Abstract
Background
Group B Streptococcus (GBS) and Escherichia coli (E. coli) cause serious bacterial infections (SBIs) and are associated with morbidity and mortality in newborn infants. Intrapartum antibiotic prophylaxis (IAP) reduces early-onset SBIs caused by GBS. The effect of IAP on late-onset SBIs caused by these organisms is unknown.
Methods
We examined all blood, urine, and cerebrospinal fluid culture results from infants admitted from 1997–2010 to 322 neonatal intensive care units managed by the Pediatrix Medical Group. We identified infants with positive cultures for GBS or E. coli and compared the incidence of early- and late-onset SBI for each organism in the time period before (1997–2001) and after (2002–2010) universal IAP recommendations.
Results
We identified 716,407 infants with cultures, 2520 (0.4%) with cultures positive for GBS and 2476 (0.3%) with cultures positive for E. coli. The incidence of GBS early-onset SBI decreased between 1997–2001 and 2002–2010 from 3.5 to 2.6 per 1000 admissions, and the incidence for E. coli early-onset SBI remained stable (1.4 per 1000 admissions in both time periods). Over the same time period, the incidence of GBS late-onset SBI increased from 0.8 to 1.1 per 1000 admissions, and incidence of E. coli late-onset SBI increased from 2.2 to 2.5 per 1000 admissions.
Conclusions
In our cohort, the incidence of early-onset GBS SBI decreased, while the incidence of late-onset SBI for E. coli and GBS increased.
Keywords: infection, infant, sepsis, group B Streptococcus, Escherichia coli
Serious bacterial infections (SBIs) are a significant cause of infant morbidity and mortality. Based on disease onset, SBIs are categorized as early-onset or late-onset.1,2 Group B Streptococcus (GBS) and Escherichia coli (E. coli) are the 2 most common organisms associated with early-onset SBI.3–5 Intrapartum antibiotic prophylaxis (IAP) reduces the incidence of early-onset GBS infections; however, invasive disease still occurs and is an important contributor to infant mortality.6,7 Since the recommendations for risk-based screening for GBS and use of IAP in 1996 and universal screening in 2002, the incidence of GBS early-onset SBIs has decreased from 1.7/1000 to 0.34/1000 live births.8–11 Increased administration of IAP has led to a change in the epidemiology of early-onset SBI among very low birth weight (VLBW, <1500 g birth weight) infants; E. coli is now the most common cause of early-onset SBI in this population.12 Although IAP reduces GBS early-onset SBI, there are conflicting data regarding the relationship between IAP and the incidence of GBS late-onset SBI.4,13
Previous studies of infants with early- and late-onset SBI included only VLBW infants, the evaluation of a single center, or excluded urine as a source of infection.4,12 We hypothesized that the widespread use of IAP has changed the incidence of GBS and E. coli as causes of early-and late-onset SBI. We examined neonatal SBIs, including bloodstream infections, urinary tract infections, and meningitis, in a large, multicenter cohort of neonatal intensive care units (NICUs) to examine the incidence of GBS and E. coli SBIs before and after the recommendation for universal IAP.
METHODS
Study Population
This was an observational, retrospective, cohort study of all infants admitted to 322 NICUs managed by the Pediatrix Medical Group in the United States from 1997–2010. Data from clinical progress notes were used to populate the data set. We reviewed neonatal and maternal demographics, as well as the results of all cultures (blood, urine, and cerebrospinal fluid [CSF]) in the first 120 days of life. We excluded urine cultures obtained from bag specimens.
Definitions
We defined early-onset SBI as a positive blood, urine (obtained from a catheterization or suprapubic tap), or CSF culture within the first 3 postnatal days. Late-onset SBI was defined as a positive culture from 4–120 postnatal days. We considered cultures that were positive for the same organism within a 21-day period as a single episode of infection. Gestational age was based on the best estimate of last menstrual period, obstetrical record, and clinical exam. The clinician recorded exposure to antenatal antibiotics and antenatal steroids in the infant’s clinical progress note upon admission to the NICU. Type of antibiotic given prior to delivery was not recorded.
Statistical Analysis
The unit of measurement for analysis was the infant. We used standard summary statistics including means, medians, and ranges to describe continuous variables, and frequency counts and percentages to describe categorical variables. Cumulative incidences of GBS and E. coli SBIs were calculated separately for early- and late-onset disease by dividing the total number of SBIs by the total number of infants hospitalized during the period of early-onset and late-onset sepsis, respectively. We compared across groups using chi-square test of association and Wilcoxon rank sum test for categorical and continuous variables, respectively.
We used univariable logistic regression to evaluate the association between the 4 separate outcomes: GBS early- and late-onset SBI and E. coli early- and late-onset SBI and the following predictor variables: gestational age, sex, race, inborn status, 5-minute Apgar, ventilator support on the first postnatal day, prenatal steroid exposure, prenatal antibiotic exposure, mode of delivery, and admission during 2 time periods of 1997–2001 and 2002–2010. These 2 time periods were chosen to indicate the periods prior to and after the institution of universal recommendations for IAP. All variables significant at the univariable level were included in the final multivariable model, which included the following predictors: gestational age, sex, race, inborn status, 5-minute Apgar, ventilator support on the first postnatal day, prenatal steroid exposure, prenatal antibiotic exposure, and mode of delivery. Birth weight was removed from the final model due to collinearity with gestational age. A P-value <0.05 was considered statistically significant, and all analyses were performed using Stata 12 (College Station, TX). The Duke University Institutional Review Board and the Western Institutional Review Board granted permission to conduct this analysis on the de-identified data set.
RESULTS
Overall
We identified 716,407 infants during the study period; 2520 (0.4%) infants had 2551 episodes of GBS SBI, 2476 (0.3%) infants had 2532 episodes of E. coli SBI, and 18 infants (0.003%) were infected with both. Compared to infants with E. coli SBI, infants with GBS SBI were more mature (median gestational age 38 weeks [25th, 75th percentile: 30, 39] vs. 30 weeks [26, 35], P<0.001), larger (2927 g [1405, 3452] vs. 1356 g [845, 2370], P<0.001), and less often exposed to maternal antibiotics (25% vs. 55%, P<0.001).
Early-onset SBI
Of the 2551 GBS infections, 1869 (73%) were early-onset SBI. Of the 2532 E. coli infections, 971(38%) were early-onset SBI. When compared to infants with E. coli early-onset SBI, infants with GBS early-onset SBI had a greater gestational age (38 weeks [36, 40] vs. 32 weeks [28, 38], P<0.001), higher birth weights (3196 g [2670, 3570] vs. 1855 g [1110, 2950], P<0.001), and fewer were exposed to antenatal antibiotics (19% vs. 59%, P<0.001) (Table 1). Of the 2839 early-onset SBI, 2257 (80%) infections occurred from 2002–2010 (Table 2).
Table 1.
Demographics of study patients at enrollment (N=248)
| No. | % | |
|---|---|---|
| Sex | ||
| Male | 135 | 54.4 |
| Female | 113 | 45.6 |
|
| ||
| Ageα (months) | ||
| Mean | 6.9 | |
| Range | 5.8–15.2 | |
|
| ||
| Race | ||
| Non-Hispanic White | 147 | 59.3 |
| African American | 48 | 19.4 |
| Asian | 2 | 0.8 |
| Hispanic | 12 | 4.8 |
| Other | 39 | 15.7 |
|
| ||
| Family History of OM | ||
| Maternal | 34 | 13.8 |
| Paternal | 15 | 6.1 |
| Sibling | 24 | 9.7 |
| >1 Family Member | 20 | 8.1 |
| None/Missing | 155 | 62.3 |
|
| ||
| Daycareα | ||
| Home | 49 | 60.5 |
| Center | 29 | 35.8 |
| Combination (Home and Center) | 3 | 3.7 |
|
| ||
| Breast-feedingα | ||
| <6 months | 28 | 11.3 |
| ≥6 months | 33 | 13.3 |
| Formula | 86 | 34.7 |
| Combination (Formula and breast milk) | 81 | 31.6 |
|
| ||
| Antibiotic Exposure (Number of visits) | ||
| Use in 0–30 days prior to visit | 106 | 8.6 |
| No antibiotic Use or 30+ days prior to visit | 1133 | 91.4 |
Variables collected at enrollment; daycare had 167 missing values, breast-feeding had 20 missing values
Table 2.
Predicted outcome of colonization with AHS in young children (N=1146 visits)
| Parameters | OR (95% CI) | P-value |
|---|---|---|
| Colonization by S. pneumoniae | ||
| No (reference) | 1.0 | |
| Yes | 0.172 (0.125–0.238) | <0.001 |
|
| ||
| Colonization by H. influenzae | ||
| No (reference) | 1.0 | |
| Yes | 1.254 (0.803–1.957) | 0.320 |
|
| ||
| Colonization by M. catarrhalis | ||
| No (reference) | 1.0 | |
| Yes | 1.271 (0.963–1.676) | 0.090 |
|
| ||
| Visit type | ||
| β AOM | 1.0 | |
| Non-AOM | 2.135(1.355–3.365) | 0.001 |
| Follow Up | 2.122(0.966–4.665) | 0.061 |
|
| ||
| Presence of URI symptoms | ||
| No (reference) | 1.0 | |
| Yes | 1.139 (0.782–1.660) | 0.498 |
|
| ||
| Sex | ||
| Male | 1.0 | |
| Female | 1.161 (0.868–1.554) | 0.313 |
|
| ||
| Exposure to tobacco smoke | ||
| No (reference) | 1.0 | |
| Yes | 0.820 (0.533–1.263) | 0.369 |
|
| ||
| Age (months) | 1.010 (0.990–1.032) | 0.316 |
|
| ||
| Breastfed | ||
| Formula(reference) | 1.0 | |
| Less than 6 months | 1.787 (0.992–3.219) | 0.053 |
| More than 6 months | 1.164 (0.761–1.780) | 0.483 |
| Combination (Formula + Breastfeeding) | 0.883 (0.631–1.237) | 0.471 |
|
| ||
| Daycare | ||
| Home | 1.0 | |
| Center | 0.817 (0.477–1.401) | 0.463 |
| Both | 0.362 (0.088–1.486) | 0.158 |
|
| ||
| Antibiotic Exposure in the last 30 days | ||
| No (reference) | 1.0 | |
| Yes | 0.969 (0.533–1.761) | 0.920 |
|
| ||
| Family History | ||
| No (reference) | 1.0 | |
| Yes | 1.150 (0.862–1.535) | 0.342 |
Associations with a significant p-value (p ≤ 0.05) are indicated in bold lettering
When the model included Non-AOM as the reference for visit type, the OR and p-value were; 0.488, p=0.002
Nearly all episodes of GBS and E. coli early-onset SBI were bloodstream infections: 1839 (98%) of GBS and 956 (98%) of E.coli SBIs (Table 3). SBI co-infections were uncommon in GBS early-onset SBI: 4 (<1%) episodes were bloodstream infections and urinary tract infections; 15 (<1%) were bloodstream infections and meningitis. Few infants with E. coli early-onset SBI had meningitis alone (4, <1%). Co-infections were uncommon in E. coli early-onset SBI: 2 episodes (<1%) with bloodstream infections and meningitis and no episodes with bloodstream infections and urinary tract infections.
TABLE 3.
Site of Infection for Infants with Early-onset and Late-onset Serious Bacterial Infection
| Early-onset SBI, n (%) | Late-onset SBI, n (%) | |||
|---|---|---|---|---|
|
| ||||
| GBS | E. coli | GBS | E. coli | |
| n | 1869 | 971 | 663 | 1522 |
| Blood | 1839 (98) | 956 (98) | 626 (92) | 1344 (86) |
| Urine | 5 (<1) | 9 (<1) | 13 (2) | 174 (11) |
| CSF | 6 (<1) | 4 (<1) | 11 (2) | 32 (2) |
| Blood & urine | 4 (<1) | 0 (0) | 1 (<1) | 6 (<1) |
| Blood & CSF | 15 (<1) | 2 (<1) | 31 (5) | 5 (<1) |
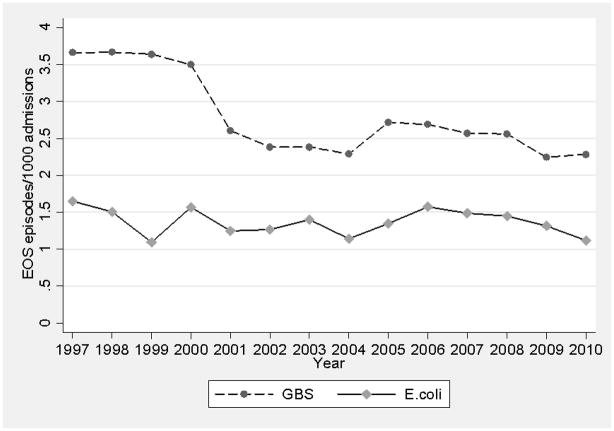
The incidence of GBS early-onset SBI from 1997–2001 to 2002–2010 decreased from 3.5 to 2.6 per 1000 admissions (Figure 1). On multivariable regression, the odds of developing GBS early-onset SBI were lower (odds ratio [OR]=0.69; 95% confidence interval [CI]: 0.59, 0.80]; P<0.001) in the later time period.
FIGURE 1.
Incidence of early-onset serious bacterial infections over time. E. coli indicates Escherichia coli, EOS, early-onset sepsis, GBS, group B Streptococcus.
The incidence of E. coli early-onset SBI was 1.4 per 1000 admissions during both time periods (Figure 1). On multivariable regression, the odds of developing E. coli early-onset SBI were not significantly different between the 2 time periods (OR=0.95; 95% CI 0.78, 1.16; P=0.63).
Mortality among infants with E. coli early-onset SBI was higher than in those with GBS early-onset SBI (10% vs. 4%, P<0.001) (Table 4). Among infants who died with GBS early-onset SBI, 53% (38/72) died within 7 days of a positive culture. Among infants who died with E. coli early-onset SBI, 44% (38/86) died within 7 days of a positive culture.
TABLE 4.
Mortality of Infants with Early-onset and Late-onset Serious Bacterial Infection Before and After Universal Intrapartum Antibiotic Prophylaxis Recommendations
| Early-onset SBI, n (%) | Late-onset SBI, n (%) | |||
|---|---|---|---|---|
|
| ||||
| GBS | E. coli | GBS | E. coli | |
| Mortality | 72/1751 (4) | 86/882 (10) | 41/584 (7) | 184/1307 (14) |
| 1997–2001 | 17/381 (4) | 14/156 (9) | 3/71 (4) | 16/212 (8) |
| 2002–2010 | 55/1370 (4) | 72/726 (10) | 38/513 (7) | 168/1095 (15) |
Late-onset SBI
Of the 2551 GBS SBIs, 682 (27%) were late-onset SBI. Of the 2532 E. coli SBIs, 1561 (62%) were late-onset SBI. There was no significant difference between the infants with GBS late-onset SBI compared to infants with E. coli late-onset SBI in gestational age (28 weeks [26, 31] vs. 28 weeks [25, 33]; P=0.63), birth weight (1090 g [816, 1533] vs. 1124 g [770, 1905]; P=0.17), exposure to antenatal steroids (63% vs. 61%, P=0.52), or the need for mechanical ventilation on the first postnatal day (66% vs. 65%, P=0.55) (Table 1). Of the 2185 late-onset SBI, 1858 (85%) infections occurred from 2002–2010 (Table 2).
Nearly all episodes of GBS late-onset SBI were bloodstream infections (626 [92%]; Table 3). Co-infections in GBS late-onset SBIs were rare: 1 (<1%) with bloodstream and urinary tract infections and 31 (5%) with bloodstream and CSF infections. Similarly, nearly all episodes of E. coli late-onset SBI were bloodstream infections (1344 [86%]; Table 3). However, E. coli late-onset SBI included 174 (11%) urinary tract infections; 6 (<1%) had both a bloodstream infection and a urinary tract infection. A small number of infants had meningitis (32 [2%]).
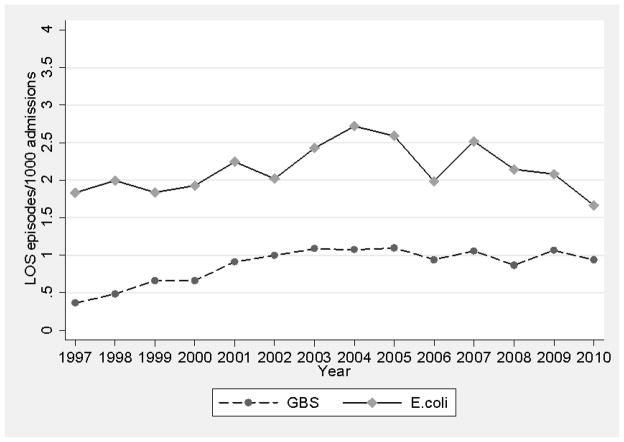
The incidence of GBS late-onset SBI from1997–2001 to 2002–2010 increased from 0.8 to 1.1 per 1000 admissions (Figure 2). On multivariable regression, the odds of developing GBS late-onset SBI were higher (OR=1.67 [95% CI 1.23, 2.25], P=0.001) in the later time period.
FIGURE 2.
Incidence of late-onset serious bacterial infections over time. E. coli indicates Escherichia coli, GBS, group B Streptococcus, LOS, late-onset sepsis.
The incidence of E. coli late-onset SBI from 1997–2001 to 2002–2010 increased from 2.2 to 2.5 per 1000 admissions (Figure 2). On multivariable regression, the odds of developing E. coli late-onset SBI were not significantly different between the 2 time periods (OR=1.17 [95% CI 0.99, 1.39], P=0.06).
Mortality was higher in infants infected with E. coli late-onset SBI compared with GBS late-onset SBI (14% vs. 7%, P<0.001; Table 4). Among infants who died with GBS late-onset SBI, 44% (19/43) died within 7 days of a positive culture. Among infants who died with E. coli late-onset SBI, 10% (19/190) died within 7 days of a positive culture.
Infants in the latter time period were smaller with lower gestational ages: among all infants who died from 1997–2001, mean gestational age was 27.6 weeks (24, 36) and mean birth weight 1261 g (580, 3218) versus those who died from 2002–2010, whose mean gestational age was 26.7 weeks (23, 35) and mean birth weight 1011 g (520, 2610).
DISCUSSION
In our cohort of admissions to a large number of NICUs, the incidence of GBS early-onset SBI decreased after the institution of universal IAP, and no change was observed in the incidence of E. coli early-onset SBI. We observed an increase in the incidence of GBS late-onset SBI from 1997–2001 to 2002–2010. Mortality in infants infected by late-onset SBI increased over time, and we speculate that changes in neonatal practice, including improved survival of VLBW infants with lower gestational ages, have contributed to increased susceptibility to infection and a resulting increase in mortality.
Our data are consistent with previous reports of a declining incidence of GBS early-onset SBI. The majority of previous studies are limited to infections in the bloodstream12,14 or bloodstream and CSF15; however, our study included urinary tract infections. In a report of bloodstream infections among VLBW infants, the National Institute of Child Health and Human Development Neonatal Research Network found a lower incidence (1.7 per 1000 live-born VLBW infants) of early-onset GBS bloodstream infections in infants born between 1998 and 2000 when compared to the incidence (5.9 per 1000 live-born VLBW infants) in infants born from 1991–1993.12 The Centers for Disease Control and Prevention reported an annual incidence of GBS early-onset SBI 33% lower from 2003–2005 compared to 2000–2001 among all infants.15 Our data, including full-term and premature infants, demonstrate a similar decline in incidence in GBS early-onset SBIs.
Previous studies on the changes in the incidence of E. coli early-onset SBI are less clear. Some studies report stable incidence of E. coli early-onset SBI,16–18 while others have observed an increased incidence of E. coli early-onset SBI, particularly among VLBW infants.1,12 A review of infection data of VLBW infants in the Neonatal Research Network demonstrated an increase in the incidence of E. coli bloodstream infections from 3.2 per 1000 live births from 1991–1993 to 6.8 per 1000 live births from 1998–2000.1 In contrast, a retrospective review of blood cultures obtained from 647 infected infants in a single-site NICU population showed stable incidence of E. coli early-onset SBI.4 In our population of term and premature infants, we observed a stable incidence of E. coli early-onset SBI over time (1.4 per 1000 admissions).
We observed an increased incidence of GBS late-onset SBIs after 2002, when the recommendations for universal IAP were released. Previously published data conflict in regards to changes in the incidence of late-onset SBI; some studies report an unchanged incidence of late-onset bloodstream infections,17,19 while others have reported an increasing incidence.4,13,20,21 E. coli late-onset SBI increased among term and premature infants after a single center adopted universal IAP recommendations.13
Our study included urine cultures obtained by catheterization or suprapubic tap, an important source of neonatal infectious morbidity and mortality. Urinary tract infections are common, occurring in 8% in VLBW infants, and repeated infections are associated with renal scarring that can lead to end-stage renal SBI.22,23 Hematogenous seeding of the urinary tract may be responsible for the increasing incidence of late-onset urinary tract infections in the immune-compromised infant, with increased susceptibility in very low birth weight infants.23
One potential cause of the observed increased incidence of late-onset SBI after universal IAP is that IAP has “shifted” the SBI onset of GBS and E. coli into the period beyond the first postnatal days.24 In an epidemiologic study of full-term, previously healthy infants, IAP was found to be associated with late-onset SBI.25 Microbiologic pressure from the increasing use of IAP might be another explanation for our findings, leading to a change in pathogenic organisms. Previous studies have reported an increase in the incidence of gram-negative rod infections as the use of IAP has increased.12,26 Increased use of IAP may promote the emergence of antibiotic-resistant organisms that become clinically evident after the first few postnatal days. Antibiotic use has been associated with changes in pathogenic organism patterns in neonatal populations. For example, increased fungal infections have been reported in NICUs that widely use cephalosporins.27 Our study population included more low birth weight infants over time, and this variation might also account for a portion of the increased incidence of late-onset SBI.28
This study is among the largest reported evaluations of early- and late-onset SBI caused by GBS and E. coli. The strengths of this report include the large sample size, a diverse patient population drawn from academic and community institutions, inclusion of maternal and infant risk factors for sepsis, and the use of data prior to and after universal IAP recommendations. The study is limited to infants admitted to NICUs and may under-represent the total burden of late-onset SBI by excluding infants presenting to pediatric intensive care units, emergency departments, and inpatient pediatric units or over-represent hospital-acquired nosocomial infections. Urine and CSF cultures were obtained at the discretion of the clinician, which might have limited our ability to determine true co-infection rates. The study is also limited by the lack of documentation of clinical signs of sepsis, lack of information about which antibiotic was administered as IAP, and resistance patterns of the bacterial isolates.
In conclusion, we found that the incidence of GBS late-onset SBI increased in the era following IAP recommendations. This may represent an epidemiological shift in pathogenic organisms, or it could represent increased vulnerability to infections in very premature infants. Further examination is needed to determine whether IAP has resulted in an overall decrease in SBI burden and mortality or merely shifted the burden to a later time point or to a different site of infection. Future research should examine a population-based cohort that includes early- and late-onset SBI, bloodstream infections, urinary tract infections, and meningitis. Trends in mortality from sepsis, particularly among low birth weight infants, should also continue to be monitored at the level of the population as pathogenic organisms shift.
Acknowledgments
Sources of funding: Dr. Laughon receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C [PI: Benjamin] under the Best Pharmaceuticals for Children Act and 1K23HL092225-01). Dr. Benjamin receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C under the Best Pharmaceuticals for Children Act), the non-profit organization Thrasher Research Foundation for his work in neonatal candidiasis, and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Smith receives support from the U.S. government for his work in pediatric and neonatal pharmacology (NICHD 1K23HD060040-01) and epidemiology (DHHS-1R18AE000028-01). Dr. Cohen-Wolkowiez receives support from the U.S. government for his work in pediatric and neonatal pharmacology (NICHD 1K23HD064814-01) and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp).
Footnotes
Disclosures: The authors have no conflicts of interest.
References
- 1.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 3.Schrag SJ, Stoll BJ. Early-onset neonatal sepsis in the era of widespread intrapartum chemoprophylaxis. Pediatr Infect Dis J. 2006;25:939–940. doi: 10.1097/01.inf.0000239267.42561.06. [DOI] [PubMed] [Google Scholar]
- 4.Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics. 2005;116:595–602. doi: 10.1542/peds.2005-0552. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker CJ, Byington CL, Polin RA. Policy statement: recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–616. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 7.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30:937–941. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B Streptococcus. N Engl J Med. 2009;360:2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 9.Goins WP, Talbot TR, Schaffner W, et al. Adherence to perinatal group B streptococcal prevention guidelines. Obstet Gynecol. 2010;115:1217–1224. doi: 10.1097/AOG.0b013e3181dd916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early-onset and late-onset neonatal group B streptococcal disease—United States, 1996–2004. MMWR Morb Mortal Wkly Rep. 2005;54:1205–1208. [PubMed] [Google Scholar]
- 11.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 12.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 13.Lukacs SL, Schoendorf KC, Schuchat A. Trends in sepsis-related neonatal mortality in the United States, 1985–1998. Pediatr Infect Dis J. 2004;23:599–603. doi: 10.1097/01.inf.0000131633.74921.90. [DOI] [PubMed] [Google Scholar]
- 14.Puopolo KM, Madoff LC, Eichenwald EC. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics. 2005;115:1240–1246. doi: 10.1542/peds.2004-2275. [DOI] [PubMed] [Google Scholar]
- 15.Perinatal group B streptococcal disease after universal screening recommendations—United States, 2003–2005. MMWR Morb Mortal Wkly Rep. 2007;56:701–705. [PubMed] [Google Scholar]
- 16.Moore MR, Schrag SJ, Schuchat A. Effects of intrapartum antimicrobial prophylaxis for prevention of group B streptococcal disease on the incidence and ecology of early-onset neonatal sepsis. Lancet Infect Dis. 2003;3:201–213. doi: 10.1016/s1473-3099(03)00577-2. [DOI] [PubMed] [Google Scholar]
- 17.Schrag SJ, Zywicki S, Farley MM, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 18.Alarcon A, Pena P, Salas S, Sancha M, Omenaca F. Neonatal early onset Escherichia coli sepsis: trends in incidence and antimicrobial resistance in the era of intrapartum antimicrobial prophylaxis. Pediatr Infect Dis J. 2004;23:295–299. doi: 10.1097/00006454-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Phares CR, Lynfield R, Farley MM, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA. 2008;299:2056–2065. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 20.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 21.Ecker KL, Donohue PK, Kim KS, Shepard JA, Aucott SW. The impact of group B Streptococcus prophylaxis on late-onset neonatal infections. J Perinatol. 2012 Jun 14; doi: 10.1038/jp.2012.76. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Nowell L, Moran C, Smith PB, et al. Prevalence of renal anomalies after urinary tract infections in hospitalized infants less than 2 months of age. J Perinatol. 2010;30:281–285. doi: 10.1038/jp.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer S, Eliakim A, Pomeranz A, et al. Urinary tract infection in very low birth weight preterm infants. Pediatr Infect Dis J. 2003;22:426–430. doi: 10.1097/01.inf.0000065690.64686.c9. [DOI] [PubMed] [Google Scholar]
- 24.Jordan HT, Farley MM, Craig A, et al. Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr Infect Dis J. 2008;27:1057–1064. doi: 10.1097/INF.0b013e318180b3b9. [DOI] [PubMed] [Google Scholar]
- 25.Glasgow TS, Young PC, Wallin J, et al. Association of intrapartum antibiotic exposure and late-onset serious bacterial infections in infants. Pediatrics. 2005;116:696–702. doi: 10.1542/peds.2004-2421. [DOI] [PubMed] [Google Scholar]
- 26.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24:635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 27.Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK., Jr The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118:717–722. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 28.Edmond KM, Kortsalioudaki C, Scott S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012;379:547–556. doi: 10.1016/S0140-6736(11)61651-6. [DOI] [PubMed] [Google Scholar]