Abstract
An important role in the regulation of apoptotic calcium release is played by the ubiquitously expressed family of inositol 1,4,5-trisphosphate receptor (IP3R) channels. One model for IP3R activation during apoptosis is cleavage by the apoptotic protease caspase 3. Here we show that early elevations in cytosolic calcium during apoptosis do not require caspase 3 activity. We detected a robust increase in calcium levels in response to staurosporine treatment in primary human fibroblasts and HeLa cells in the presence of the caspase inhibitor Z-VAD, indicating that calcium release during the initiation of apoptosis occurs independently of caspase 3. Similar results were obtained with MCF-7 cells which lack caspase 3 expression. Stable expression of caspase 3 in MCF-7 cells and TAT-based transduction of the active recombinant caspase 3 directly into living MCF-7 cells had marginal effects on the early events leading to cytosolic calcium elevations and irreversible commitment to apoptotic cell death. Significantly, blocking IP3 binding to the IP3R with an IP3 sponge resulted in suppression of staurosporine-induced calcium release and cell death. Collectively, our results suggest that generation of IP3 is sufficient for the initiation of apoptotic calcium signaling, and caspase 3-mediated truncation of IP3R channel is a consequence, not causative, of apoptotic calcium release.
Keywords: Calcium; Apoptosis; Caspase 3; Inositol 1,4,5-trisphosphate receptor; Staurosporine
1. Introduction
Calcium release into the cytoplasm is a critical regulator of almost all cellular processes including activation of cell death signaling pathways. Calcium functions as a second messenger during both early and late stages of apoptosis, orchestrating almost all facets of the apoptotic cascade. In particular, calcium release from the endoplasmic reticulum (ER) leads to increased permeability of the outer mitochondrial membrane resulting in dissipation of the mitochondrial transmembrane potential, generation of reactive oxygen species and release of pro-apoptotic proteins from mitochondria [1]. Inositol 1,4,5-trisphosphate receptor (IP3R) channels play a primary role in apoptotic calcium release from ER calcium stores in almost all cell types. Previous studies demonstrated that suppression of IP3R activity in different cell types inhibits apoptosis induced by variety of stimuli, including Fas, B and T cell receptor stimulation [2–4].
A direct role for caspase 3 in regulation of IP3R-mediated calcium release was proposed upon finding that IP3R-1 has a consensus caspase 3 cleavage site which is not present on the other two isoforms, and cleavage at this site abolishes IP3-dependent calcium release [5]. IP3R-1 can be specifically cleaved by caspase 3 in response to multiple apoptotic stimuli, including staurosporine, anti-IgM, and TNF-α [5, 6]. Supporting a direct role for caspase 3 in IP3R cleavage, caspase 3 null MCF-7 cells do not generate cleaved IP3R-1 in response to apoptotic stimuli [5]. As caspase cleavage removes the Nterminal suppressor domain and the IP3 binding domain, it has been hypothesized that cleavage at this site may cause “leaky” channels which allow calcium diffuse from the ER to the cytosol [6–10]. Consistent with this hypothesis, expression the IP3R channel domain without the ligand binding and suppressor domains results in constitutively open channels [11]. Cleavage of the receptor renders the channel insensitive to IP3, and recombinant IP3R-1 with the DEVD sequence changed to IEVA does not support caspase activation and cell death in triple IP3R knockout DT40 cells [5, 6]. Other reports suggest that the IP3R-3 specifically supports apoptotic calcium release [2, 3, 12, 13]. Furthermore, caspase 3 activation and cleavage of intracellular substrates is a relatively late event in apoptosis, and we have shown that caspase activities cannot explain the initial changes in intracellular calcium in response to apoptotic stimuli such as staurosporine and Fas ligand [14, 15]. Another group has recently confirmed that cleavage of IP3R-1 by caspase occurs late in apoptosis and, therefore, it is not a key event in apoptotic signaling [16].
In the present study we addressed the functional significance of caspase 3-mediated IP3R-1 cleavage in the initiation of staurosporine-induced apoptotic signaling. We found that pharmacological inhibition of caspase activity in primary human fibroblasts and HeLa cells has no effect on the robust staurosporine-induced calcium release into the cytoplasm. Similarly, caspase 3 deficiency in MCF-7 cells is not associated with defects in activation of pro-apoptotic calcium signaling induced by staurosporine. Furthermore, direct introduction of active recombinant caspase 3 into untreated cells does not result in calcium leak into the cytosol. On the other hand, blocking IP3 binding to the IP3R with the IP3 sponge [17] completely abrogates calcium release from ER stores in response to staurosporine treatment, suggesting that IP3-mediated channel opening is the primary mechanism for calcium release during apoptosis. Taken together, our findings strongly support a caspase 3-independent model for IP3R-mediated calcium release during both initiation and early progression of apoptotic cell death.
2. Materials and methods
2.1 Cell lines
Dermal Human Fibroblast, MCF-7 and HeLa cell lines were obtained from ATCC. Fibroblasts were cultured in minimum essential media (Gibco) supplemented with 15% fetal bovine serum (FBS), 1× MEM non-essential amino acid solution (Sigma), 1mM sodium pyruvate, and 1% penicillin-streptomycin. MCF-7 cells were cultured in minimum essential media (Gibco) supplemented with 10% fetal bovine serum (FBS), 1× MEM non-essential amino acid solution (Sigma), 1mM sodium pyruvate, 10µg/ml insulin and 1% penicillin-streptomycin. HeLa cells were cultured in Dulbecco's Eagle's modified medium (Gibco) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and 1% penicillin-streptomycin.
2.2 Antibodies
An antibody generated against the unique C-terminal sequence RIGLLGHPPHMNVNPQQPA (single amino acid code) of the type 1 IP3R has been described previously [14, 18]. Mouse monoclonal anti-α-fodrin clone AA6 and anti-PARP1 clone C-2–10 were from Millipore. Mouse monoclonal Anti-SERCA-2 clone 2A7-A1 was purchased from Thermo Scientific.
2.3 Stable and Transient Transfections
For stable and transient transfections, cells were transfected using Lipofectamine 2000 as indicated by the manufacturer (Invitrogen). For generation of caspase-3 stable transfectants, MCF-7 cells were selected with 800 µg/ml G418 in the culture medium 24 h after transfection with a pcDNA 3.1-based expression vector. For transient transfections, HeLa cells were plated in 6-well dishes and assessed for transfection efficiency 24 h later by visualization of green fluorescent protein.
2.4 Caspase-3 Expression and Purification
Expression vector containing full length caspase-3 cDNA fused to His tag was obtained from Addgene. Recombinant caspase-3 protein was purified using TALON (Clontech) resin as described [19]. Purify of the protein was analyzed by SDS-PAGE followed by Coomassie staining (Supplementary Fig. 1). Enzymatic activity of the fractions was determined as described below and shown in Supplementary Fig. 1B). Fractions 1–3 were pooled, aliquoted, and frozen in 10% glycerol until use.
2.5 Green fluorescent protein (GFP) Expression and Purification
BL21 (DE3) E. coli cells transformed with plasmid pGFPuv, encoding the so-called Cycle 3 mutant version of GFP [20] under control the phage T7 promoter were grown to A600 = 0.8 in LB medium and induced with 1 mM IPTG for 2 h at 37 °C. Cells were harvested by centrifugation and lysed in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.4) containing EDTA-free protease inhibitor cocktail (Roche) by passing them three times through an EmulsiFlex high-pressure homogenizer device (Avestin). The lysate was cleared by centrifugation (20,000 ×g for 30 min) and the supernatant was applied to a HiTrap metal-chelating column pre-charged with Ni2+ (GE). The column was washed with a gradient of 10 mm – 50 mM imidazole in PBS, followed by desalting into PBS with a HiPrep desalting column (GE). Protein aliquots were flash-frozen in liquid nitrogen and stored at −80 °C.
2.6 Caspase-3 Activity and Cell Death Determination
Cell death was determined by propidium iodide staining as described [21]. Fluorometric determination of DEVDase (caspase-3-like) activity was performed on lysates prepared from Jurkat cells as described [21].
2.7 Protein delivery
For protein transduction, the Chariot transfection reagent (Active Motif) was used according to manufacturer’s instructions. Transduction efficiency was determined by separately transducing purified recombinant green fluorescent protein (GFP). Typically 100 percent of cells had intracellular GFP within 30 minutes (Supplementary Fig. 2). Calcium imaging, caspase-3 and Western blot assays were performed 3 h after Chariot/protein complex applications.
2.8 Calcium imaging
Measurements of intracellular calcium concentration were performed on Fura-2 loaded cells 14–18 hours after plating on glass coverslips as described previously [22]. For long term imaging in Figure 1E, multiple coverslips were treated simultaneously and were sequentially imaged as described [14].
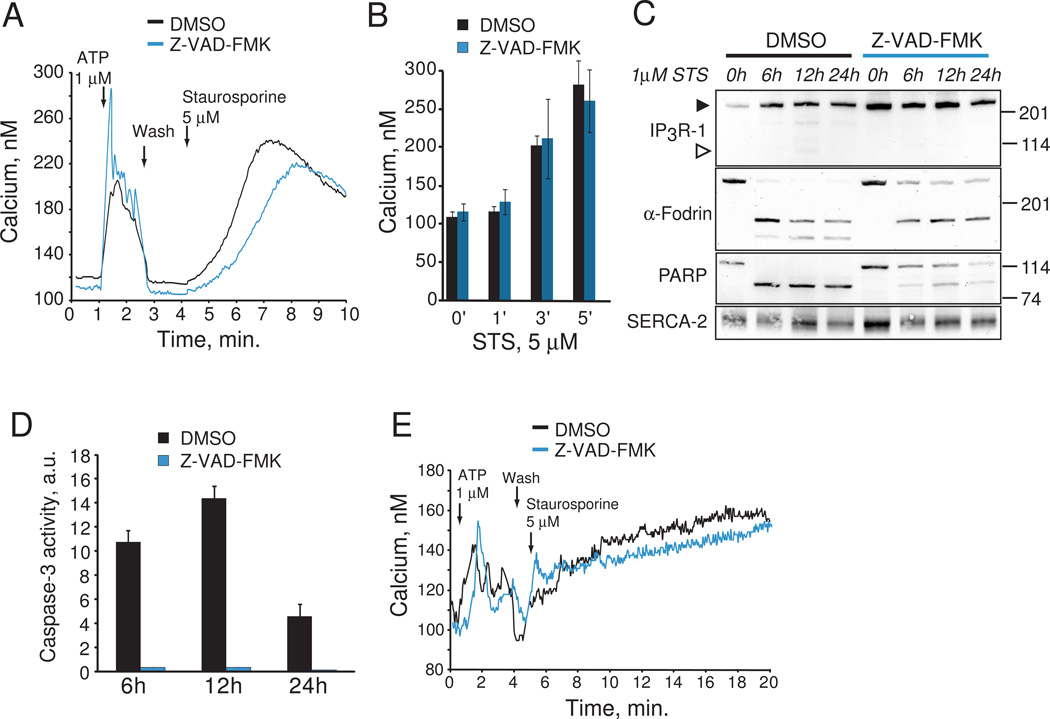
Fig. 1.
Requirement of caspase 3 for ATP- and staurosporine-induced calcium release. A. Fura-2 calcium imaging of DMSO and Z-VAD-FMK pre-treated HeLa cells. Averaged time courses for calcium release in response to 1µM NaATP and 5µM staurosporine are shown from one experiment each. B. Pooled data from three experiments showing mean +/− s.e.m. calcium release induced by staurosporine (STS) at the indicated times. C. IP3R-1, PARP and α-fodrin cleavage in response to 1µM staurosporine treatment in HeLa cells incubated with DMSO or Z-VAD-FMK. Full length IP3R is indicated by a closed arrowhead, and the 95 kDa caspase 3 cleaved C-terminal fragment by an open arrowhead. This experiment was repeated three times with similar results. D. Caspase 3-like activity (DEVDase activity) in response to 1µM staurosporine treatment in HeLa cells incubated with DMSO or Z-VAD-FMK. Data is pooled and averaged from three experiments. E. Fura-2 calcium imaging of DMSO and Z-VAD-FMK pre-treated primary human fibroblasts. Averaged time courses for calcium release in response to 1µM NaATP and. 5µM staurosporine are shown.
3. Results
3.1 Inhibition of caspase activity does not affect staurosporine-induced calcium release
To assess the role of caspase activity in the initiation of agonist and staurosporine-induced calcium release we pre-incubated HeLa cells with 20µM of the suicide caspase inhibitor Z-VAD-FMK to block all caspase activity. We observed unaltered calcium flux into cytoplasm in response to purinergic agonist ATP (1µM) in cells treated with either DMSO or Z-VAD-FMK (Fig. 1A). Furthermore, inhibition of caspase activity did not alter kinetics and duration of staurosporine-induced calcium release (Fig. 1B). We next examined whether staurosporine treatment was associated with degradation of the IP3R or other established substrates of caspase 3. Degradation of the IP3R was not evident even after 24 h staurosporine treatment (closed arrowhead, Fig. 1C), and production of the C-terminal 95 kDa caspase 3 fragment was barely detectable (open arrowhead, Fig. 1C). To examine whether staurosporine treatment resulted in cleavage of well-established caspase 3 substrates, we monitored the degradation of α-fodrin and PARP-1. α-Fodrin is cleaved during apoptosis by calpain into a 150 kDa fragment, and caspase 3 into 150 and 120 kDa fragments [23]. We found that staurosporine treatment of HeLa cells resulted in rapid production of 150 and 120 kDa fragments, and production of the 120 kDa fragment was inhibited by Z-VAD-FMK (Fig. 1C). Furthermore, the proteolysis of PARP was rapid after staurosporine treatment and significantly inhibited by Z-VAD-FMK (Fig 1C). The ER resident Ca2+ ATPase SERCA-2, which is not known to be a substrate for caspase 3, was used as a loading control. Caspase 3 enzymatic activity was robustly induced by staurosporine treatment, and completely inhibited by Z-VAD-FMK (Fig. 1D). Thus, at least in HeLa cells, caspase 3 activation has no effect on staurosporine-induced calcium release or degradation of the IP3R. Furthermore, activation of the calcium-dependent apoptotic protease calpain (as determined by α-fodrin cleavage) was a prominent and caspase 3-independent early event in staurosporine-induced apoptosis. To determine if our findings were specific to HeLa cells, we next examined the effect of inhibiting caspase 3 activity on calcium release in primary human fibroblasts. Similar to HeLa cells, no changes in calcium flux into the cytoplasm in response to ATP or staurosporine were detected after pre-treatment of fibroblasts with Z-VAD-FMK (Fig. 1E). Thus, caspase 3-mediated cleavage of IP3R channel is an unlikely mechanism to regulate staurosporine-dependent early elevations in cytosolic calcium in HeLa cells and primary human fibroblasts.
2.1 Caspase 3 does not support staurosporine-induced calcium release in MCF-7 cells
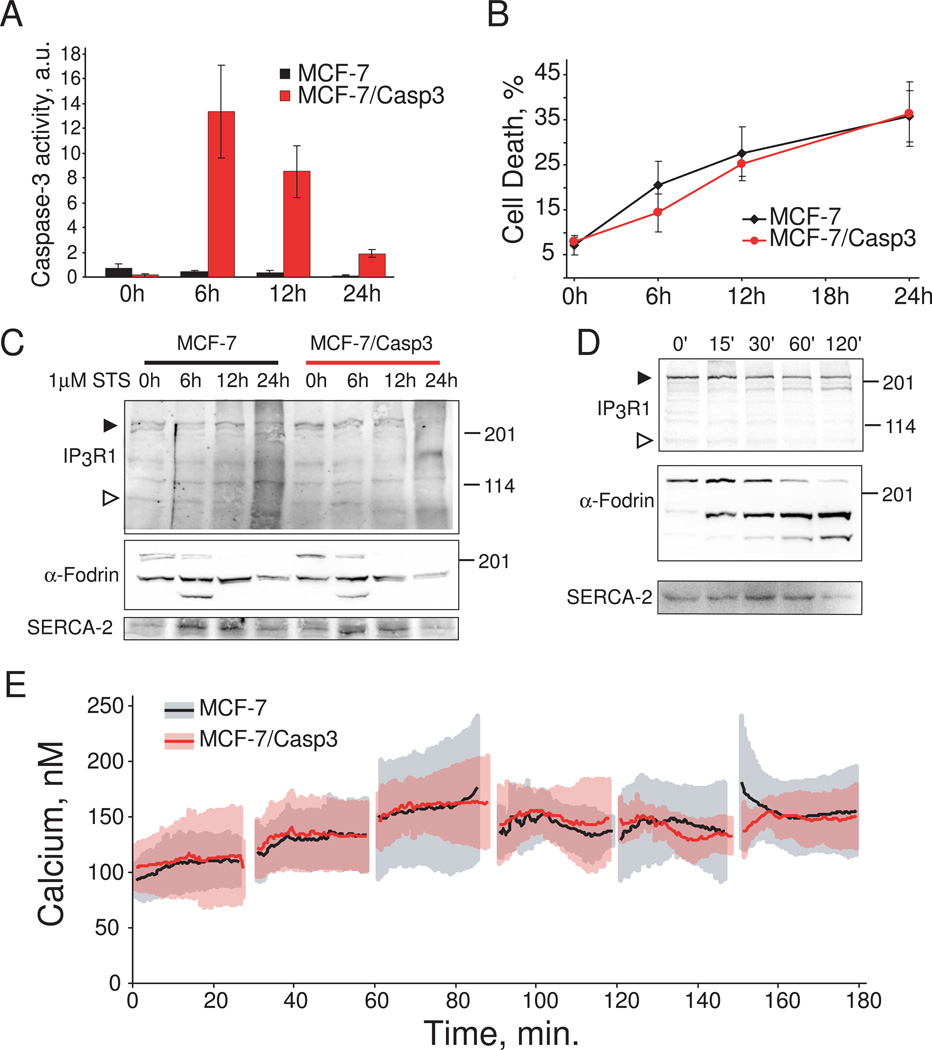
To further elucidate the role of caspase 3 in apoptotic calcium release we took advantage of the human MCF-7 breast carcinoma cell line which does not express caspase 3 as a result of a mutation within exon 3 of the CASP3 gene [24]. Caspase 3 expression was reconstituted by stable transfection of MCF-7 cells with full-length procaspase 3. Staurosporine challenge of MCF-7 cells stably expressing caspase 3 (MCF-7/Casp3) resulted in robust induction of caspase 3 activity peaking at ~6 h after treatment, whereas activity in wild type MCF-7 cells was undetectable (Fig. 2A). Next, we examined whether activation of caspase 3 would result in increased sensitivity of MCF-7 cells towards induction of apoptosis. Both MCF-7 and MCF-7/Casp3 cell lines demonstrated highly similar cell death kinetics in response to 1µM staurosporine treatment at 6, 12 and 24 hours, indicating that execution of staurosporine-induced cell death is not dependent on caspase 3 (Fig. 2B). This observation is further supported by similar patterns of α-fodrin cleavage in both MCF-7 and MCF-7/Casp3 cells (Fig.2C). Both of these results are consistent with a previous study demonstrating that deficiency of caspase 3 activity in MCF-7 cells can be partially substituted by caspase-7 during apoptosis [25]. Examination of IP3R-1 expression levels revealed that caspase 3 expression results in partial degradation of IP3R protein after 24 h of staurosporine treatment (closed arrow head, Fig. 2C). However, this was not associated with production of the 95 kDa C-terminal caspase 3 fragment (open arrow head, Fig. 2C), and was also associated with reduced levels of α-fodrin and SERCA-2 suggesting breakdown of total cellular protein. The delayed proteolysis of the IP3R might also be explained by low substrate preference of caspase 3 towards IP3R-1. To explore this hypothesis, we treated cerebellum microsomes enriched in IP3R-1 protein with recombinant caspase 3. As shown in Fig. 2D, treatment of microsomes with active recombinant caspase 3 resulted in significantly faster kinetics of α-fodrin cleavage as compared to IP3R-1. Furthermore, production of the 95 kDa C-terminal fragment was not evident in these experiments (open arrowhead, Fig. 2D), which is consistent with a recently published report [26]. The slow kinetics of IP3R-1 degradation in vitro and in intact cells would make it unlikely that caspase 3-mediated truncation of IP3R-1 contributes to the rapid mobilization of intracellular calcium seen with staurosporine treatment [14]. To address this possibility directly, we compared changes in intracellular calcium levels upon staurosporine stimulation in wild type MCF-7 and MCF-7/Casp3 cells. There were no differences in the calcium release kinetics between these two cell types up to three hours after staurosporine treatment (Fig 2E).
Fig. 2.
Requirement of caspase 3 for staurosporine-induced apoptosis and IP3R-1 cleavage. A. Caspase 3-like activity (DEVDase activity) in wild type MCF-7 and MCF-7/Casp3 cells after 1µM staurosporine treatment. B. Cell death in wild type MCF-7 and MCF-7/Casp3 cells after 1µM staurosporine treatment detected by propidium iodide staining. Data in panels A and B were pooled and averaged from three separate experiments. C. IP3R-1, α-fodrin, and SERCA-2 levels in wild type MCF-7 and MCF-7/Casp3 cells after 1µM staurosporine treatment. Full length IP3R is indicated by a closed arrowhead, and the 95 kDa caspase 3 cleaved C-terminal fragment by an open arrowhead. D. In vitro cleavage of IP3R-1 and α-fodrin by recombinant caspase 3. Rat cerebellar microsomes were incubated with recombinant caspase 3 protein for 0–120 minutes at 37°C. Arrowhead indicate full length and cleaved IP3R as in panel C. E. Averaged time course for calcium release in response to 1µM staurosporine in wild type MCF-7 and MCF-7/Casp3 cells. Breaks in the data are the results of coverslip changes. Data were pooled and averaged from three separate experiments.
2.2 Direct introduction of active caspase 3 into MCF-7 cells does not affect calcium levels
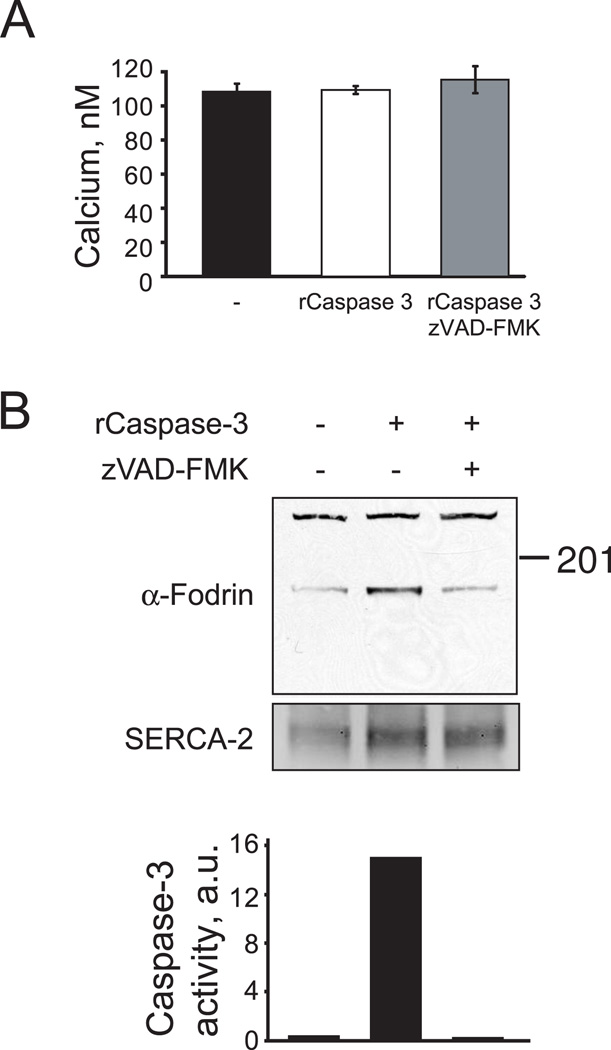
We transduced MCF-7 cells with the recombinant caspase 3 protein to determine whether active caspase 3, in absence of other IP3R regulators, would result in permeabilization of the channel and subsequent calcium release from ER stores. Three hours after introduction of active caspase 3, MCF7 cells had no changes in cytosolic calcium when compared to control cells or cells transduced with caspase 3 pre-incubated with Z-VAD-FMK (Fig 3A). To confirm that active caspase 3 was delivered into the cells, we examined α-fodrin cleavage and caspase 3 enzymatic activity. A significant increase in α-fodrin cleavage and caspase 3 activity was detected 3 hours after transduction with active caspase 3, but not in cells transduced with inactivated caspase 3 (Fig. 3B–C).
Fig. 3.
Intracellular calcium concentration in MCF-7 cells transduced with active recombinant caspase 3. A. Averaged calcium levels in non- transduced MCF-7 cells (left), transduced with recombinant caspase 3 (center) and recombinant caspase 3 pre-treated with Z-VAD-FMK (right). Calcium concentrations were measured 3 h after transduction. Similar results were obtained with real-time imaging as in Fig. 1E. Data is pooled and averaged from three separate experiemnts. B. α-Fodrin cleavage and caspase 3-like activity (DEVDase activity) before and after MCF-7 cell transduction with recombinant caspase 3. The results are representative of three separate determinations.
2.3 Staurosporine-induced calcium release depends on IP3 generation
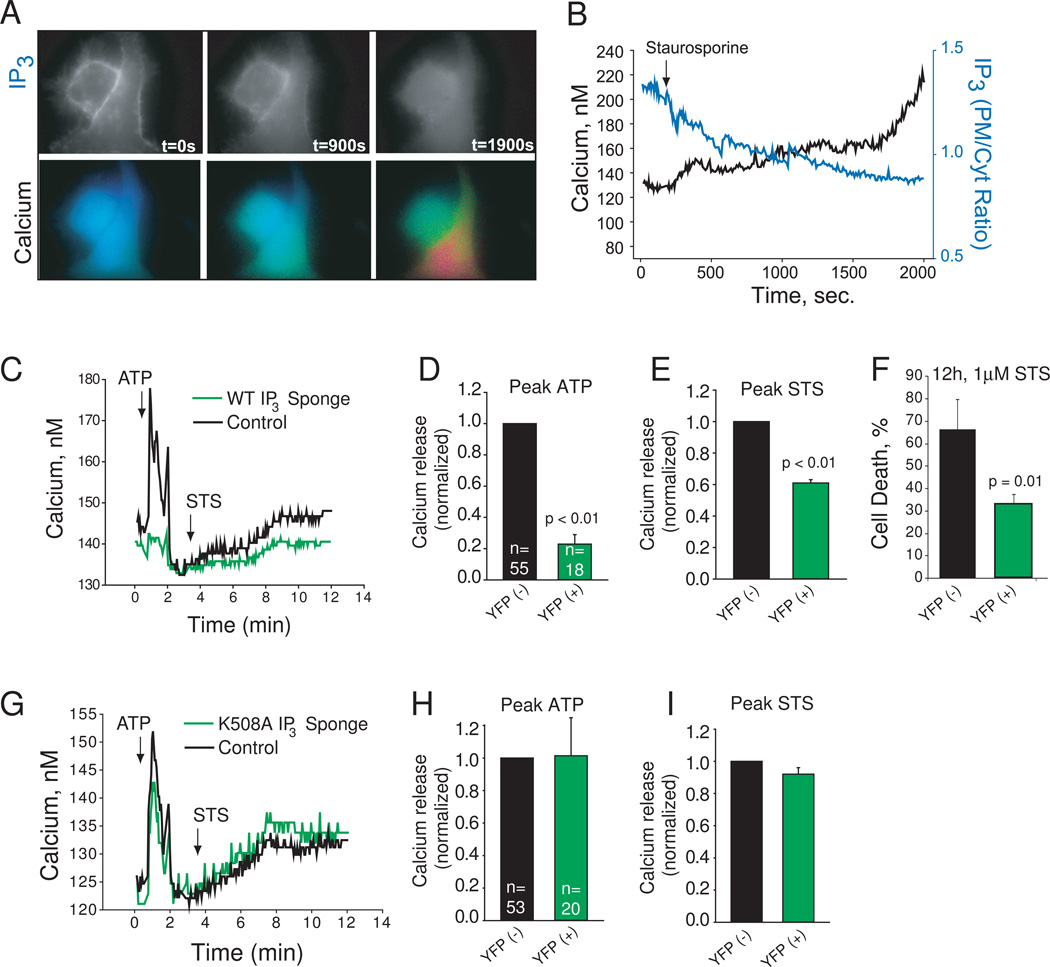
These results prompted us to investigate an alternative model of IP3R channel activation during initiation of apoptotic signaling. We hypothesized that apoptotic calcium release absolutely depends upon IP3 generation, as has been shown for death receptor signaling [21, 27]. To examine this possibility, we measured IP3 levels in intact HeLa cells upon staurosporine treatment using the IP3 biosensor PLCδ1PH-GFP [28]. As shown in Fig. 4A, staurosporine treatment resulted in dissipation of PLCδ1PH-GFP fluorescence from the plasma membrane and increased cytosolic fluorescence indicative of IP3 production. Concomitant measurement of cytosolic calcium revealed a strong correlation between the production of IP3 and calcium release, suggesting that IP3 generation is associated with staurosporine treatment (Fig. 4B). To evaluate more directly the contribution of IP3 to staurosporine-induced calcium release, we overexpressed in HeLa cells the ligand binding domain of the IP3R-1 protein fused to YFP (IP3 sponge), which is known to suppress IP3-evoked calcium release [17]. As a control, we also overexpressed the IP3 sponge with a point mutation eliminating IP3 binding (K508A sponge). We transiently transfected each sponge construct into HeLa cells and monitored calcium release sequentially in response to a purinergic agonist (1µM NaATP) and 1µM staurosporine. Sponge expressing cells were identified by simultaneously imaging YFP. We found that expressing the WT sponge suppressed calcium release in response to ATP resulting in severely reduced peak amplitude of calcium release following agonist stimulation (Fig. 4C,D), confirming the ability of this fusion protein to suppress IP3-evoked calcium release. Furthermore, WT sponge expression significantly inhibited calcium release in response to staurosporine treatment (Fig. 4C, E). Consistent with the requirement of IP3 production for staurosporine-induced cell death, expression of the WT sponge significantly inhibited cell death after staurosporine treatment (Fig. 4F). In contrast, expression of K508A sponge had no effects on calcium release kinetics or amplitude in response to ATP or staurosporine stimulation (Fig. 4G–I). These results suggest that IP3 gating of the IP3R, not caspase 3 cleavage, mediates apoptotic calcium release induced by staurosporine.
Fig. 4.
Requirement of IP3 for ATP- and staurosporine-induced calcium release. A. PLCδ1PH-GFP fluorescence (grayscale) and Fura-2 ratio (pseudocolor) imaged simultaneously in two HeLa cells. Time in seconds after staurosporine treatment (1µM) is indicated. B. Quantified calcium (black) and IP3 levels (blue) of the cell on the right in Panel A. C. Fura-2 calcium imaging of a HeLa cells expressing wild type IP3 sponge (as determined by YFP fluorescence; here an average of 5 cells from one experiment), and adjacent cells which are YFP-negative (in black; here an average of 20 cells from the same field). Agonist additions of 1µM NaATP and 2µM staurosporine are indicated. D. Peak ATP release averaged from three experiments. YFP(+) indicates sponge expressing cells. Total number of cells analyzed listed within the bar. E. Peak staurosporine (STS) release averaged from three experiments. YFP(+) indicates sponge expressing cells. F. Cell death as determined by propidium iodide staining. Averages of three separated determinations are shown. G–I. HeLa cells expressing the IP3 binding defective mutant sponge K508A and imaged and analyzed as in C–E.
4. Conclusions
We have found little evidence in this model system or others [14, 21, 27] for direct evidence to support the notion that caspase 3 contributes significantly to IP3R-dependent calcium release at physiologically relevant time scales during apoptosis. In particular, IP3R-1 is a very weak substrate for caspase 3, and we find no evidence that caspase 3 affects the permeability of the IP3R in intact cells. Thus, our results argue against a physiological role of caspase 3 in calcium homeostasis. Instead, our observations strongly indicate that the initiation of apoptotic calcium release relies entirely on IP3 generated by one of phospholipase C isoforms in response to cytotoxic stimuli.
Only the type 1 IP3R contains a consensus sequence for caspase 3 cleavage. Our group and others have shown that IP3R isoforms type 1, 2, and 3 can form hetero-oligomers [29–31]. Indeed, most cell types express varying amounts of all three IP3R isoforms [32] with the exception of the certain neuronal subpopulations which express primarily IP3R-1. A question arises whether heterotetrameric channels containing the type 1 IP3R as a subunit would be susceptible to caspase 3 cleavage and subsequent “leak” of calcium from the ER to the cytosol. This question may be answered by expression of various amounts of recombinant IP3R channel isoforms into triple IP3R knockout DT40 cells. This system has been instrumental investigating various aspects of IP3R function during apoptosis, including addressing whether caspase 3 cleavage of the channel is important. Interesting, using this system it was found that various IP3R constructs including wild type, caspase truncated, and channel dead (D2550A) forms of the channel all supported staurosporine-mediated calcium release and cell death, primarily by stimulating calcium entry [33]. Somewhat inconsistent with this finding, recombinant type I IP3R with the DEVD sequence changed to IEVA does not support caspase activation and cell death in triple IP3R knockout DT40 cells [6]. Regardless, our findings suggest that the IP3R-1 is such a poor substrate for caspase 3 that cleavage of the channel is unlikely to contribute to early calcium release events during apoptosis. It is also possible that cleavage by other proteases could contribute “leaky” IP3R channels, and in particular it was recently suggested that calpain cleavage of the IP3R may contribute to apoptotic calcium release [34, 35]. However, in our experimental systems described here and elsewhere [14] we find little or no degradation of the IP3R until very late in apoptosis, and this is despite robust calpain activation as determined by α-fodrin cleavage in the presence of Z-VAD-FMK (Fig. 1C).
Our results complement a study which was published while this paper was under review demonstrating that the IP3R-1 in not cleaved using several apoptotic paradigms, and that it is a poor substrate for caspase 3 in vitro [26]. We confirm and extend these findings in this study to show that caspase 3 does not contribute to apoptotic calcium release. Furthermore, we show that IP3 production is necessary and sufficient for apoptotic calcium release induced by staurosporine in HeLa cells (Fig. 4). This is consistent with the absolute requirement of PLC-γ1 activity and IP3 production for death receptor-mediated apoptotic calcium release [21, 36]. Future studies will examine how phospholipase C isoforms may be activated during initiation of the intrinsic apoptotic caspase.
Supplementary Material
Acknowledgements
We thank Tamas Balla (National Institutes of Health, Bethesda) for providing PLCδ1PH-GFP construct, Stephen Burke for helpful suggestions and critical reading of the manuscript and Xinmin Wang for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants 1R01GM081685-01 and 3R01GM081685-03S1 and Shiners Hospitals for Children grant 86900 (to D.B.).
Abbreviations
- GFP
green fluorescent protein
- IP3R
inositol 1,4,5-trisphosphate receptor
- PARP
poly (ADP-ribose) polymerase
- SERCA-2
sarco-endoplasmic reticulum calcium ATPase-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that there are no conflicts of interest
References
- 1.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. Embo J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- 4.Jayaraman T, Marks AR. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol Cell Biol. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirota J, Furuichi T, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J Biol Chem. 1999;274:34433–34437. doi: 10.1074/jbc.274.48.34433. [DOI] [PubMed] [Google Scholar]
- 6.Assefa Z, Bultynck G, Szlufcik K, Nadif Kasri N, Vermassen E, Goris J, Missiaen L, Callewaert G, Parys JB, De Smedt H. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J Biol Chem. 2004;279:43227–43236. doi: 10.1074/jbc.M403872200. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- 8.Szlufcik K, Bultynck G, Callewaert G, Missiaen L, Parys JB, De Smedt H. The suppressor domain of inositol 1,4,5-trisphosphate receptor plays an essential role in the protection against apoptosis. Cell Calcium. 2006;39:325–336. doi: 10.1016/j.ceca.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Szlufcik K, Missiaen L, Parys JB, Callewaert G, De Smedt H. Uncoupled IP3 receptor can function as a Ca2+-leak channel: cell biological and pathological consequences. Biol Cell. 2006;98:1–14. doi: 10.1042/BC20050031. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama T, Hattori M, Uchida K, Nakamura T, Tateishi Y, Bannai H, Iwai M, Michikawa T, Inoue T, Mikoshiba K. The regulatory domain of the inositol 1,4,5-trisphosphate receptor is necessary to keep the channel domain closed: possible physiological significance of specific cleavage by caspase 3. Biochem J. 2004;377:299–307. doi: 10.1042/BJ20030599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-Franco J, Galvan D, Mignery GA, Fill M. Location of the permeation pathway in the recombinant type 1 inositol 1,4,5-trisphosphate receptor. J Gen Physiol. 1999;114:243–250. doi: 10.1085/jgp.114.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackshaw S, Sawa A, Sharp AH, Ross CA, Snyder SH, Khan AA. Type 3 inositol 1,4,5-trisphosphate receptor modulates cell death. Faseb J. 2000;14:1375–1379. doi: 10.1096/fj.14.10.1375. [DOI] [PubMed] [Google Scholar]
- 13.Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- 14.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 15.Boehning D, van Rossum DB, Patterson RL, Snyder SH. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc Natl Acad Sci U S A. 2005;102:1466–1471. doi: 10.1073/pnas.0409650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkoreh G, Blais V, Beliveau E, Guillemette G, Denault JB. Type 1 inositol-1,4,5-trisphosphate receptor is a late substrate of caspases during apoptosis. J Cell Biochem. doi: 10.1002/jcb.24155. [DOI] [PubMed] [Google Scholar]
- 17.Uchiyama T, Yoshikawa F, Hishida A, Furuichi T, Mikoshiba K. A novel recombinant hyperaffinity inositol 1,4,5-trisphosphate (IP(3)) absorbent traps IP(3), resulting in specific inhibition of IP(3)-mediated calcium signaling. J Biol Chem. 2002;277:8106–8113. doi: 10.1074/jbc.M108337200. [DOI] [PubMed] [Google Scholar]
- 18.Joseph SK, Samanta S. Detergent solubility of the inositol trisphosphate receptor in rat brain membranes. Evidence for association of the receptor with ankyrin. J Biol Chem. 1993;268:6477–6486. [PubMed] [Google Scholar]
- 19.Denault JB, Salvesen GS. Expression, purification, and characterization of caspases. Curr Protoc Protein Sci. 2003;Chapter 21(Unit 21):13. doi: 10.1002/0471140864.ps2113s30. [DOI] [PubMed] [Google Scholar]
- 20.Crameri A, Whitehorn EA, Tate E, Stemmer WP. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol. 1996;14:315–319. doi: 10.1038/nbt0396-315. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak AL, Wang X, Stieren ES, Scarbrough SG, Elferink CJ, Boehning D. Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J Cell Biol. 2006;175:709–714. doi: 10.1083/jcb.200608035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeschke MG, Gauglitz GG, Song J, Kulp GA, Finnerty CC, Cox RA, Barral JM, Herndon DN, Boehning D. Calcium and ER stress mediate hepatic apoptosis after burn injury. J Cell Mol Med. 2009;13:1857–1865. doi: 10.1111/j.1582-4934.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem. 1998;273:15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 24.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 25.Walsh JG, Cullen SP, Sheridan C, Luthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci U S A. 2008;105:12815–12819. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elkoreh G, Blais V, Beliveau E, Guillemette G, Denault JB. Type 1 inositol-1,4,5-trisphosphate receptor is a late substrate of caspases during apoptosis. J Cell Biochem. 2012;113:2775–2784. doi: 10.1002/jcb.24155. [DOI] [PubMed] [Google Scholar]
- 27.Akimzhanov AM, Wang X, Sun J, Boehning D. T-cell receptor complex is essential for Fas signal transduction. Proc Natl Acad Sci U S A. 107:15105–15110. doi: 10.1073/pnas.1005419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph SK, Bokkala S, Boehning D, Zeigler S. Factors determining the composition of inositol trisphosphate receptor hetero-oligomers expressed in COS cells. J Biol Chem. 2000;275:16084–16090. doi: 10.1074/jbc.M000506200. [DOI] [PubMed] [Google Scholar]
- 30.Joseph SK, Boehning D, Pierson S, Nicchitta CV. Membrane insertion, glycosylation, and oligomerization of inositol trisphosphate receptors in a cell-free translation system. J Biol Chem. 1997;272:1579–1588. doi: 10.1074/jbc.272.3.1579. [DOI] [PubMed] [Google Scholar]
- 31.Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto-Hino M, Furuichi T, Saruta T, Hasegawa M, Mikoshiba K. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J Biol Chem. 1995;270:14700–14704. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- 32.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 33.Khan MT, Bhanumathy CD, Schug ZT, Joseph SK. Role of inositol 1,4,5-trisphosphate receptors in apoptosis in DT40 lymphocytes. J Biol Chem. 2007;282:32983–32990. doi: 10.1074/jbc.M705183200. [DOI] [PubMed] [Google Scholar]
- 34.Kopil CM, Siebert AP, Kevin Foskett J, Neumar RW. Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor impairs ER Ca(2+) buffering and causes neurodegeneration in primary cortical neurons. J Neurochem. 2012;123:147–158. doi: 10.1111/j.1471-4159.2012.07859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopil CM, Vais H, Cheung KH, Siebert AP, Mak DO, Foskett JK, Neumar RW. Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor (InsP(3)R1) has InsP(3)-independent gating and disrupts intracellular Ca(2+) homeostasis. J Biol Chem. 2011;286:35998–36010. doi: 10.1074/jbc.M111.254177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akimzhanov AM, Wang X, Sun J, Boehning D. T-cell receptor complex is essential for Fas signal transduction. Proc Natl Acad Sci U S A. 2010;107:15105–15110. doi: 10.1073/pnas.1005419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.