Abstract
Rationale
Several studies have documented impairments in memory processes as a result of ketamine administration; however, few studies have compared the profile of cognitive effects of ketamine to other drugs.
Objectives
The aim of this study was to compare the cognitive effects of ketamine with those of triazolam in healthy volunteers.
Methods
Doses of ketamine (0.2, 0.4 mg/kg intramuscular (i.m.)), triazolam (0.2, 0.4 mg/70 kg p.o.), and double-dummy placebos were administered to 20 volunteers under repeated measures, counterbalanced, double-blind conditions. Peak physiological, psychomotor, subjective, and cognitive effects were examined.
Results
Ketamine impaired balance when balance was assessed early in the task order, whereas triazolam impaired psychomotor coordination and divided attention irrespective of task order. Triazolam also tended to produce greater effects on working memory and episodic memory tasks than ketamine at doses that produced lower subjective effects and higher estimates of performance.
Conclusions
Ketamine produces less cognitive impairment than triazolam at doses that produced greater subjective effects. Thus ketamine does not produce the underestimation of cognitive impairment typically seen with triazolam.
Keywords: Ketamine, Triazolam, Cognitive, Memory, Psychomotor, Metacognition
Introduction
Ketamine is an N-methyl-D-aspartate receptor antagonist that is used therapeutically to induce anesthesia prior to the administration of a general anesthetic or for brief surgical procedures that do not require skeletal muscle relaxation (Ketamine Package Insert). Ketamine is also used recreationally for its mood-altering properties. Frequent or chronic ketamine use reportedly impairs working memory, source memory, and recognition memory (Morgan et al. 2004b, 2010), and users report impaired cognitive function and mental health problems (Muetzelfeldt et al. 2008).
Studies that have examined the acute effects of ketamine provide evidence for dose-related impairment of the encoding, but not retrieval, of episodic memory (Morgan et al. 2004a; Honey et al. 2005; Parwani et al. 2005; Rowland et al. 2005; Lofwall et al. 2006). Although many studies have documented the effect of ketamine on memory, relatively few have compared its effects to other drugs with different mechanisms of action. Given that different types of drug effects (e.g., amnesia, sedation, and dissociative effects) may lead to similar overt impairments in cognitive performance, it is important to understand how drugs with different mechanisms might lead to such impairments. The few studies that compare ketamine to other drugs have methodological limitations. For example, in one study, ketamine (0.26 mg/kg iv followed by 0.65 mg/kg/h) produced effects similar to lorazepam (2 mg) on an episodic word recall task, but the study was limited by the inclusion of only one dose of each drug (Krystal et al. 1998). In another study, ketamine (0.23 mg/kg iv followed by 0.5 mg/kg/h) impaired delayed recall whereas amphetamine (0.25 mg/kg) did not; however, that study was also limited by the inclusion of only a single dose of each drug (Krystal et al. 2005).
The primary purpose of the present study was to compare the cognitive and subjective effects of ketamine and triazolam, using two doses of both drugs in the same individuals. This study was designed to extend previous findings from our laboratory in which similar doses of ketamine (Lofwall et al. 2006) and triazolam (Carter et al. 2006, 2009) were examined separately in different studies. Data from those studies suggest that the pattern of effects observed with ketamine is distinct from what has been observed with the benzodiazepines; however, the effects of ketamine have not been rigorously compared to those of a benzodiazepine in the same study (Mintzer et al. 1997; Lofwall et al. 2006). Thus, the purpose of this study was to further examine these potentially distinct profiles of effects using a complete crossover design to control for individual differences. Doses of each drug were selected on the basis of results from previous studies in an attempt to produce similar magnitudes of psychomotor performance impairment at each dose level. Our hypothesis was that doses of ketamine that impair psychomotor performance to a similar extent as doses of triazolam would result in higher ratings of subjective effects and estimates of performance impairment with less actual cognitive impairment relative to triazolam.
Materials and methods
Participants
Twenty adult volunteers (ten males; eight Caucasian) completed this study. Participants ranged in age from 19 to 42 years (median 24 years), in weight from 52 to 91 kg (median 67 kg), and in education from 13 to 22 years (median 16 years). Participants reported consuming 0 to 12 alcoholic beverages per week (median one alcoholic beverage/week) and 0 to 467 mg caffeine/day (median 87 mg caffeine/day). One participant reported smoking cigarettes regularly (<20 cigarettes per day), but was comfortable with abstaining from tobacco use for the duration of the sessions.
Key exclusion criteria included a personal or family history of a severe psychiatric condition, a positive urine drug test, pregnancy, and nursing (more detailed criteria are provided in the “Electronic supplemental materials” section). The Johns Hopkins University School of Medicine Institutional Review Board approved this study. Participants gave their written informed consent before beginning the study and were paid for their participation.
General procedures
Five conditions (placebo, 0.2 and 0.4 mg/kg ketamine i.m., and 0.2 and 0.4 mg/70 kg triazolam p.o.) were studied during five separate outpatient sessions at the Behavioral Pharmacology Research Unit using a double-blind, double-dummy, crossover design. Prior to the first session, participants practiced the experimental tasks to achieve a stable level of performance. Before drug administration, participants’ urine was tested for the presence of cocaine, benzodiazepines, and opioids using an EMIT system (Syva Co., Palo Alto, CA, USA) and expired air was tested for the presence of alcohol using a breathalyzer test. Twice during each session, an i.m. injection (containing ketamine or placebo) and a capsule (containing triazolam or placebo) was administered. When triazolam was administered, it always occurred during the first administration event, whereas when ketamine was administered it always occurred during the second administration event. The two administration events were separated by 75 min so that the first post-drug assessment battery would capture the peak effects of each drug (additional methodological details are provided in the “Electronic supplemental materials” section).
The assessment battery (see below and “Electronic supplemental materials” section) was administered three times per session: prior to drug administration, following drug administration (approximately 80–85 min after triazolam and 5–10 min after ketamine), and then again approximately 120–130 min after the first post-drug battery (approximately 200 min after triazolam and 125 min after ketamine). To control for the dissipation of ketamine’s effects over the course of the 45-min battery, the task order was counterbalanced between participants. One task order (Form A) consisted of: blood pressure, heart rate, mean arterial pressure, respiration, balance, circular lights, digit-symbol substitution task, Sternberg Maintenance task, Sternberg Manipulation task, subjective effects questionnaire, and divided attention task. A second task order (Form B) consisted of the same tasks in the reverse order as described above. The working memory tasks (primary outcome measures) were placed in the middle of the battery to reduce the likelihood of an interaction between task order and drug effects on these measures. Episodic memory measures (recall and recognition) were assessed as described below. The Hallucinogen Rating Scale and Hood Mysticism Scale were administered at the end of the session (Strassman et al. 1994; Hood et al. 2001).
Drugs
Triazolam (Pharmacia Corp./Pfizer Inc., Kalamazoo, MI) and lactose monohydrate (placebo; Amend Drug and Chemical Company, Irvington, NJ) were orally administered (p.o.) in opaque capsules. Racemic ketamine HCl (50 mg/ml; Bedford Laboratories, Bedford, OH) and 0.9 % saline solution (placebo; Abbott Laboratories, Abbott Park, IL) were administered as a 1 ml i.m. injection in the participant’s non-dominant arm.
Physiological and psychomotor effects
Systolic and diastolic blood pressure, heart rate, and mean arterial pressure were taken using a Criticare monitor (Criticare Systems Inc., Waukesha, WI). Respiration was measured as the number of breaths per minute.
Balance
The time that a participant remained on one foot without opening his or her eyes or touching the floor or another part of his or her body with the raised foot was measured for up to 30 s with each foot (60 s total).
Circular lights
This task is a measure of psychomotor speed and coordination (Mumford et al. 1995). The dependent measure was the number of correct presses (i.e., lights extinguished) in 60 s.
Digit-symbol-substitution task (DSST)
This task was a computer version of the digit-symbol-substitution task (McLeod et al. 1982). Dependent measures were the number of correct patterns reproduced within 90 s. Before completion of the DSST, participants estimated how quickly and how accurately they expected to perform on the DSST task using a 100-mm visual analog scale (VAS). Participant estimates of performance were then compared to the actual task scores (see “Electronic supplemental materials” section).
Subjective effects measures
This questionnaire was based upon a previously described questionnaire in which participants were instructed to rate how they felt at present in response to 36 questions (e.g., “Do you feel sleepy?”) on 100-mm VASs (Rush et al. 1999; Carter et al. 2009).
Working memory measure
The two working memory tasks (modified Sternberg maintenance task and modified Sternberg manipulation task) are variants of the classic Sternberg task (Sternberg 1969) and were administered using procedures similar to those described by Mintzer and Griffiths (2007b) and described in greater detail in the “Electronic supplemental materials” section. Accuracy and reaction time were the primary dependent variables.
Before and after completion of each of the Sternberg working memory tasks, participants estimated how well they expected to perform or how well they thought they had just performed, respectively, using a 100-mm VAS scale. Participant estimates of performance were calculated as described above.
Divided attention task
Details associated with the divided attention task are described in greater detail in the “Electronic supplemental materials” section. The primary dependent measure associated with the digit monitoring component was proportion correct (number of times a mouse press was made when the target digit was presented in the corner of the screen out of a total possible of 24).
Episodic memory measures
During each session, participants studied a list of 36 words at the expected time of peak drug effects. To maintain their attention on the task, they were asked to categorize each word as “artificial” (i.e., man-made) or “natural” (Carter et al. 2009).
Free recall
120 min after they had studied the list of words, participants were given 5 min to write down all the words they could remember. The dependent measure was the number of correct words recalled within 5 min.
Recognition memory
Details associated with recognition memory are described in the “Electronic supplemental materials” section. Dependent measures were the proportion of old words correctly identified as old (collapsed across definitely old, probably old, and maybe old; this is the hit rate), the proportion of new words incorrectly identified as old (collapsed across definitely old, probably old, and maybe old; this is the false alarm rate), and signal detection measures of sensitivity in distinguishing between old and new words (d′) and response bias (C) (Snodgrass and Corwin 1988). Metamemory (participants’ awareness of the state of their memory) was assessed by the Goodman–Kruskal gamma correlation (a correlation between confidence and correctness in recognition; Goodman and Kruskal 1954) for the recognition memory task; this approach has been shown to be sensitive to detecting impairments in metamemory (Nelson 1984; Koriat and Goldsmith 1996; Mintzer et al. 2010).
Statistical analyses
Data were analyzed using repeated measures regression models with an AR(1) or CS covariance structure (as appropriate for the data set) in PROC MIXED in SAS version 9 (SAS Institute Inc., Cary, NC). The mean±standard error of the mean (SEM) is presented throughout. Data for measures that were assessed repeatedly are presented as a percentage of pre-drug (baseline) scores or difference scores (subjective effects measures). Data are presented for the first post-administration assessment only because drug effects had largely dissipated at the second post-administration assessment and data from the first post-administration assessment represent the peak drug effects observed in this study. Additional statistical details are provided in the “Electronic supplemental materials” section. When the F statistic for an effect was significant (p≤0.05), comparisons among means were conducted using simple effects tests. The false-positive rate for these comparisons was protected by a significant F statistic as specified by Fisher’s LSD (Keppel 1991).
Results
Effects as a function of task order
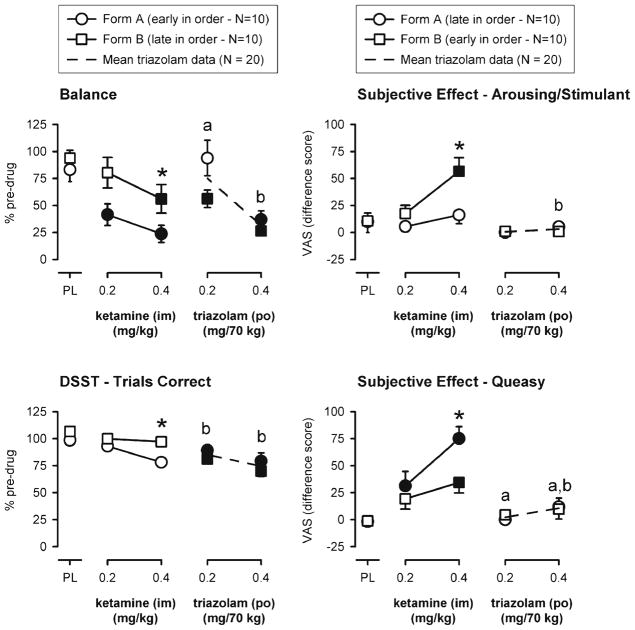
There was a significant task order by dose interaction for balance, DSST, and several subjective effects (alert, arousing or stimulant effect, sleepy, tired or lazy, and queasy). As can be seen in Fig. 1 (left panels), 0.4 mg/kg ketamine (i.m.) had significantly greater impairing effects on balance and DSST performance relative to placebo when those tasks were administered near the beginning of the battery (form A) as compared to the end of the battery (form B). The subjective effects questionnaire was administered near the end of the form A battery and near the beginning of the form B battery. Similarly, ratings of feeling an arousing or stimulant drug effect in the 0.4 mg/kg ketamine (i.m.) condition were significantly greater than placebo and a corresponding dose of triazolam when ratings were collected soon after ketamine administration (form B) relative to later in the session (Fig. 1, upper right panel). In contrast, ratings of subjective queasiness were higher later in session (i.e., form A as compared to form B; Fig. 1, lower right panel). Subjective ratings of sleepy, tired or lazy, and alert were somewhat mixed with numerically greater effects (higher ratings of sleepy and tired or lazy and lower ratings of alertness) of the low dose of ketamine occurring earlier (i.e., form B vs. form A) and greater effects of the high dose of ketamine occurring later (i.e., form A vs. form B; data not shown).
Fig. 1.
Representative measures on which there was an effect of task order. Task order was designated by form A or form B (see “Materials and methods” section for additional detail); balance and DSST occurred early in the task order on form A, whereas subjective effects measures occurred early in the task order on form B. Y-axes: score expressed as percent pre-drug (left panels) or rating expressed as a difference score from baseline (right panels). X-axes: dose in milligrams per kilogram (ketamine) or milligrams per 70 kg (triazolam) (log scale). PL designates placebo. Data points show means, brackets show ±1 SEM, and the absence of brackets indicates that 1 SEM fell within the area of the data symbol. Circles show data from form A, squares show data from form B, and filled symbols indicate values that are significantly different from their respective placebo value. Asterisks indicate a significant effect of task order within a dose. Letters (a or ) indicate a significant difference between ketamine and triazolam for the respective form (A or B) at that dose
There were no significant differences in the effects of triazolam as a function of task order and no significant task order by dose interactions for the remaining physiological, psychomotor performance, subjective effects, and working memory measures. Thus, data from the remaining assessments presented below collapsed across task order to allow for optimum statistical power.
Effects collapsed across task order
Working memory
Modified Sternberg maintenance task
Significant main effects of dose, but not delay, were observed on the percent of trials correct [F(4,72)<2.70, p<0.05] and on the median response time [F(4,72)<3.93, p<0.01] for the maintenance task with the control condition (prompt remained on screen) entered as a covariate. As shown in Fig. 2 (top, left panels), the larger doses of ketamine and triazolam significantly decreased working memory accuracy compared to placebo under the 0- and 12-s delay conditions. In contrast, only triazolam significantly increased median response times on the task. The increase in response time following 0.2 mg/70 kg triazolam (p.o.) was significantly greater than that of 0.2 mg/kg ketamine (i.m.; Fig. 2, lower left panels).
Fig. 2.
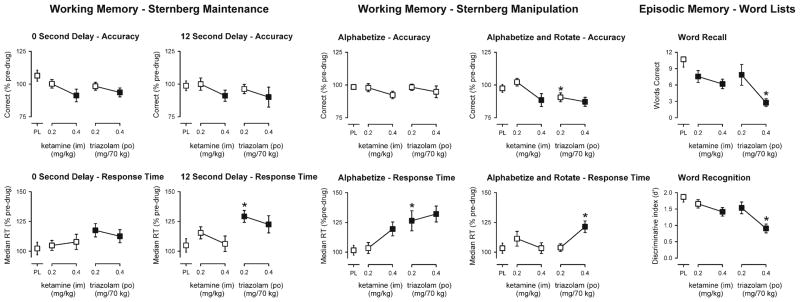
Peak effects of ketamine and triazolam on working memory and episodic memory tasks. Working Memory tasks: Y-axes represent the number of trials correct or the median reaction time expressed as a percent of pre-drug responding. Episodic Memory task: Y represent the number of correct words recalled or the ability to discriminate (discriminative index, d′) between old and new words. X-axes represent dose in milligrams per kilogram (ketamine) or milligrams per 70 kg (triazolam) (log scale). PL designates placebo. Data points show means, brackets show ±1 SEM. Filled symbols indicate a significant difference from placebo. An asterisk indicates a statistically significant difference between a dose of triazolam and the corresponding (i.e., low or high) dose of ketamine
Modified Sternberg manipulation task
Significant main effects of dose, but not manipulation condition, were observed on the percent of trials correct [F(4,72)<3.50, p<0.05] on the manipulation task with the control condition (no manipulation required) entered as a covariate. In contrast, there were significant main effects of dose [F(4,72)<4.71, p< 0.005], manipulation condition [F(1,18)<5.90, p<0.05], and a significant dose by manipulation interaction [F(4,72)<2.94, p<0.05], on the median response time. As shown in Fig. 2 (top, center panels), the larger doses of ketamine and triazolam significantly decreased working memory accuracy compared to placebo under the alphabetize and rotate condition, but not under the alphabetize condition. Neither of the smaller doses was significantly different from placebo; however, accuracy after 0.2 mg/70 kg triazolam was significantly lower than that after 0.2 mg/kg ketamine (Fig. 2). In contrast to the effects on accuracy, the effects of ketamine and triazolam on response times appeared to be greater under the alphabetize condition as compared to the alphabetize and rotate condition. The larger dose of ketamine (0.4 mg/kg, i.m.) significantly increased response times under the alphabetize condition and not under the alphabetize and rotated condition. Similarly, both doses of triazolam significantly increased response times under the alphabetize condition, whereas only the larger dose of triazolam significantly increased response times under the alphabetized and rotated condition (Fig. 2, bottom, center panels). There were significant differences between the smaller doses of each drug in the alphabetize condition and between the larger doses of each drug in the alphabetize and rotate condition with triazolam producing greater increases in response time in each case (Fig. 2, bottom, center panels).
Episodic memory
Significant main effects of dose were observed on the number of words correctly recalled in the free recall task [F(4,76)< 10.94, p<0.0001] and on the discriminative index (d′) for word recognition [F(4,76)<17.49, p<0.0001] (Fig. 2, right panels; Table 1). The number of words correctly recalled was significantly decreased by both doses of ketamine and triazolam; however, the larger dose of triazolam (0.4 mg/70 kg, p.o.) had a significantly greater amnesic effect than the larger dose of ketamine (0.4 mg/kg, i.m.; Fig. 2, upper right panel). The mean (±1 SEM) number of words recalled under the placebo condition was decreased from 10.7±1.4 to 6.2±0.9 words after the dose of 0.4 mg/kg ketamine, whereas only 2.8±0.7 words were recalled after the dose of 0.4 mg/70 kg triazolam.
Table 1.
Psychomotor and cognitive effects
| Dose condition | Circular lights | Divided attention
|
Word recognition
|
Metamemory | ||||
|---|---|---|---|---|---|---|---|---|
| Number completed | Tracking deviation | Proportion correct | Hit rate | False alarm rate | d′ | C | Gamma correlation | |
| Placebo | 102.9 (2.1) | 103.5 (2.8) | 104.1 (3.0) | 0.82 (0.02) | 0.21 (0.03) | 1.87 (0.15) | −0.03 (0.07) | 0.57 (0.05) |
| Ketamine | ||||||||
| 0.2 mg/kg (i.m.) | 94.7 (2.5) | 101.6 (2.2) | 97.2 (3.0) | 0.78 (0.02) | 0.22 (0.03) | 1.66 (0.13) | 0.02 (0.06) | 0.60 (0.05) |
| 0.4 mg/kg (i.m.) | 82.1 (3.0) | 118.4 (4.4) | 85.4 (4.5) | 0.73 (0.04) | 0.26 (0.03) | 1.41 (0.13) | 0.02 (0.11) | 0.59 (0.06) |
| Triazolam | ||||||||
| 0.2 mg/70 kg (p.o.) | 87.8 (2.3)a | 129.7 (5.6)a | 92.1 (2.6) | 0.74 (0.03) | 0.25 (0.04) | 1.53 (0.18) | 0.04 (0.10) | 0.51 (0.07) |
| 0.4 mg/70 kg (p.o.) | 73.2 (2.5)a | 172.1 (9.1) | 79.2 (3.9) | 0.62 (0.03)a | 0.32 (0.04) | 0.91 (0.14)a | 0.13 (0.09) | 0.41 (0.06)a |
Values in bold indicate significant differences from placebo
Indicates a significant difference between the low or high doses of ketamine and triazolam, respectively
Participants’ ability to discriminate between “old” and “new” words in the recognition task was significantly decreased after the larger dose of 0.4 mg/kg ketamine and after both doses of triazolam (Fig. 2, lower right panel). Like the effects of triazolam on free recall, the impairment of recognition by the larger dose of triazolam was significantly greater than that after the larger dose of ketamine. The effects of both drugs on the word recognition task appear to be a primary result of participants failing to identify “old” words as such because hit rates (old words correctly identified as old) were also significantly lower in cases in which the discriminative index was significantly decreased, whereas the false alarm rate (new or unstudied words incorrectly identified as old) was only significantly increased after the 0.4 mg/70 kg dose of triazolam (Table 1). There was no significant main effect of dose on response bias (C) (Table 1).
A significant main effect of dose was observed for gamma correlations (metamemory) [F(4,68)<3.50, p<0.05] and individual dose comparisons revealed that 0.4 mg/70 kg of triazolam was significantly different from placebo and the larger dose of 0.4 mg/kg ketamine (Table 1).
Divided attention
Significant main effects of dose were observed on both components of the divided attention task as measured by tracking deviation (distance in pixels between the diamond stimulus and cross hair) [F(4,72)<28.23, p<0.0001] and the proportion of targets correctly identified [F(4,72)<8.47, p< 0.0001] (Table 1). Triazolam (both doses), but not ketamine, significantly increased tracking deviation relative to placebo, although the larger dose of ketamine approached significance (p<0.055). The effects of the smaller and larger doses of triazolam on tracking deviation were significantly greater than those of the smaller and larger doses of ketamine, respectively (Table 1). The proportion of targets correctly identified was significantly decreased by both doses of triazolam and the larger dose of ketamine (Table 1). The effects of ketamine and triazolam on this measure were not significantly different from each other; however, there was a main effect of response time to identify correct targets [F(4,72)<3.17, p<0.05] and there was a significant difference between the two smaller doses of the drugs with significantly longer response times observed after 0.2 mg/70 kg triazolam as compared to 0.2 mg/kg ketamine (data not shown).
Subjective ratings of somatic effects, cognitive effects, and performance estimates
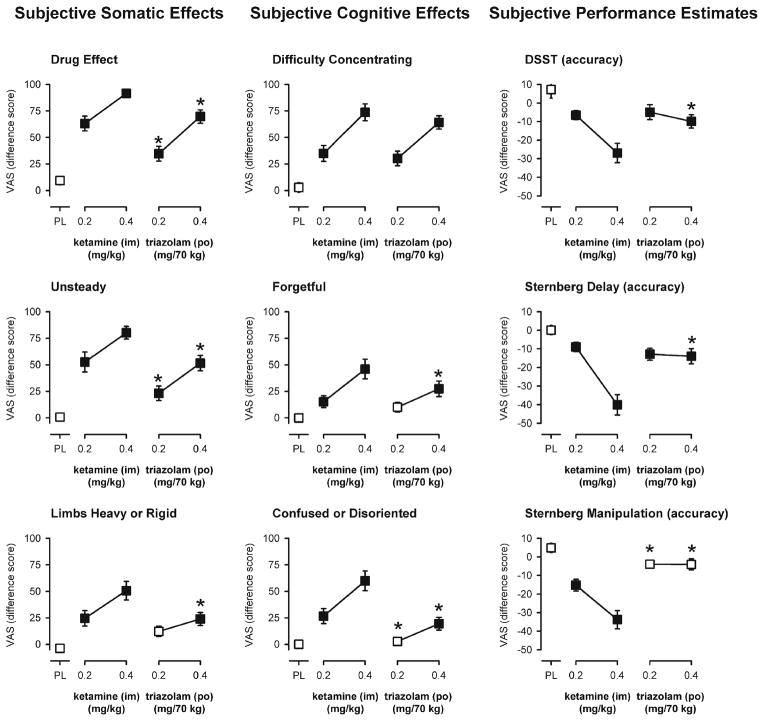
Participant ratings of “drug effect” were significantly increased by both doses of each drug relative to placebo; however, comparison of the two lower doses or the two higher doses of ketamine and triazolam showed that the magnitude of drug effect was rated significantly lower after doses of triazolam as compared to ketamine (Fig. 3, top left panel).
Fig. 3.
Peak effects of ketamine and triazolam on subjective effects measures or subjective performance estimates. Y-axes represent subjective ratings on a 100-mm visual analog scale expressed as a difference score from baseline (somatic and cognitive effects) or as a percent pre-drug estimate minus task performance (performance estimates). X-axes represent dose in milligrams per kilogram (ketamine) or milligrams per 70 kg (triazolam) (log scale). PL designates placebo. Data points show means, brackets show ±1 SEM, and the absence of brackets indicates that 1 SEM fell within the area of the data symbol. Filled symbols indicate a significant difference from placebo. An asterisk indicates a statistically significant difference between a dose of triazolam and the corresponding (i.e., low or high) dose of ketamine. Performance estimates from the Sternberg Manipulation task are collapsed across manipulation task condition
The profile of somatic subjective effects of the two drugs was somewhat similar with both drugs increasing feelings of subjective effects such as: light headed or dizzy, unsteady, limbs heavy or rigid, queasy, sleepy, and ratings of bad effects and disliking of the drug effect (Table 2). However, ketamine produced greater ratings on each of these measures as compared to triazolam (Fig. 3, left panels) with the exception of “sleepy” on which the effects of the larger dose of triazolam were significantly greater than those of the larger dose of ketamine (Table 2). There were also a number of somatic subjective effects that were not shared by the two drugs and were only increased after ketamine. These measures included feeling nervous or anxious, numbness or tingling, headache, dry mouth, hot or flushed, shaky or jittery, speech slurred, or arousing or stimulant drug effect (Table 2). Subjective ratings of feeling less comfortable and less relaxed were also significantly increased after ketamine (and not triazolam) as compared to placebo (Table 2).
Table 2.
Subjective measures on which there was a significant difference between the two drugs
| Subjective effect | KET | TRZ | Low dose | High dose |
|---|---|---|---|---|
| Drug effect | >PL | >PL | KET>TRZ | KET>TRZ |
| Confused/disoriented | >PL | >PL | KET>TRZ | KET>TRZ |
| Forgetful | >PL | >PL | NS | KET>TRZ |
| Mentally slowed down | >PL | >PL | NS | KET>TRZ |
| Bad effects | >PL | >PL | KET>TRZ | KET>TRZ |
| Dislike drug | >PL | >PL | KET>TRZ | KET>TRZ |
| Light headed/dizzy | >PL | >PL | KET>TRZ | KET>TRZ |
| Unsteady | >PL | >PL | KET>TRZ | KET>TRZ |
| Limbs heavy/rigid | >PL | >PL | NS | KET>TRZ |
| Queasya | >PL | >PL | NS | KET>TRZ |
| Nervous/anxious | >PL | KET>TRZ | KET>TRZ | |
| Numbness/tingling | >PL | KET>TRZ | KET>TRZ | |
| Headache | >PL | NS | KET>TRZ | |
| Dry mouth | >PL | NS | KET>TRZ | |
| Hot/flushed | >PL | NS | KET>TRZ | |
| Shaky or jittery | >PL | NS | KET>TRZ | |
| Speech slurred | >PL | NS | KET>TRZ | |
| Arousing/stimulantb | >PL | NS | KET>TRZ | |
| Sleepy | >PL | >PL | NS | TRZ>KET |
| Comfortable | <PL | NS | TRZ>KET | |
| Relaxed | <PL | NS | TRZ>KET |
TRZtriazolam, KET ketamine, PL placebo, NS not significant
Significant effect of task order on this measure. Significant difference when questionnaire occurs late in the assessment battery
Significant effect of task order on this measure. Significant difference when questionnaire occurs early in the assessment battery
The profile of subjective ratings related to participant’s own cognitive functioning were also generally similar for the two drugs, but tended to be greater in magnitude after ketamine relative to triazolam (Fig. 3, center panels). Participants reported feeling significantly more confused or disoriented, forgetful, and mentally slowed down after the larger dose of ketamine as compared to the larger dose of triazolam (Table 2; Fig. 3, center panels). Consistent with participants’ subjective ratings of their cognitive functioning, participants’ estimates of their performance on the DSST and both working memory tasks were significantly lower (indicating an estimate of worse performance) after administration of the larger dose of ketamine as compared to the larger dose of triazolam (Fig. 3, right panels). The negative performance estimate scores for the larger dose of ketamine indicate an over-estimation of performance impairment (i.e., under-confidence). Performance estimates assessed before and after completion of the modified Sternberg delay task or prior to each of the modified Sternberg manipulation conditions were not significantly different from each other, respectively. However, there was some evidence that completing the modified Sternberg manipulation task affected performance estimates for triazolam as the performance estimates after both doses of triazolam were not significantly different from placebo prior to task completion (Fig. 3, bottom, right panel), but were significantly less than those after administration of placebo (p<0.039 and p<0.013 for the smaller and larger doses, respectively) after task performance.
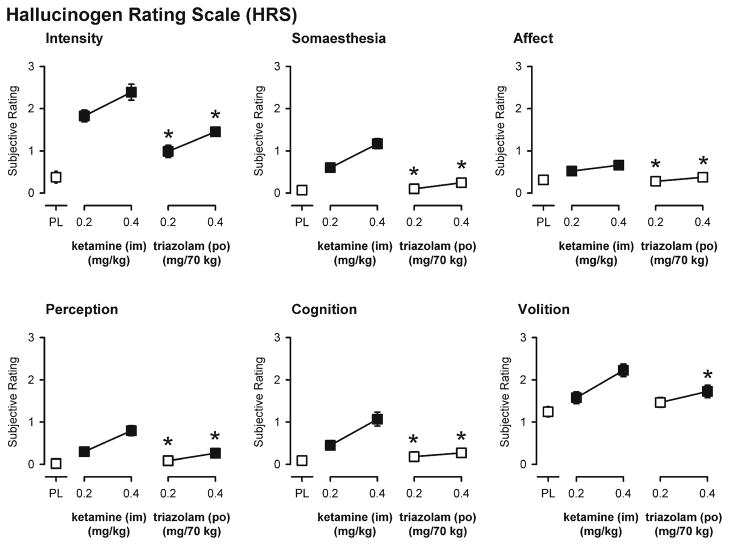
Psychedelic-like effects
There were significant main effects of dose on each of the six subscales of the Hallucinogen Rating Scale (Fig. 4). Both doses of ketamine resulted in ratings significantly greater than placebo on each scale, whereas triazolam only significantly increased ratings on the intensity, perception, and volition subscales and did so to a lesser extent than ketamine (Fig. 4). There were no significant main effects of dose on either of the three subscales or the total composite score of the Hood Mysticism Scale (data not shown).
Fig. 4.
End-of-day ratings on the six subscales of the Hallucinogen Rating Scale. Y-axes represent subjective ratings. X-axes represent dose in milligrams per kilogram (ketamine) or milligrams per 70 kg (triazolam) (log scale). PL designates placebo. Data points show means, brackets show ±1 SEM, and the absence of brackets indicates that 1 SEM fell within the area of the data symbol. Filled symbols indicate a significant difference from placebo. An asterisk indicates a statistically significant difference between a dose of triazolam and the corresponding (i.e., low or high) dose of ketamine
Discussion
This study was designed to compare the cognitive effects of ketamine and triazolam to each other and to a placebo condition using several different measures of working memory and episodic memory in healthy individuals without a history of drug abuse to test the hypothesis that doses of ketamine would result in greater subjective ratings of drug effects and estimates of cognitive performance impairment with less actual cognitive impairment relative to triazolam. There are several important findings from this study. First, at these doses, triazolam was more likely to decrease accuracy and increase response time across a variety of outcomes (i.e., psychomotor performance, divided attention, working memory, and episodic memory). Second, subjective ratings of the drug effects, including ratings of cognitive effects and performance estimates, did not correspond to objective performance impairment in the triazolam condition as ratings of impairment tended to be lower for triazolam relative to ketamine. Third, the finding that impairments in psychomotor performance (balance, DSST) produced by ketamine corresponded with the stimulant-like effects that were reported early in its duration of action, but not with the sedative-like effects that were reported later, suggest that the psychomotor impairing effects of ketamine are more closely related to its dissociative effects as opposed to its sedative effects. That is, when balance was assessed early in the task order (Form A condition), the effects of 0.2 mg/kg ketamine were significantly greater than those of 0.2 mg/70 kg triazolam (Fig. 1, top left panel, circles), which is consistent with a previous study of ketamine i.m. in which marked effects on balance were observed 5 min after administration (Lofwall et al. 2006). Fourth, neither drug (of abuse) significantly increased ratings of liking, good effects, or mysticism scores, but both drugs increased ratings of disliking and bad effects in these participants, thereby supporting previous findings that the population of participants (e.g., drug users vs. non-users) and the environment in which drug administration takes place (i.e., the set and setting) can dramatically impact the type and magnitude of the drug effects that are observed and reported (cf., Carter et al. 2006, 2009; Johnson et al. 2008; Carter and Griffiths 2009).
The findings reported here are largely consistent with previous studies of the cognitive effects of ketamine and triazolam. Ketamine (0.4 mg/kg i.m.) significantly decreased episodic word recall, word recognition, and working memory accuracy in two modified Sternberg tasks (Fig. 2), which is consistent with the effects we have previously reported (Lofwall et al. 2006) and with impairments in episodic memory and working memory using other tasks and a different route of administration (i.v.; Morgan et al. 2004a; Honey et al. 2005, 2006; LaPorte et al. 2005; Rowland et al. 2005). The profile of triazolam effects is also consistent with previous studies (Mintzer and Griffiths 2002; Carter et al. 2009; Kleykamp et al. 2010) in that triazolam produced significant impairments in psychomotor and cognitive performance, which were slightly overestimated, but to a much lesser extent as compared to other drugs such as ketamine (cf., Figs. 2 and 3).
Although there were a few significant interactions between task order and dose, such interactions were generally observed for tasks that occurred at the beginning or end of the task order (Fig. 1). The apparent interaction between task order and dose could be a result of the task order, the tasks occurring at different times during the day, or a potential interaction between the different tasks. Data from the word recall and recognition tasks (which were administered at fixed times following drug administration) show that the effects of ketamine were significantly less than those of triazolam on episodic memory (Fig. 2). In contrast, ratings of subjective effects and performance estimates were significantly greater after ketamine as compared to triazolam (Fig. 3). The profile of effects that was observed in this study for ketamine (greater subjective effects ratings relative to actual impairment) appears to be distinct from the profile of effects that has been observed with triazolam (greater cognitive impairment with less awareness of the magnitude of one’s impairment) in this and in previous studies (Mintzer and Griffiths 2002; Carter et al. 2009; Kleykamp et al. 2010). This is consistent with previous studies that have reported greater effects of benzodiazepines such as triazolam or lorazepam on metacognition (i.e., participants are less aware of their cognitive impairment) relative to other classes of drugs (Mintzer and Griffiths 2002, 2003a).
The finding that impairments in psychomotor performance produced by ketamine corresponded with the stimulant-like effects that were reported early in its duration of action, but not with the sedative-like effects that were reported later, suggest that the psychomotor- and perhaps cognitive-impairing effects of ketamine might be more closely related to its dissociative effects as opposed to its sedative effects. Subjective ratings of feeling “confused or disoriented” (ratings potentially indicative of dissociative effects) were significantly greater after each dose of ketamine as compared to the respective doses of triazolam (Fig. 3, bottom center panel). In contrast, the only subjective effects measures in which ratings of triazolam were significantly greater than those of ketamine were for “sleepy,” “comfortable,” and “relaxed” (ratings associated with sedative effects; Table 2). Likewise, impairments in the accuracy of participants’ working memory performance was only apparent after doses of triazolam that also significantly increased response time (potentially indicative of sedation), whereas this was not the case for ketamine (impairments in accuracy were observed in the absence of increased response times; Fig. 2). Taken together, these results suggest that dissociative effects might play a greater role in the cognitive-impairing effects of ketamine, whereas sedative effects might play a greater role in the cognitive-impairing effects of triazolam. However, previous studies have shown that triazolam can produce memory deficits independent of overt sedative effects (Mintzer and Griffiths 2003b, 2007a).
In this study, the drugs of abuse ketamine and triazolam did not significantly increase ratings of liking, good effects, or mysticism scores, and did significantly increase ratings of disliking and bad effects. This finding appears to be consistent with the notion that the “set and setting” can dramatically impact the type and magnitude of the drug effects that are observed and reported. For example, the history of the individual appears to be important as triazolam has been shown to significantly increase ratings of “liking” and “good effects” in individuals with a history of drug abuse (Carter et al. 2006), but not in individuals without a history of drug abuse (Carter et al. 2009). Likewise, moderate drinkers (of alcohol) have been shown to self-administer larger doses of diazepam in the laboratory as compared to (lighter) social drinkers (deWit et al. 1989). The setting, or the environment and its associated contingencies also appear to be important moderators of drug effects. For example, data from our laboratory have shown that the reinforcing effects of oral cocaine are greater in an environmental context that demands vigilance as compared to an environmental context that demands relaxation (Jones et al. 2001). Thus, it is possible that the environmental context of the cognitive laboratory and the repeated cognitive testing employed in this study might have mitigated some of the psychedelic effects that have been reported previously from studies in which ketamine was administered to individuals without histories of drug abuse (Bowdle et al. 1998).
Future studies are likely to better elucidate the precise pharmacological and cognitive mechanism(s) responsible for the memory-impairing effects of ketamine observed in this study. Here, we show that cognitive effects of ketamine are evident within 5 min, but dissipate rapidly within 1–2 h after i.m. administration, thereby providing a less than ideal model for an extended study of ketamine effects. Taken together, the results of this study and of previous research demonstrate that there are reliable differences between drugs that impair cognitive function through glutamatergic or GABAergic mechanisms. The extent to which actual impairments in cognitive performance are dissociable from subjective or perceived impairments in cognitive performance (i.e., the extent to which one might underestimate his/her impairment) after the administration of different drugs of abuse, is an important public health issue. Drugs that impair an individual’s performance to a greater extent than his/her awareness of the impairment might increase the likelihood of risky behaviors such as choosing to drive while impaired.
Ethical standards
The study described in this manuscript was approved by the Johns Hopkins University Institutional Review Board and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All persons gave their informed consent prior to their inclusion in the study.
Supplementary Material
Acknowledgments
The authors thank Crystal Barnhouser, Julia Kane, and John Yingling for technical assistance, and Paul Nuzzo and Linda Felch for data analysis. This study was supported by National Institute on Drug Abuse Research Grants R01 DA03889 and T32 DA07209
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-012-2883-x) contains supplementary material, which is available to authorized users.
Conflict of interest In the past 3 years, Lawrence Carter has served as a Special Government Employee for FDA and as a consultant for Dan’s Plan, LLC, Jazz Pharmaceuticals, Inc., KemPharm, Inc., and UCB, SA. He is currently employed by Jazz Pharmaceuticals, Inc. and owns stock options in the company. During the past 3 years, on issues related to drug abuse liability, Roland Griffiths has been a consultant to or has received contracts or grants from: Abbott Laboratories, Alexza Pharmaceuticals, Bristol-Myers Squibb, Hoffman-La Roche Inc., Jazz Pharmaceuticals, Merck & Co., Neurocrine Biosciences, Novartis, Pharmacia Corporation, Pfizer, Sanofi-Aventis, Somaxon Pharmaceuticals, Transcept Pharmaceuticals Inc., Vanda Pharmaceuticals, and Wyeth Pharmaceuticals.
Contributor Information
Lawrence P. Carter, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, 4301 W. Markham Street, Little Rock, AR 72205, USA
Bethea A. Kleykamp, Hayes, Inc, 157 S. Broad Street, Lansdale, PA 19446, USA
Roland R. Griffiths, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224, USA. Department of Neuroscience, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224, USA
Miriam Z. Mintzer, Email: mmintzer@jhmi.edu, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, 5510 Nathan Shock Drive, Baltimore, MD 21224, USA
References
- Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 1998;88:82–88. doi: 10.1097/00000542-199801000-00015. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105(Suppl 1):S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacol (Berl) 2009;206:141–154. doi: 10.1007/s00213-009-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: a comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacol. 2006;31:2537–2551. doi: 10.1038/sj.npp.1301146. [DOI] [PubMed] [Google Scholar]
- deWit H, Pierri J, Johanson CE. Reinforcing and subjective effects of diazepam in nondrug-abusing volunteers. Pharmacol Biochem Behav. 1989;33:205–213. doi: 10.1016/0091-3057(89)90451-6. [DOI] [PubMed] [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross-classifications. JASA. 1954;49:732–764. [Google Scholar]
- Honey GD, Honey RA, O’Loughlin C, Sharar SR, Kumaran D, Suckling J, Menon DK, Sleator C, Bullmore ET, Fletcher PC. Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study. Cereb Cortex. 2005;15:749–759. doi: 10.1093/cercor/bhh176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, O’loughlin C, Turner DC, Pomarol-Clotet E, Corlett PR, Fletcher PC. The effects of a subpsychotic dose of ketamine on recognition and source memory for agency: implications for pharmacological modelling of core symptoms of schizophrenia. Neuropsychopharmacology. 2006;31:413–423. doi: 10.1038/sj.npp.1300846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood RW, Jr, Ghorbani N, Watson PJ, Ghramaleki AF, Bing MN, Davison HK, Morris RJ, Williamson WP. Dimensions of the mysticism scale: confirming the three-factor structure in the United States and Iran. J Sci Study Relig. 2001;40:691–705. [Google Scholar]
- Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–620. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Reinforcing effects of oral cocaine: contextual determinants. Psychopharmacol (Berl) 2001;154:143–152. doi: 10.1007/s002130000626. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher’s handbook. 3. Prentice Hall; Englewood Cliffs, NJ: 1991. [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp Clin Psychopharmacol. 2010;18:1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriat A, Goldsmith M. Monitoring and control processes in the strategic regulation of memory accuracy. Psychol Rev. 1996;103:490–517. doi: 10.1037/0033-295x.103.3.490. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Bennett A, D’Souza DC, Abi-Dargham A, Morrissey K, Abi-Saab D, Bremner JD, Bowers MB, Jr, Suckow RF, Stetson P, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacol (Berl) 1998;135:213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D’Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- LaPorte DJ, Blaxton TA, Michaelidis T, Robertson DU, Weiler MA, Tamminga CA, Lahti AC. Subtle effects of ketamine on memory when administered following stimulus presentation. Psychopharmacol (Berl) 2005;180:385–390. doi: 10.1007/s00213-005-2179-5. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Griffiths RR, Mintzer MZ. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Exp Clin Psychopharmacol. 2006;14:439–449. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–466. [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Alcohol and triazolam: differential effects on memory, psychomotor performance and subjective ratings of effects. Behav Pharmacol. 2002;13:653–658. doi: 10.1097/00008877-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: a single-dose comparison of effects on human memory and attentional processes. Exp Clin Psychopharmacol. 2003a;11:56–72. doi: 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Triazolam–amphetamine interaction: dissociation of effects on memory versus arousal. J Psychopharmacol. 2003b;17:17–29. doi: 10.1177/0269881103017001689. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. A triazolam/amphetamine dose-effect interaction study: dissociation of effects on memory versus arousal. Psychopharmacol (Berl) 2007a;192:425–440. doi: 10.1007/s00213-007-0726-y. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Differential effects of scopolamine and lorazepam on working memory maintenance versus manipulation processes. Cogn Affect Behav Neurosci. 2007b;7:120–129. doi: 10.3758/cabn.7.2.120. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Kleykamp BA, Griffiths RR. Dose effects of triazolam and scopolamine on metamemory. Exp Clin Psychopharmacol. 2010;18:17–31. doi: 10.1037/a0018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose–response study. Psychopharmacol (Berl) 2004a;172:298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105:121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug Alcohol Depend. 2004b;75:301–308. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJ, Curran HV. Journey through the K-hole: phenomenological aspects of ketamine use. Drug Alcohol Depend. 2008;95:219–229. doi: 10.1016/j.drugalcdep.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Rush CR, Griffiths RR. Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther. 1995;272:570–580. [PubMed] [Google Scholar]
- Nelson TO. A comparison of current measures of the accuracy of feeling-of-knowing predictions. Psychol Bull. 1984;95:109–133. [PubMed] [Google Scholar]
- Parwani A, Weiler MA, Blaxton TA, Warfel D, Hardin M, Frey K, Lahti AC. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacol (Berl) 2005;183:265–274. doi: 10.1007/s00213-005-0177-2. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA. Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology. 2005;30:633–639. doi: 10.1038/sj.npp.1300642. [DOI] [PubMed] [Google Scholar]
- Rush CR, Frey JM, Griffiths RR. Zaleplon and triazolam in humans: acute behavioral effects and abuse potential. Psychopharmacology. 1999;145:39–51. doi: 10.1007/s002130051030. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sternberg S. The discovery of processing stages: extensions of Donder’s method. Acta Psychologica. 1969;30:276–315. [Google Scholar]
- Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose response study of N, N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.