BMDC (Liang et al., 2009b), consistent with the requirement for MyD88 in TLR2 immunostimulatory signaling. These findings implied that LT-IIb-B5 may enhance the Ag-presenting function of BMDC. Indeed, it was shown that LT-IIb-B5, but importantly not the TLR2-nonbinding mutant S74D, stimulates TLR2-dependent activation of BMDC, which in turn provide functional costimulation to CD4+ T cells. Specifically, LT-IIb-B5 induces cytokine production (e.g., IL-6 and TNFα) and upregulates the expression of class II MHC and costimulatory molecules (CD40, CD54, CD80, and CD86) in BMDC, resulting in costimulation of co-cultured CD4+ T cells as evidenced by their enhanced proliferation and IL-2 production (Liang et al., 2009a; Liang et al., 2009b).
To determine whether LT-IIb-B5 exerts mucosal adjuvant activity in vivo, groups of mice were immunized intranasally with a streptococcal protein immunogen– the AgI/II adhesin of Streptococcus mutans (10 μg) – in the absence or presence of LT-IIb-B5 or intact LT-IIb (both at 1 μg) (Liang et al., 2009b). Mice given AgI/II with either LT-IIb-B5 or LT-IIb elicited significantly higher mucosal and systemic AgI/II-specific Ab responses than mice immunized with AgI/II alone (Liang et al., 2009b). Strikingly, the capacity of LT-IIb-B5 to stimulate salivary IgA Ab responses to AgI/II was comparable to that of LT-IIb, although the holotoxin was more potent in augmenting vaginal IgA or serum IgG Ab responses (Liang et al., 2009b). As discussed above, LT-IIb does not activate TLR2 (Liang et al., 2007b) and its powerful adjuvant properties clearly indicate that induction of TLR signaling is not an obligatory mechanism for adjuvanticity. Thus, LT-IIb and LT-IIb-B5 appear to function through distinct adjuvant mechanisms, although the intrinsic enterotoxicity of LT-IIb precludes its use in human vaccines.
Similarly to LT-IIb-B5, LT-IIa-B5 also induced TLR2-dependent activation of Ag-presenting function resulting in enhanced CD4+ T cell proliferation in vitro. Specifically, the expression of co-stimulatory molecules (CD40, CD80, CD86, and MHC-II) and cytokines (IL-12p40, TNF-α, and IFN-γ) was increased in LT-IIa-B5-treated BMDC. Moreover, LT-IIa-B5 enhanced the uptake of a model Ag (OVA-FITC) by BMDC, in contrast to CT- B5 which had no effect (Lee et al., 2010). The ability of LT-IIa-B5 to enhance Ag uptake and to induce expression of co-stimulatory molecules and cytokines by BMDC was strictly dependent upon cellular expression of TLR2. Importantly, the improved Ag uptake induced by LT-IIa-B5 was correlated with increased Ag-specific proliferation of CD4+ T cells in an in vitro syngeneic DO11.10 CD4+ T cell proliferation assay (Lee et al., 2010). These experiments suggested that LT-IIa-B5 exhibits potent immunostimulatory properties which could be exploitable as a non-toxic mucosal adjuvant.
This notion was subsequently confirmed in vivo as LT-IIa-B5 was shown to enhance mucosal immune responses by modulating the activities of DC. Specifically, intranasal (i.n.) immunization of mice with OVA, in the presence of LT-IIa-B5, increased the uptake of the Ag by DC in the NALT in wt but not TLR2-deficient mice (Lee et al., 2011). Furthermore, LT-IIa-B5 enhanced the migration of DC from the NALT to the draining cervical lymph nodes (CLN) in a manner that required cellular expression of TLR2 and the C-C chemokine receptor 7 (CCR7). LT-IIa-B5 additionally augmented enhanced maturation of DC in the CLN, as shown by elevated expression of costimulatory molecules (CD40, CD80, and CD86). This was accompanied by Ag-specific CD4+T cell proliferation in the CLN of mice that had received i.n. LT-IIa-B5. Finally, LT-IIa-B5 caused a dramatic increase in the levels of OVA-specific salivary IgA and OVA-specific serum IgG in wild-type but not in TLR2-deficient mice. These data firmly established that the immunomodulatory properties of LT-IIa-B5 depend on appropariate immunomodulation of mucosal DCs (Lee et al., 2011).
Recently, the B pentamer of the third member of the LT-II enterotoxins, the LT-IIc-B5 was also shown to enhance the uptake of Ag by BMDC. In fact, LT-IIc-B5 achieved this while exceeding the levels attained by use of LT-IIa-B5,LT-IIb-B5, or LPS. It is not known yet whether this activity of LT-IIc-B5 is similarly TLR2-dependent. In this regard, the ability of LT-IIc-B5 to interact with TLR2 has not been addressed yet, although this is likely since it shares the TLR2-interactive region (M69, A70, L73, and S74) of the other LT-II B pentamers.
7. Conclusions
The HLTs are fascinating molecular tools for discovering basic mechanisms of adjuvanticity or immunomodulation. The divergence in the sequences of their B polypeptides has led to distinct binding activities for gangliosides, Toll-like receptors, and perhaps still unidentified immunomodulatory receptors. This in turn determines, in great part, their distinct immunomodulatory activities, outlined in this review. From a translational viewpoint, recent discoveries have shown that catalytically defective holotoxin mutants retain significant adjuvanticity, whereas the B pentamers of Type II HLTs can activate TLR2-dependent adjuvant action. Although it is unlikely that every useful adjuvant property of HLTs can be dissociated from toxicity, available data indicate that it is feasible to construct HLT derivatives, with minimal or no toxic effects, that can be used in vaccine formulations against mucosal infections in the near future.
Acknowledgments
Studies performed in the authors’ lab and cited in this review were supported by U.S. Public Health Service Grants DE015254 and DE017138 (to GH) and DE013833 (to TDC).
References
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Aman AT, Fraser S, Merritt EA, Rodigherio C, Kenny M, Ahn M, Hol WGJ, Williams NA, Lencer WI, Hirst TR. A mutant cholera toxin B subunit that binds GM1- ganglioside but lacks immunomodulatory or toxic activity. Proc Natl Acad Sci USA. 2001;98:8536–8541. doi: 10.1073/pnas.161273098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce S, Nawar HF, Muehlinghaus G, Russell MW, Connell TD. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infect Immun. 2007;75:1413–1423. doi: 10.1128/IAI.01367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce S, Nawar HF, Russell MW, Connell TD. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation of lymphoid cells. Infect Immun. 2005;73:2718–2727. doi: 10.1128/IAI.73.5.2718-2727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H, Thompson AH, Iwasaki T, Sato Y, Inaba Y, Aizawa C, Kurata T, Tamura S. Isolation and characterization of mouse nasal-associated lymphoid tissue. J Immunol Meth. 1997;202:123–131. doi: 10.1016/s0022-1759(96)00243-8. [DOI] [PubMed] [Google Scholar]
- Berenson CS, Nawar HF, Yohe HC, Castle SA, Ashline DJ, Reinhold VN, Hajishengallis G, Connell TD. Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I) Glycobiology. 2010;20:41–54. doi: 10.1093/glycob/cwp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J Biol Chem. 2000;275:8633–8640. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell T, Holmes R. Molecular genetic analysis of ganglioside GD1b-binding activity of Escherichia coli type IIa heat-labile enterotoxin by use of random and site-directed mutagenesis. Infect Immun. 1992;60:63–70. doi: 10.1128/iai.60.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell TD. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev Vaccines. 2007;6:821–834. doi: 10.1586/14760584.6.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell TD, Holmes RK. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol Microbiol. 1995;16:21–31. doi: 10.1111/j.1365-2958.1995.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Connell TD, Metzger D, Sfintescu C, Evans RT. Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol Lett. 1998;62:117–120. doi: 10.1016/s0165-2478(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Cox E, Verdonck F, Vanrompay D, Goddeeris B. Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Vet Res. 2006;37:511–539. doi: 10.1051/vetres:2006014. [DOI] [PubMed] [Google Scholar]
- Critchley DR, Magnani JL, Fishman PH. Interaction of cholera toxin with rat intestinal brush border membranes. Relative roles of gangliosides and galactoproteins as toxin receptors. J Biol Chem. 1981;256:8724–8731. [PubMed] [Google Scholar]
- Czerkinsky C, Anjuere F, McGhee JR, George-Chandy A, Holmgren J, Kieny MP, Fujiyashi K, Mestecky JF, Pierrefite-Carle V, Rask C, Sun JB. Mucosal immunity and tolerance: relevance to vaccine development. Immunol Rev. 1999;170:197–222. doi: 10.1111/j.1600-065X.1999.tb01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Dertzbaugh MT. Mucosal adjuvants. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, Strober W, McGhee JR, editors. Mucosal Immunology. Elsevier/Academic Press; San Diego: 2005. pp. 967–986. [Google Scholar]
- Fishman PH. Recent advances in identifying the functions of gangliosides. Chem Phys Lipids. 1986;42:137–151. doi: 10.1016/0009-3084(86)90049-6. [DOI] [PubMed] [Google Scholar]
- Fukuta S, Magnani JL, Twiddy EM, Holmes RK, Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing AJH. Targeting toll-like receptors for drug development: a summary of commercial approaches. Immunol Cell Biol. 2007;85:490–494. doi: 10.1038/sj.icb.7100102. [DOI] [PubMed] [Google Scholar]
- Gill DM, Clements JD, Robertson DC, Finkelstein RA. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981a;33:677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DM, Clements JD, Robertson DC, Finkelstein RA. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981b;33:677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeringer GC, Vidic B. The embryogenesis and anatomy of Waldeyer’s ring. Otolaryngol Clin North Am. 1987;20:207–217. [PubMed] [Google Scholar]
- Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J Dent Res. 2005a;84:1104–1116. doi: 10.1177/154405910508401205. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Nawar H, Tapping RI, Russell MW, Connell TD. The Type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect Immun. 2004;72:6351–6358. doi: 10.1128/IAI.72.11.6351-6358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Nikolova E, Russell MW. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A antibodies to the cell surface protein antigen I/II: Reversal by IgA1 protease cleavage. Infect Immun. 1992;60:5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Russell MW. Molecular approaches to vaccination against oral infections. In: Rogers A, editor. Molecular Oral Microbiology. Caister Academic Press; Norfolk, UK: 2008. pp. 257–285. [Google Scholar]
- Hajishengallis G, Tapping RI, Martin MH, Nawar H, Lyle EA, Russell MW, Connell TD. Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect Immun. 2005b;73:1343–1349. doi: 10.1128/IAI.73.3.1343-1349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Structure and function of sphingoglycolipids in transmembrane signalling and cell-cell interactions. Biochem Soc Trans. 1993;21 ( Pt 3):583–595. doi: 10.1042/bst0210583. [DOI] [PubMed] [Google Scholar]
- Hakomori S, Igarashi Y. Functional role of glycosphingolipids in cell recognition and signaling. J Biochem (Tokyo) 1995;118:1091–1103. doi: 10.1093/oxfordjournals.jbchem.a124992. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Linardic CM. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993;1154:223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- Heritage PL, Underdown BJ, Arsenault AL, Snider DP, McDermott MR. Comparison of murine nasal-associated lymphoid tissue and Peyer’s patches. Am J Respir Crit Care Med. 1997;156:1256–1262. doi: 10.1164/ajrccm.156.4.97-03017. [DOI] [PubMed] [Google Scholar]
- Holmes RK, Jobling MG, Connell TD. Cholera toxin and related enterotoxins of gram-negative bacteria. In: Moss J, BI, Vaughn M, Tu AT, editors. Bacterial toxins and virulence factors in disease. Handbook of natural toxins. Marcel Dekker, Inc; New York: 1995. pp. 225–255. [Google Scholar]
- Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine. 2003;21(Suppl 2):S89–95. doi: 10.1016/s0264-410x(03)00206-8. [DOI] [PubMed] [Google Scholar]
- Holmgren J, Fredman P, Lindblad M, Svennerholm AM, Svennerholm L. Rabbit intestinal glycoprotein receptor for Escherichia coli heat-labile enterotoxin lacking affinity for cholera toxin. Infect Immun. 1982;38:424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J, Svennerholm A-M. Mucosal immunity to bacteria. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, Strober W, McGhee JR, editors. Mucosal Immunology. 3. Elsevier/Academic Press; Amsterdam: 2005. pp. 783–797. [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik SG, Lee H, Lee JO. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Jobling MG, Holmes RK. Analysis of structure and function of the B subunit of cholera toxin by the use of site-directed mutagenesis. Mol Microbiol. 1991;5:1755–1767. doi: 10.1111/j.1365-2958.1991.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Jobling MG, Holmes RK. Mutational analysis of ganglioside GM(1)-binding ability, pentamer formation, and epitopes of cholera toxin B (CTB) subunits and CTB/heat-labile enterotoxin B subunit chimeras. Infect Immun. 2002;70:1260–1271. doi: 10.1128/IAI.70.3.1260-1271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nature Reviews Immunology. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper CF, Koornstra PJ, Hameleers DM, Biewenga J, Spit BJ, Duijvestijn AM, van Breda Vriesman PJ, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- Kuziemko GM, Stroh M, Stevens RC. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochem. 1996;35:6375–6384. doi: 10.1021/bi952314i. [DOI] [PubMed] [Google Scholar]
- Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev Vaccines. 2007;6:673–684. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- la Sala A, He J, Laricchia-Robbio L, Gorini S, Iwasaki A, Braun M, Yap GS, Sher A, Ozato K, Kelsall B. Cholera toxin inhibits IL-12 production and CD8alpha+ dendritic cell differentiation by cAMP-mediated inhibition of IRF8 function. J Exp Med. 2009;206:1227–1235. doi: 10.1084/jem.20080912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge W, Denes B, Fodor I. Cholera toxin B subunit modulation of mucosal vaccines for infectious and autoimmune diseases. Curr Opin Investig Drugs. 2010;11:919–928. [PubMed] [Google Scholar]
- Lavelle EC, Jarnicki A, McNeela E, Armstrong ME, Higgins SC, Leavy O, Mills KH. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J Leukoc Biol. 2004;75:756–763. doi: 10.1189/jlb.1103534. [DOI] [PubMed] [Google Scholar]
- Lavelle EC, McNeela E, Armstrong ME, Leavy O, Higgins SC, Mills KH. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J Immunol. 2003;171:2384–2392. doi: 10.4049/jimmunol.171.5.2384. [DOI] [PubMed] [Google Scholar]
- Lee CH, Masso-Welch P, Hajishengallis G, Connell TD. TLR2-dependent modulation of dendritic cells by LT-IIa-B5, a novel mucosal adjuvant derived from a type II heat-labile enterotoxin. J Leukoc Biol. 2011;5:911–21. doi: 10.1189/jlb.0511236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Nawar HF, Mandell L, Liang S, Hajishengallis G, Connell TD. Enhanced antigen uptake by dendritic cells induced by the B pentamer of the type II heat-labile enterotoxin LT-IIa requires engagement of TLR2. Vaccine. 2010;28:3696–3705. doi: 10.1016/j.vaccine.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levery SB. Glycosphingolipid structural analysis and glycosphingolipidomics. Methods Enzymol. 2005;405:300–369. doi: 10.1016/S0076-6879(05)05012-3. [DOI] [PubMed] [Google Scholar]
- Liang S, Hajishengallis G. Heat-labile enterotoxins as adjuvants or anti-inflammatory agents. Immunol Invest. 2010;39:449–467. doi: 10.3109/08820130903563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Hosur KB, Lu S, Nawar HF, Weber BR, Tapping RI, Connell TD, Hajishengallis G. Mapping of a microbial protein domain involved in binding and activation of the TLR2/TLR1 heterodimer. J Immunol. 2009a;182:2978–2985. doi: 10.4049/jimmunol.0803737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Hosur KB, Nawar HF, Russell MW, Connell TD, Hajishengallis G. In vivo and in vitro adjuvant activities of the B subunit of Type IIb heat-labile enterotoxin (LT-IIb-B5) from Escherichia coli. Vaccine. 2009b;27:4302–4308. doi: 10.1016/j.vaccine.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Wang M, Tapping RI, Stepensky V, Nawar HF, Triantafilou M, Triantafilou K, Connell TD, Hajishengallis G. Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. J Biol Chem. 2007a;282:7532–7542. doi: 10.1074/jbc.M611722200. [DOI] [PubMed] [Google Scholar]
- Liang S, Wang M, Triantafilou K, Triantafilou M, Nawar HF, Russell MW, Connell TD, Hajishengallis G. The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J Immunol. 2007b;178:4811–4819. doi: 10.4049/jimmunol.178.8.4811. [DOI] [PubMed] [Google Scholar]
- Lycke N, Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010;3:556–566. doi: 10.1038/mi.2010.54. [DOI] [PubMed] [Google Scholar]
- Martin M, Metzger DJ, Michalek SM, Connell TD, Russell MW. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4(+) T cells. Infect Immun. 2001;69:4486–4492. doi: 10.1128/IAI.69.7.4486-4492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias-Santos C, Rodrigues JF, Sbrogio-Almeida ME, Connell TD, Ferreira LCS. Distinctive immunomodulatory and inflammatory properties of the Escherichia coli type II heat-labile enterotoxin LT-IIa and its B pentamer following intradermal administration. Clin Vaccine Immunol. 2011;18:1243–1251. doi: 10.1128/CVI.00012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos JJ, Collier RJ, Romig WR. Enzymic activity of cholera toxin. II Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979;254:5855–5861. [PubMed] [Google Scholar]
- Mudrak B, Kuehn MJ. Heat-labile enterotoxin: Beyond GM1 binding. Toxins. 2010;2:1445–70. doi: 10.3390/toxins2061445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J, Nguyen H, Czerkinsky C, Kiyono H. Oral immunization: an update. Curr Opin Gastroenterol. 2008;24:713–719. doi: 10.1097/MOG.0b013e32830d58be. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Iwamori M. Ganglioside distribution at different levels of organization and its biological implications. Adv Exp Med Biol. 1984;174:135–146. doi: 10.1007/978-1-4684-1200-0_12. [DOI] [PubMed] [Google Scholar]
- Nawar HF, Arce S, Russell MW, Connell TD. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect Immun. 2005;73:1330–1342. doi: 10.1128/IAI.73.3.1330-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawar HF, Arce S, Russell MW, Connell TD. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect Immun. 2007;75:621–633. doi: 10.1128/IAI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawar HF, Berenson CS, Hajishengallis G, Takematsu H, Mandell L, Clare RL, Connell TD. Binding to gangliosides containing N-acetylneuraminic acid is sufficient to mediate the immunomodulatory properties of the nontoxic mucosal adjuvant LT-IIb(T13I) Clin Vaccine Immunol. 2010a;17:969–978. doi: 10.1128/CVI.00076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawar HF, Greene CJ, Lee CH, Mandell LM, Hajishengallis G, Connell TD. LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb. Vaccine. 2011;29:721–727. doi: 10.1016/j.vaccine.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawar HF, King-Lyons ND, Hu JC, Pasek RC, Connell TD. LT-IIc, a new member of the type II heat-labile enterotoxin family encoded by an Escherichia coli strain obtained from a nonmammalian host. Infect Immun. 2010b;78:4705–4713. doi: 10.1128/IAI.00730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi M, Matsumoto M, Takao T, Hoshino M, Shimonishi Y, Tsuji S, Begum NA, Takeuchi O, Akira S, Toyoshima K, Seya T. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J Immunol. 2001;166:2610–2616. doi: 10.4049/jimmunol.166.4.2610. [DOI] [PubMed] [Google Scholar]
- Nohara K, Ozawa H, Tai T, Saji H, Fujimaki H. Gangliosides involved in activation of rat T lineage cells. Biochim Biophys Acta. 1997;1345:207–214. doi: 10.1016/s0005-2760(96)00181-6. [DOI] [PubMed] [Google Scholar]
- Nohara K, Suzuki M, Inagaki F, Sano T, Kaya K. A novel disialoganglioside in rat spleen lymphocytes. J Biol Chem. 1992;267:14982–14986. [PubMed] [Google Scholar]
- Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infect Immun. 2004;72:1657–1665. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi PA, Critchley DR, Fishman PH. The heat-labile enterotoxin of Escherichia coli binds to polylactosaminoglycan-containing receptors in CaCo-2 human intestinal epithelial cells. Biochemistry. 1994;33:12886–12895. doi: 10.1021/bi00209a021. [DOI] [PubMed] [Google Scholar]
- Pickett CL, Twiddy EM, Belisle BW, Holmes RK. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986;165:348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MW, Bergmeier LA, Zanders ED, Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980;28:486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EJ, McNeela E, Pizza M, Rappuoli R, O’Neill L, Mills KH. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro-and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J Immunol. 2000;165:5750–5759. doi: 10.4049/jimmunol.165.10.5750. [DOI] [PubMed] [Google Scholar]
- Satchell KJ. Activation and suppression of the proinflammatory immune response by Vibrio cholerae toxins. Microbes Infect. 2003;5:1241–1247. doi: 10.1016/j.micinf.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Sixma TK, Kalk KH, van Zanten BA, Dauter Z, Kingma J, Witholt B, Hol WG. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J Mol Biol. 1993;230:890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- Sixma TK, Pronk SE, Kalk KH, Wartna ES, van Zanten BA, Witholt B, Hol WG. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Acquotti D, Riboni L, Giuliani A, Kirschner G, Tettamanti G. New chemical trends in ganglioside research. Chem Phys Lipids. 1986;42:3–26. doi: 10.1016/0009-3084(86)90040-x. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. The dynamics of LPS recognition: complex orchestration of multiple receptors. J Endotoxin Res. 2005;11:5–11. doi: 10.1179/096805105225006641. [DOI] [PubMed] [Google Scholar]
- van den Akker F, Sarfaty S, Twiddy EM, Connell TD, Holmes RK, Hol WG. Crystal structure of a new heat-labile enterotoxin, LT-IIb. Structure. 1996;4:665–678. doi: 10.1016/s0969-2126(96)00073-1. [DOI] [PubMed] [Google Scholar]
- Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Critchley DR, Fishman PH, Moss J. Exogenous gangliosides enhance the interaction of fibronectin with ganglioside-deficient cells. Exp Cell Res. 1983;143:295–302. doi: 10.1016/0014-4827(83)90054-x. [DOI] [PubMed] [Google Scholar]
- Yohe HC, Ye S, Reinhold BB, Reinhold VN. Structural characterization of the disialogangliosides of murine peritoneal macrophages. Glycobiology. 1997;7:1215–1227. doi: 10.1093/glycob/7.8.1215. [DOI] [PubMed] [Google Scholar]
- Yura M, Takahashi I, Terawaki S, Hiroi T, Kweon MN, Yuki Y, Kiyono H. Nasal administration of cholera toxin (CT) suppresses clinical signs of experimental autoimmune encephalomyelitis (EAE) Vaccine. 2001;20:134–139. doi: 10.1016/s0264-410x(01)00278-x. [DOI] [PubMed] [Google Scholar]
- Zeller CB, Marchase RB. Gangliosides as modulators of cell function. Am J Physiol. 1992;262:C1341–1355. doi: 10.1152/ajpcell.1992.262.6.C1341. [DOI] [PubMed] [Google Scholar]
- Zhang RG, Scott DL, Westbrook ML, Nance S, Spangler BD, Shipley GG, Westbrook EM. The three-dimensional crystal structure of cholera toxin. J Mol Biol. 1995;251:563–573. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]