Abstract
Background/Aims
While chemoradiotherapy (CRT) is considered to be a reasonable treatment for locally advanced pancreatic cancer (LAPC), there is little information about the associated risk of gastrointestinal (GI) hemorrhage. We investigated the clinical features of GI toxicity after CRT in patients with LAPC and examined the effect of GI hemorrhage on survival.
Methods
Patients enrolled in this study had received CRT for pathologically proven LAPC. Their medical records were retrospectively reviewed.
Results
A total of 156 patients with LAPC (median age, 65 years; range, 39 to 90 years) who received treatment between August 2005 and March 2009 were included in this study. The most common GI toxicities were ulcer formation (25.6%) and hemorrhage (25.6%), and the most common grade 3 to grade 5 GI toxicity was hemorrhage (65%). The origins of GI hemorrhage were gastric ulcer (37.5%), duodenal ulcer (37.5%), and radiation gastritis (15.0%). The independent risk factor for GI hemorrhage was tumor location in the pancreatic body. The median overall survival of the patients with a GI hemorrhage was 13.8 months (range, 2.8 to 50.8 months) and was not significantly different from that of patients without GI hemorrhage.
Conclusions
GI hemorrhage was common in patients with LAPC after CRT. Although GI hemorrhage was controlled with endoscopic hemostasis, preventive measures should be investigated to reduce needless suffering.
Keywords: Chemoradiotherapy, Gastrointestinal hemorrhage, Toxicities, Pancreatic neoplasms
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States.1 Surgical resection is the only curative treatment for pancreatic cancer.2 However, only 5% to 25% of patients with pancreatic cancer are candidates for curative pancreatectomy.
Locally advanced pancreatic cancer (LAPC) is surgically unresectable nonmetastatic disease, which includes the cases of extensive peripancreatic lymph node involvement and major vasculatures involvement.3 The median survival time for patients with LAPC is only 9 to 10 months.4
Chemoradiotherapy (CRT) is a reasonable treatment modality for LAPC because it is known to increase survival in patients with LAPC.5-7 CRT has been frequently used instead of chemotherapy alone or radiotherapy alone. However, overall toxic effects of CRT are greater than those of chemotherapy alone.8 Such adverse effects of CRT limit the maximum dose of chemotherapy and radiotherapy, and lead to unfavorable treatment results.9
CRT for treatment of LAPC produces unique gastrointestinal (GI) toxicities, including ulcer and hemorrhage in the stomach and duodenum that are included in the radiation field. However, to our knowledge, there are a few data focusing on GI hemorrhage of CRT for treatment of LAPC. In addition, the effect of GI hemorrhage on survival of patients with LAPC has not been evaluated. GI hemorrhage of CRT needs to be examined to prevent adverse events and to develop methods to reduce the severity of such events. Indeed, in clinical practice, we experienced many patients with CRT-induced GI hemorrhage, which led us to perform this study. Thus, we examined clinical features of GI hemorrhage of CRT in patients with LAPC, and the effect of GI hemorrhage on survival.
MATERIALS AND METHODS
1. Patients
Patients who received concurrent CRT for treatment of LAPC at Severance Hospital in Seoul, Korea, between August 2005 and March 2009 were selected for this study. Inclusion criteria included pathologically-proven pancreatic adenocarcinoma, age of over 20 years, and the Eastern Cooperative Oncology Group performance status of 0 to 2. Exclusion criteria included patients who had received chemotherapy or surgery before CRT, and patients with scheduled radiotherapy less than 4,000 cGy. Patients who had not completed their scheduled radiation therapy were also excluded for per protocol analysis.
2. Treatment for LAPC
For regression analysis, regimens of chemotherapy and radiotherapy were classified into several groups. All chemotherapy regimens of CRT performed for LAPC were classified into three groups: gemcitabine, 5-fluorouracil (5-FU), and 5-FU plus gemcitabine. Gemcitabine group was given 1,000 mg/m2 of gemcitabine on days 1, 8, and 15 of a 4-week regimen or gemcitabine (same as above) along with 70 mg/m2 of cisplatin on day 1 of the regimen. 5-FU group received either 5-FU (1,000 mg/m2 on days 1 to 3 of a 4-week regimen) or; TS-1 (60 to 80 mg for 2 weeks); or a combination of 5-FU (1,000 mg/m2 on days 1 to 3), etoposide (100 mg/m2 on days 1 to 3), and cisplatin (70 mg/m2 on day 1). For 5-FU plus gemcitabine group, 1,000 mg/m2 of 5-FU was given on days 1 to 3, and 1,000 mg/m2 gemcitabine on days 1, 8, and 15 of a 4-week regimen.
All radiotherapy regimens of CRT performed for LAPC were either three-dimensional (3D) conformal radiotherapy (total dose, 4,000 to 5,400 cGy; one dose, 180 to 250 cGy; fraction, 28) or intensity modulated radiotherapy (total dose, 4,200 to 6,000 cGy; one dose, 200 to 293 cGy; fraction, 25).
3. GI toxicities
GI toxicities were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. In our study, radiation-induced injuries observed with endoscopy were defined as telangiectasia, diffuse erythema of mucosa, ulcers, and scar formation.10,11
4. Statistical analysis
To investigate the risk factors of GI toxicities by CRT, Pearson's chi-square test for univariate analysis and logistic regression for multivariate analysis were used. To evaluate survival effect of GI hemorrhage, Cox regression test was used. The Kaplan-Meier method and the log-rank test were used to compare survival between patients with GI hemorrhage and patients without GI hemorrhage.
All analyses were performed using statistical software SPSS version 11 (SPSS Inc., Chicago, IL, USA). A p-values lower than 0.05 indicated significance.
RESULTS
1. Patient characteristics
One hundred fifty-six patients with LAPC were eligible for analysis (Table 1). The median age at the time of the diagnosis of pancreatic cancer was 65 years, ranging from 35 to 90 years. Male patients accounted for 61.5% of the population. The tumors were mostly located at the pancreatic head (63.5%). The median size of the tumor was 2.9 cm, ranging from 1.1 to 7.0 cm. The median level of CA 19-9 was 384 U/mL (range, 0.1 to 20,000 U/mL). The 3D conformal radiotherapy was delivered to 119 patients (76.3%), and the median delivered dose was 5,040 cGy (range, 4,000 to 5,400 cGy). Intensity modulated radiotherapy was delivered to 37 patients (23.7%), and the median delivered dose was 5,842 cGy (range, 4,200 to 6,000 cGy). The median follow-up period was 13.2 months (range, 2 to 52.2 months).
Table 1.
Baseline Patient Characteristics
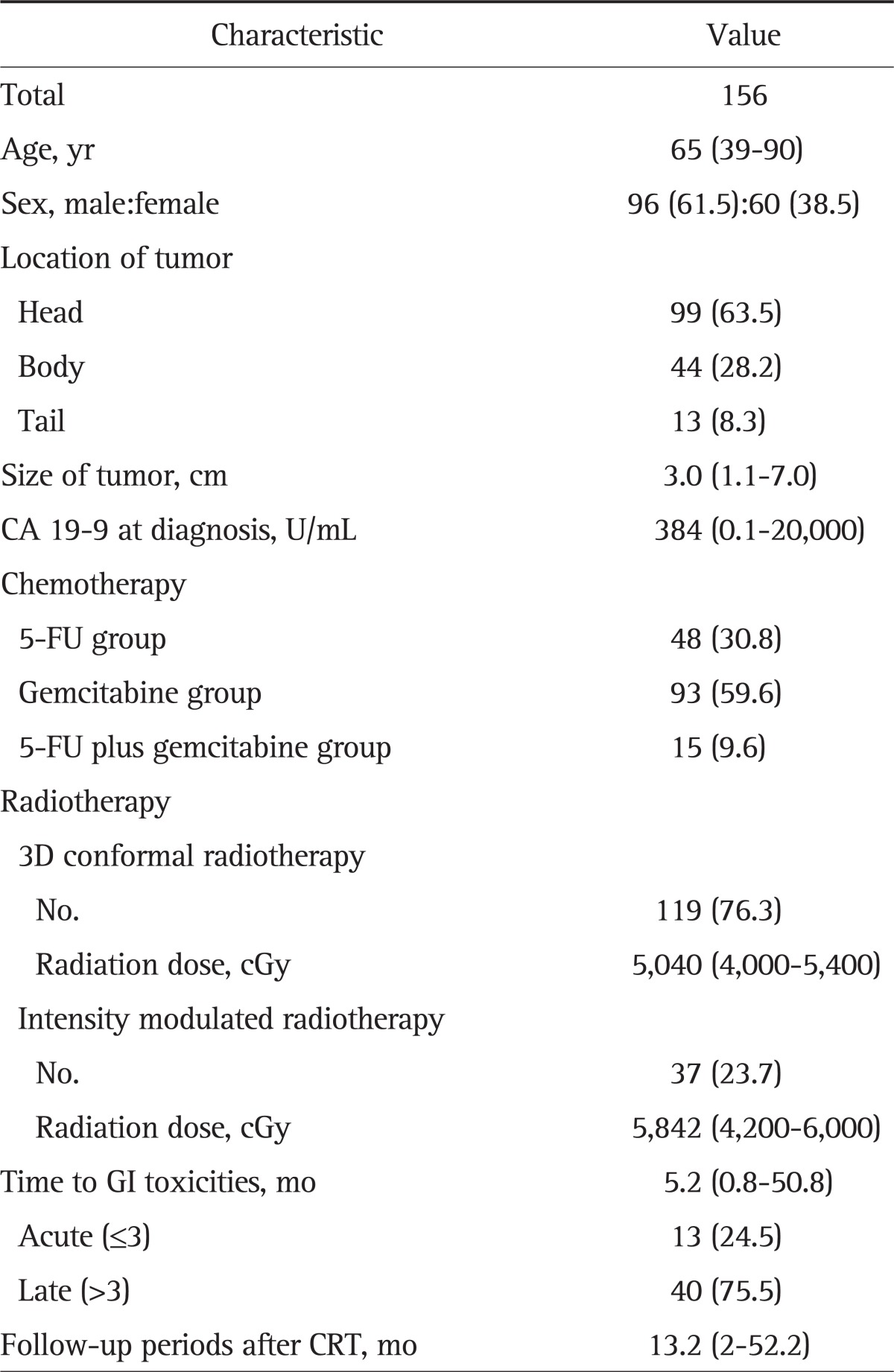
Data are presented as median (range) or number (%).
5-FU, 5-fluorouracil; GI, gastrointestinal; 3D, three-dimensional; CRT, chemoradiotherapy.
2. GI toxicities
The prevalence of GI toxicities was 57.7% (Table 2). There were 30 patients with grade 1 or 2 abdominal pain or dyspepsia. Two patients had grade 3 anorexia. Nausea and vomiting developed in four patients, and these were well controlled with appropriate medications. Forty patients (25.6%) had GI hemorrhage: nine patients with hematemesis (22.5%), 21 patients with melena (52.5%), and 10 patients with hematochezia (25%). Eighteen patients (11.5%) had grade 3 or 4 GI hemorrhage, and eight patients (5.1%) had grade 5 (death) hemorrhage.
Table 2.
Gastrointestinal Toxicities after Chemoradiotherapy According to the National Cancer Institute Common Terminology Criteria Version 4.0
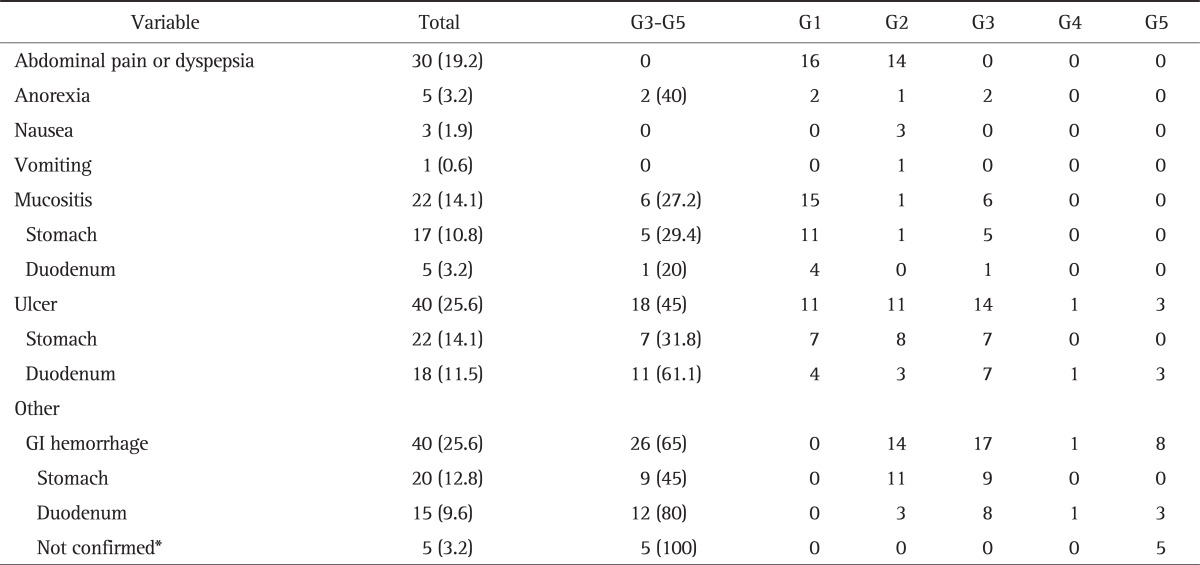
Data are presented as number (%).
GI, gastrointestinal.
*Endoscopy was not performed, and the focus of bleeding was not confirmed.
Seventy-eight patients (50%) underwent upper GI endoscopy after CRT. There were mucositis in 27.2% of patients, ulcer in 45%, and GI hemorrhage with above grade 3 toxicity in 65%. The median time of development of GI toxicities was 5.2 months.
3. GI hemorrhage
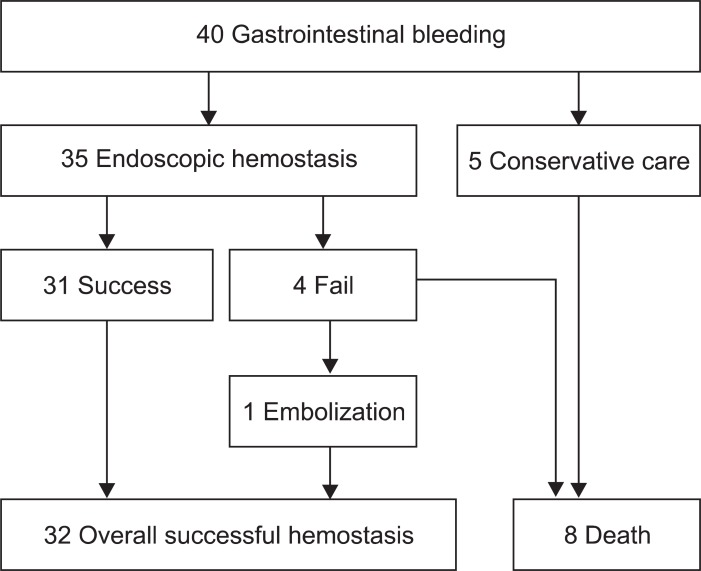
Forty patients (25.6%) suffered from GI hemorrhage after CRT (Table 3). The median initial hemoglobin was 10.1 g/dL (range, 7.1 to 15.3 g/dL), which decreased to 7.1 g/dL (range, 3.5 to 10.8 g/dL) when bleeding. Thirty-five patients underwent upper GI endoscopy. The results showed the cause of bleeding to be a gastric ulcer in 15 patients (37.5%), duodenal ulcer in 15 (37.5%), and radiation gastritis in five (15%). Upper GI endoscopy was not performed in five patients upon their guardians' rejection. As the patients were in terminal stages, the guardians did not want them to undergo any more examinations. The remaining 35 patients received endoscopic treatment. Hemorrhage was successfully stopped by endoscopic treatment in 31 patients (77.5%). The methods of endoscopic hemostasis were hypertonic saline-epinephrine injection, human plasmin thrombin injection, argon plasma coagulation, or hemoclipping. Embolization was performed in one of the four other patients, and hemorrhage was finally stopped. However, three others died due to GI hemorrhage. The mortality of GI hemorrhage was eight patients in total (Fig. 1).
Table 3.
Gastrointestinal Hemorrhage after CRT (n=40)
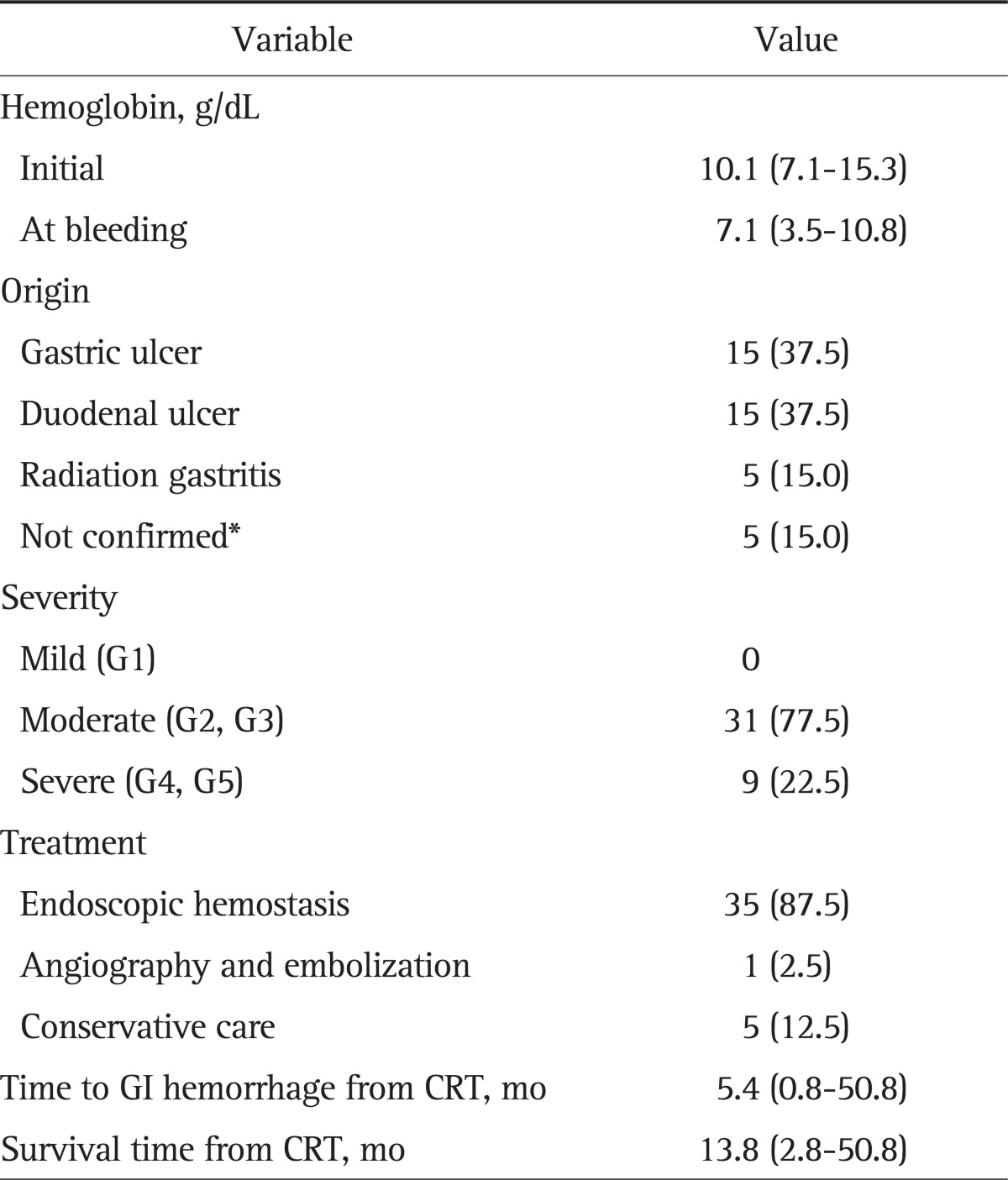
Data are presented as median (range) or number (%).
CRT, chemoradiotherapy; GI, gastrointestinal.
*Endoscopy was not performed, and the focus of bleeding was not confirmed.
Fig. 1.
Treatment flow chart. Treatment of gastrointestinal hemorrhage after chemoradiotherapy.
The median time from CRT to GI hemorrhage was 5.4 months (range, 0.8 to 50.8 months), and the median overall survival was 13.8 months (range, 2.8 to 50.8 months).
4. Risk factors for GI hemorrhage
The association between clinical parameters and the risk of GI hemorrhage were analyzed (Table 4). In univariate analysis, location of tumor (p=0.007) and size of tumor (p=0.042) were risk factors for GI hemorrhage. In multivariate analysis, only tumors located on the pancreatic body (odds ratio [OR], 2.99; 95% confidence interval [CI], 1.27 to 7.03) was a significant risk factor for GI hemorrhage. The hazard ratio (HR) was 1.28 (95% CI, 0.78 to 2.07) for the effect of GI hemorrhage on survival, but it was not significant (Table 5). Male patients had significantly higher HR than females (HR, 1.62; 95% CI, 1.07 to 2.43; p=0.020). However, there were no difference in the number of patients with hypertension or diabetes mellitus between males and females (OR, 1.26; 95% CI, 0.66 to 2.40; p=0.492).
Table 4.
Risk Factors for GI Hemorrhage in All Patients (n=156)
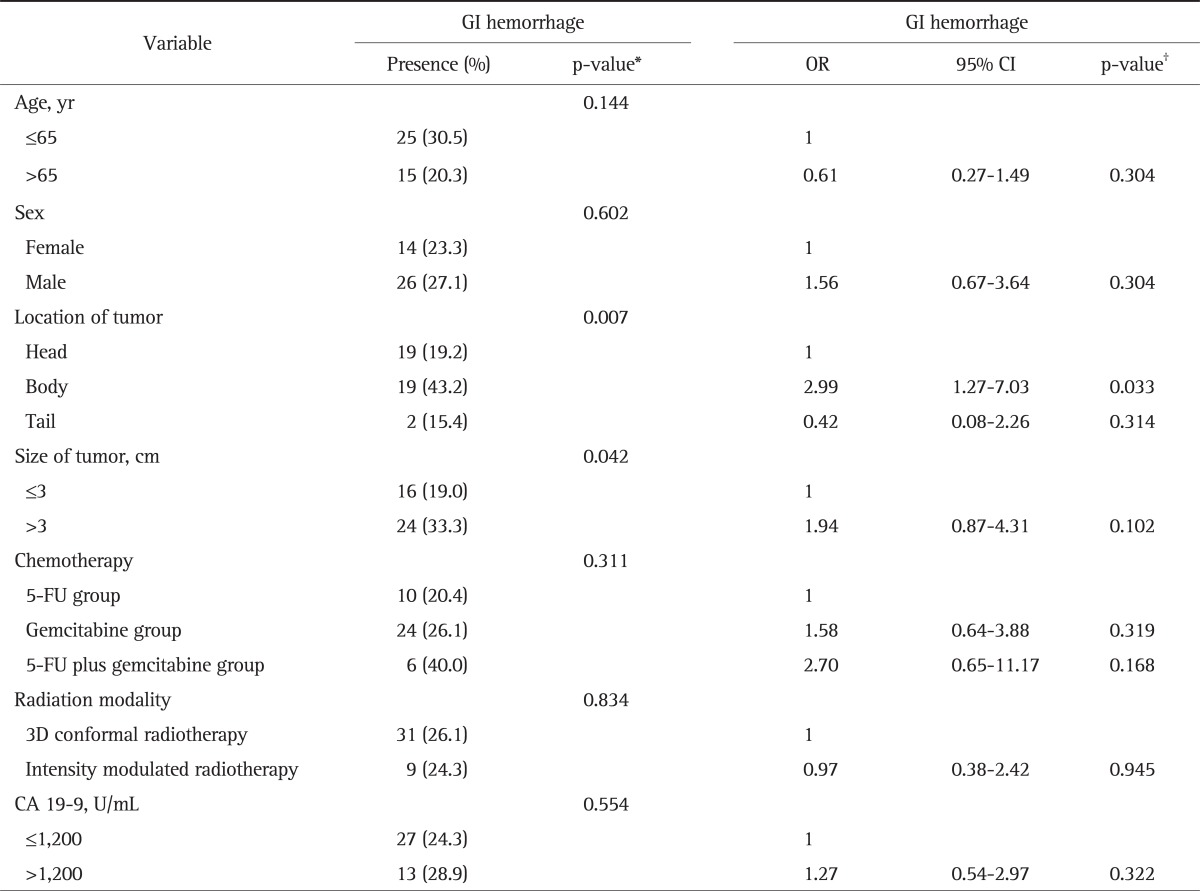
GI, gastrointestinal; OR, odds ratio; CI, confidence interval; 5-FU, 5-fluorouracil; 3D, three-dimensional.
*Chi-square test was used; †Logistic regression was used.
Table 5.
Cox Regression Analysis of the Effect of GI Hemorrhage on Survival
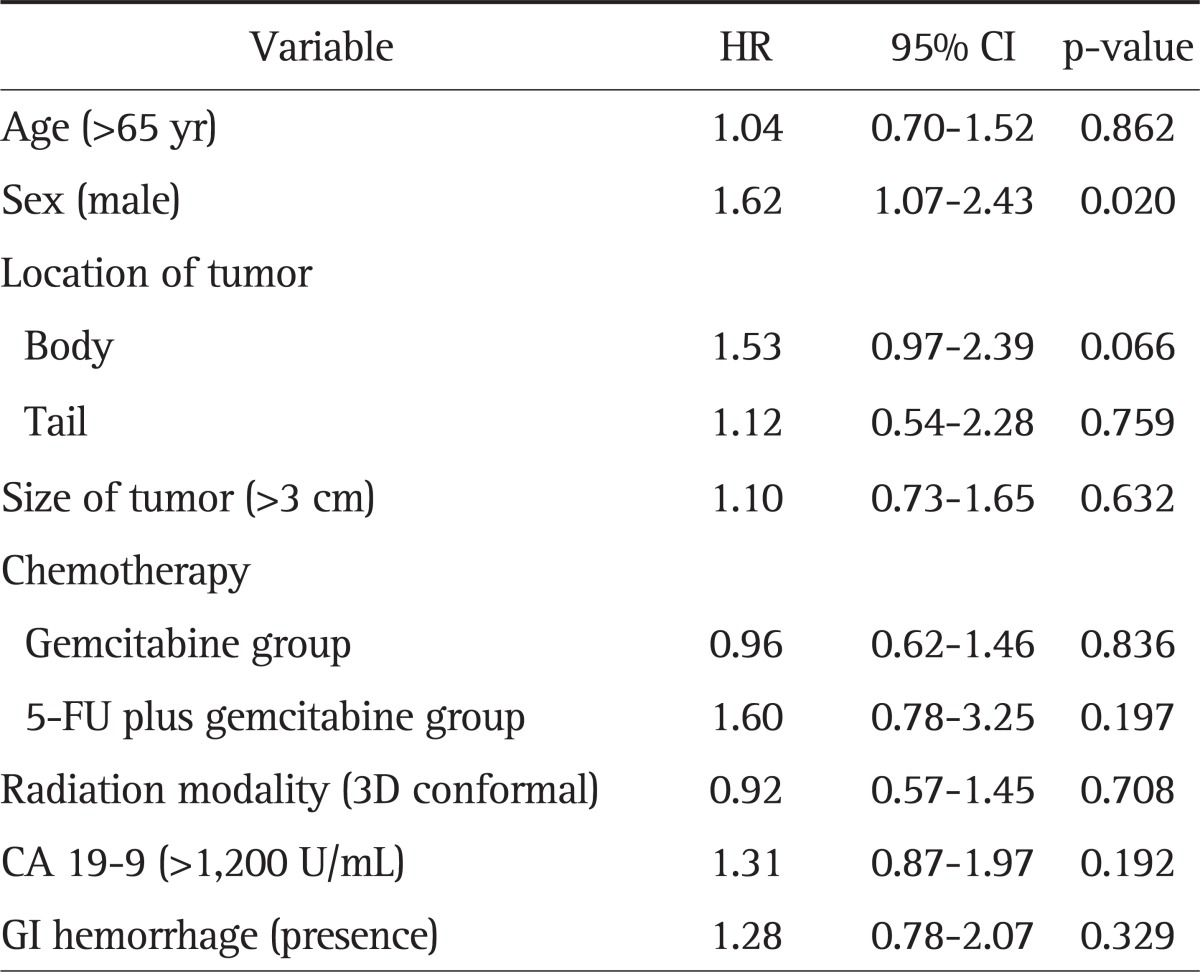
GI, gastrointestinal; HR, hazard ratio; CI, confidence interval; 5-FU, 5-fluorouracil.
5. Survival
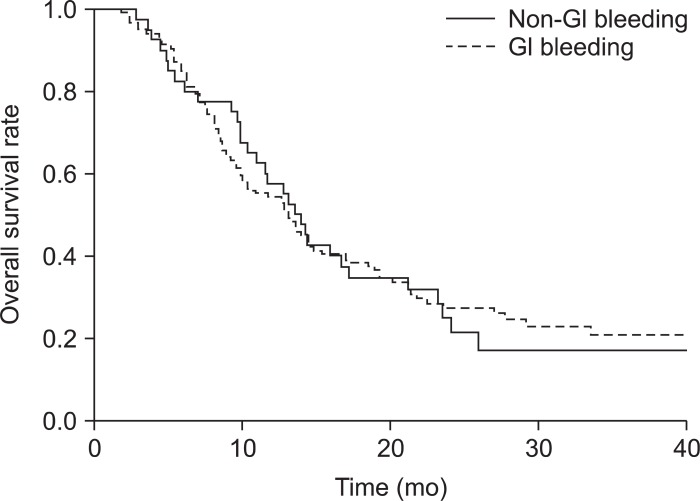
At the time of final analysis, 117 patients (75%) were dead. The median overall survival after the start of CRT was 13.1 months (range, 11.3 to 14.9 months). The median overall survival was 13.1 months (range, 9.9 to 16.3 months) in patients without GI hemorrhage and 13.8 months (range, 2.8 to 50.8 months) in patients with GI hemorrhage (Fig. 2). Although overall survival was longer in patients with GI hemorrhage, this difference was not significant.
Fig. 2.
Comparison of survival between gastrointestinal (GI) hemorrhage patients and non-GI hemorrhage patients. The overall median survival time was 13.1 months in the non-GI hemorrhage group and 13.5 months in the GI hemorrhage group.
DISCUSSION
Our analysis showed that the prevalence of CRT-induced GI hemorrhage is frequent and serious if not treated properly. The body of the pancreas was the risk factor of GI hemorrhage after CRT. The median overall survival was similar with other studies.5,8,12 This study also showed that GI hemorrhage after CRT did not reduce survival of patients with LAPC.
CRT was first introduced in the Gastrointestinal Tumor Study Group trial.13 Many studies reported the benefit of CRT, and CRT became one of the treatment options for pancreatic cancer.6,7 Based on the results of several studies, the RT dose of 50 to 60 Gy (182 cGy/day) is generally used.14,15 A study reported that the toxicity was higher in the LAPC group where the radiation dose increased up to 55 Gy than in the LAPC group with the dose up to 50 Gy; however, patient compliance was similar between the groups, and the treatment performance in the former was better than that in the latter.16 In studies comparing CRT and chemotherapy, however, more cases of toxicity were found in the CRT group; thus, care must be taken with regard to the use of CRT.17,18
Due to low awareness of GI hemorrhage, the frequency of endoscopic examination was quite low. Of 156 patients, 20 were examined with endoscopy before CRT and 78 after CRT. Very few patients who had GI hemorrhage underwent endoscopic study before the onset of bleeding. Had endoscopy also been performed in other patients, the chances of finding complications such as radiation gastritis would have been higher.
The location of the tumor was related with GI hemorrhage. As the body of the pancreas is located close to both the stomach and duodenum, radiation on the pancreas affects the two organs as well. There is no consensus over the best time to perform endoscopy after radiotherapy. After CRT, however, GI toxicites are likely to develop at anytime. Therefore, it is recommended that endoscopy be performed as was done in this study. If abnormal findings are found in endoscopy before CRT, preemptive treatment is necessary. Moreover, endoscopy as a baseline study is recommended for the comparison with post-CRT endoscopic results. Usually CRT is followed by chemotherapy or surgery about one month later. Endoscopy is recommended before such therapies as GI ulcer or hemorrhage can occur even within 90 days after CRT. As ulcerative bleeding is highly responsive to proton pump inhibitor, its early detection and treatment may prevent adverse events. Although the best frequency of endoscopy may be debatable, yearly or more frequent endoscopy, particularly in patients with a history of GI hemorrhage, is recommended considering the possibility of delayed ulcer and hemorrhage.
This study has several limitations. First, the results were obtained by retrospectively reviewing the medical charts. Second, regarding CRT, the chemotherapy-induced adverse effects could not be excluded. In this study, a greater number of patients received gemcitabine treatment than those who received 5-FU. Several studies reported that gemcitabine was more toxic than 5-FU.19,20 In addition, it was difficult to identify the cause of GI hemorrhage after surgery as well as after beginning a chemotherapy only regimen following CRT. Third, the low number of patients who received endoscopy before CRT made it impossible to determine if patients developed an ulcer before CRT. Fourth, patients who received 3D conformal radiotherapy and intensity modulated radiotherapy were analyzed together, and 37 of them received intensity-modulated radiotherapy. To the best of our knowledge, there has been no study that compared 3D conformal radiotherapy and intensity-modulated radiotherapy. Thus, a study is required to investigate if there is any difference between the two modalities.
In conclusion, the present results show that GI hemorrhage is common in LAPC after CRT. Although the median survival was similar regardless of GI hemorrhage, the clinicians need to consider the GI hemorrhage of CRT in LAPC. Extensive studies are required to compare the benefits and risks in terms of survival and complications between CRT and chemotherapy. In addition, studies are required to identify tests or treatments that can reduce CRT-induced GI hemorrahge.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Winek T, Hamre D, Mozell E, Vetto RM. Prognostic factors for survival after pancreaticoduodenectomy for malignant disease. Am J Surg. 1990;159:454–456. doi: 10.1016/s0002-9610(05)81244-9. [DOI] [PubMed] [Google Scholar]
- 3.Willett CG, Czito BG, Bendell JC, Ryan DP. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–4544. doi: 10.1200/JCO.2005.23.911. [DOI] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 5.Shinchi H, Takao S, Noma H, et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002;53:146–150. doi: 10.1016/s0360-3016(01)02806-1. [DOI] [PubMed] [Google Scholar]
- 6.Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 7.Nakachi K, Furuse J, Kinoshita T, et al. A phase II study of induction chemotherapy with gemcitabine plus S-1 followed by chemoradiotherapy for locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:527–534. doi: 10.1007/s00280-009-1193-4. [DOI] [PubMed] [Google Scholar]
- 8.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 9.Sawaki A, Hoki N, Ito S, et al. Clinical impact of radiotherapy for locally advanced pancreatic cancer. J Gastroenterol. 2009;44:1209–1214. doi: 10.1007/s00535-009-0116-9. [DOI] [PubMed] [Google Scholar]
- 10.DeCosse JJ, Rhodes RS, Wentz WB, Reagan JW, Dworken HJ, Holden WD. The natural history and management of radiation induced injury of the gastrointestinal tract. Ann Surg. 1969;170:369–384. doi: 10.1097/00000658-196909010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sell A, Jensen TS. Acute gastric ulcers induced by radiation. Acta Radiol Ther Phys Biol. 1966;4:289–297. doi: 10.3109/02841866609133149. [DOI] [PubMed] [Google Scholar]
- 12.Li CP, Chao Y, Chi KH, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys. 2003;57:98–104. doi: 10.1016/s0360-3016(03)00435-8. [DOI] [PubMed] [Google Scholar]
- 13.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Boz G, De Paoli A, Innocente R, et al. Radiotherapy and continuous infusion 5-fluorouracil in patients with nonresectable pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:736–740. doi: 10.1016/s0360-3016(01)01708-4. [DOI] [PubMed] [Google Scholar]
- 15.Mehta VK, Poen JC, Ford JM, et al. Protracted venous infusion 5-fluorouracil with concomitant radiotherapy compared with bolus 5-fluorouracil for unresectable pancreatic cancer. Am J Clin Oncol. 2001;24:155–159. doi: 10.1097/00000421-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Henry AM, Ryder WD, Moore C, et al. Chemoradiotherapy for locally advanced pancreatic cancer: a radiotherapy dose escalation and organ motion study. Clin Oncol (R Coll Radiol) 2008;20:541–547. doi: 10.1016/j.clon.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 18.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 19.Crane CH, Abbruzzese JL, Evans DB, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293–1302. doi: 10.1016/s0360-3016(01)02740-7. [DOI] [PubMed] [Google Scholar]
- 20.Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]