Abstract
Gastrointestinal leakage is one of the most serious post surgical complications and is a major source of mortality and morbidity. The insertion of a covered self-expandable metal stent could be a treatment option in selected cases. However, it is unclear how long the stent should be retained to achieve complete sealing, and membrane-covered stents have the problem of a high migration rate. We observed four cases of postsurgical leakage following the primary closure of a duodenal perforation, esophagojejunostomy, and esophagogastrostomy, each of which was successfully managed by the temporary placement of covered stents. In all cases, the optimal time of stent removal could be estimated by the markedly decreased amount of drainage, the lack of leakage observed on radiocontrast images, and the endoscopic findings. In this case series, all of the stents could be removed within 7 weeks. For those cases with a high risk of migration, stents with temporary fixations to earlobes and/or partially uncovered proximal flanges were used. These results suggest that the application of a covered stent could be a treatment option for various gastrointestinal leaks after surgery, particularly when the defect cannot be sealed by conservative care and the leakage has good external drainage.
Keywords: Stents, Anastomotic leak, Drainage
INTRODUCTION
Gastrointestinal leaks after surgery are usually associated with high rates of mortality and morbidity.1 Although the treatment strategy depends on the location and size of the defect, there is no definite guideline to manage this complication. After a delayed diagnosis, the post-operative suture can break down, and the mortality rate associated with conservative care can be comparable to that associated with surgical procedures.2
The temporary placement of a covered self-expandable metal stent (SEMS) is another treatment option for sealing surgical leaks.3 We observed four cases of leakages after various gastrointestinal surgeries that were successfully managed by the temporary placement of covered stents. In all of the cases, the leakage was completely sealed, and the stents could be removed within 7 weeks. Eight weeks is generally considered to be the maximal duration of stent maintenance for benign diseases.4
CASE REPORTS
1. Case 1
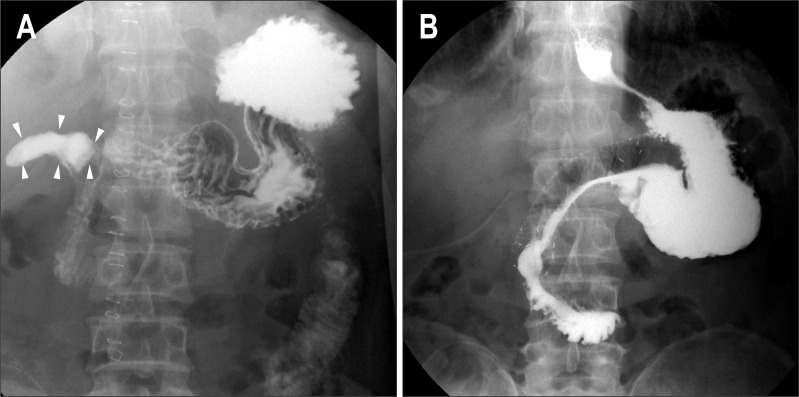
A 59-year-old man visited the emergency department due to severe epigastric pain. A perforated duodenal ulcer was diagnosed, and a primary closure with omentopexy was performed. However, radiocontrast study 6 days later revealed leakage from the primary closure site (Fig. 1A). A partially covered SEMS (HANAROSTENT® duodenum/pylorus (NCN), 2.0×11.0 cm; M. I. Tech Co., Ltd., Pyeongtaek, Korea) was applied to the leakage site through the scope. Five days later, follow-up radiocontrast study did not reveal any leakage (Fig. 1B), and the amount of peritoneal drainage decreased to less than 15 mL/day. Oral feeding was permitted immediately, and the stent was removed 13 days after the insertion. Balloon dilation was required due to duodenal stricture after 2 months. The patient was in good condition during a 2-month follow-up after the dilatation.
Fig. 1.
Radiocontrast study of case 1. (A) Leakage from the primary closure site of the perforated duodenal ulcer (arrowheads). (B) Sealing of the leak 5 days after the insertion of the stent.
2. Case 2
A 55-year-old man underwent total gastrectomy with lymphnode dissection and end-to-end Roux-en-Y esophagojejunostomy for advanced gastric cancer. Pneumoperitoneum was observed 8 days after the surgery, and a second operation revealed extensive leakage from the esophagojejunal anastomosis. Only irrigation and drainage could be performed, because a primary closure was impossible due to the friability of the operated tissue. However, most of radiocontrast still leaked out 10 days after the second surgery.
Endoscopy revealed near-total dehiscence of the anastomosis (Fig. 2A). Completely covered SEMS (Niti-S esophageal covered stent, 1.8×6.0 cm; Taewoong Medical Co., Goyang, Korea) was applied. However, the stent migrated distally 2 days after the insertion. Thus, we placed an anti-migration stent with 1.0-cm-wide uncovered proximal flanges and silk threads that linked its upper edge with the patient's earlobe (CHOOSTENT® esophageal ST (CCN), 2.2×12.0 cm; M. I. Tech Co., Ltd.)5 after removing the migrated stent. Oral feeding was started after 2 weeks, and the drainage from the catheter stopped 4 weeks after the insertion of the stent. A follow-up radiocontrast study revealed no leakage, and the stent was removed 5 weeks after the second stent insertion. Minor bleeding occurred after the removal because the uncovered portion of the proximal flange was embedded in the mucosa. Follow-up endoscopy 10 days after the removal revealed complete healing of the dehiscence and bleeding site (Fig. 2B). The patient died 3 months later from the progression of gastric cancer.
Fig. 2.
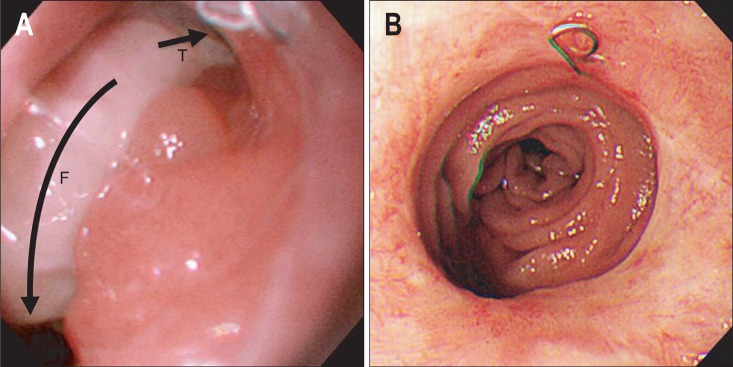
Endoscopic findings of case 2. (A) Near-total dehiscence of the esophagojejunostomy. "T" indicates the true lumen of the jejunum, and "F" indicates the false lumen of the peritoneal space. (B) Complete healing of the dehiscence 5 weeks after the insertion of the stent.
3. Case 3
A 58-year-old man received a thoraco-laparoscopic esophagectomy with a cervical esophagogastrostomy due to submucosa-invasive esophageal cancer. Hypovolemic shock and acute renal failure occurred 2 days after the surgery, and continuous renal-replacement therapy followed. Three weeks after the surgery, a radiocontrast study revealed leakage from the esophagojejunostomy, and endoscopy revealed a 1.2-cm dehiscence of the anastomosis that was located 18 cm from the incisors (Fig. 3A). The radiocontrast that was injected into the working channel of the endoscope was discharged to the cervical drainage. We applied an anti-migration stent with 0.5-cm-wide, uncovered proximal flange and silk threads (CHOOSTENT® esophageal ST, 2.2×12.0 cm; M. I. Tech Co., Ltd.). However, the attempt to perform a fixation to an earlobe failed because the threads were broken during the insertion. A nasojejunal tube (ENBD-7-LIGUORY, 7 Fr, 250 cm; Cook Medical Inc., Winston-Salem, NC, USA) was inserted through the scope for feeding. The stent did not migrate, but a follow-up endoscopy revealed that a fistula was present at the anastomosis site 4 weeks after the insertion of the stent. Repeated endoscopic examination revealed the complete closure of the fistula 7 weeks after the insertion, and the stent was removed (Fig. 3B). The patient remained stable over a 1-year follow-up period.
Fig. 3.
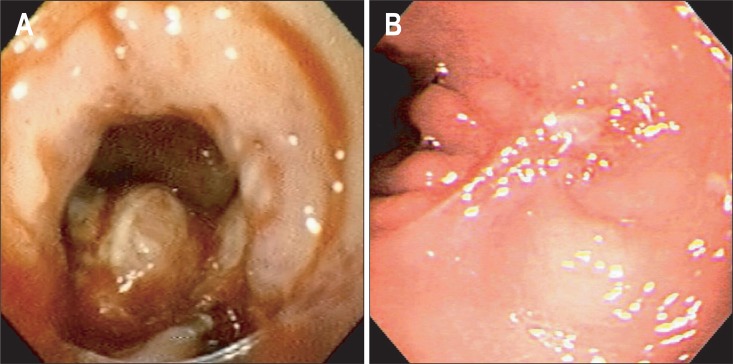
Endoscopic findings of case 3. (A) Dehiscence of the esophagojejunostomy. (B) Complete closure of the opening 7 weeks after the insertion of the stent.
4. Case 4
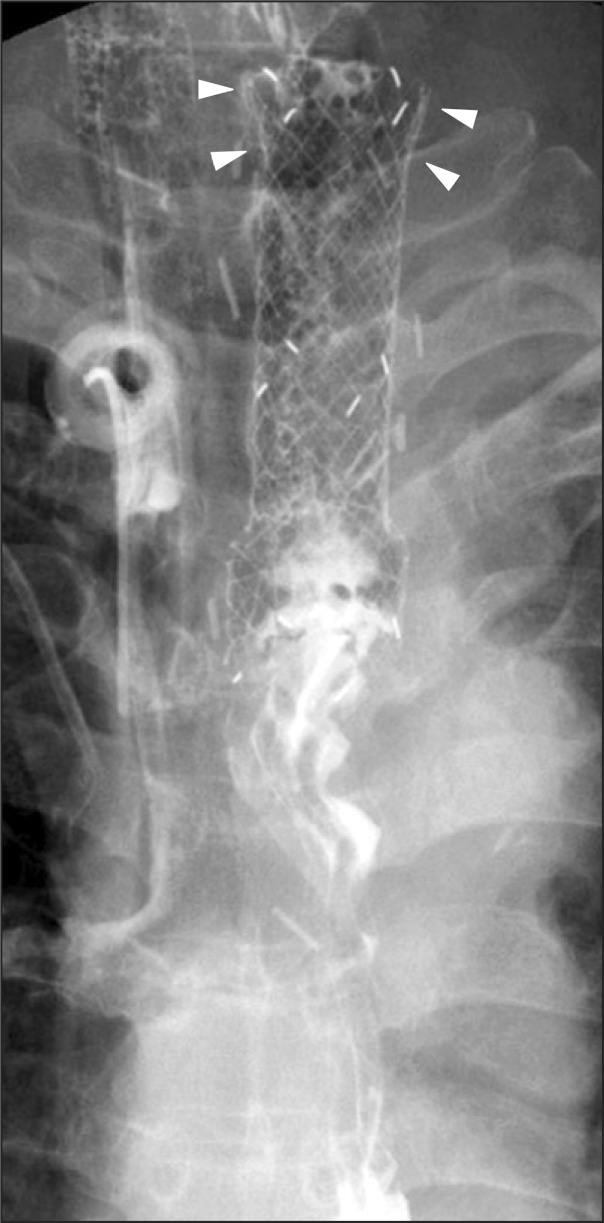
A 55-year-old male underwent laparoscopic transhiatal esophagectomy with a cervical esophagogastrostomy because of submucosa-invasive esophageal cancer. Pneumonia and a wound infection developed, and intensive care for the complications followed. Eight days after the surgery, exudative discharge was observed at the cervical drainage site. Endoscopic examination was performed 4 weeks after the surgery because the patient's vital signs were unstable, and it revealed a 1.4-cm fistula opening with necrotic material at the esophagogastrostomy, 17 cm from the incisors. We applied a fully covered stent with a funnel-shaped proximal flange (Choo Cervical Stent cervical type, 1.8×8.0 cm; M. I. Tech Co., Ltd.) because the fistula was very close to the upper esophageal sphincter (Fig. 4). Silk threads that were attached to the upper edge of the stent were successfully fixed to the patient's earlobe. Oral feeding was started 5 days after the insertion of the stent. Three weeks after the insertion, a radiocontrast study still revealed minor leakage. Thus, the stent was maintained for an additional 2 weeks and then removed. The patient improved and remained in good condition for 1 year.
Fig. 4.
Radiologic image of the fully covered stent with a funnel-shaped proximal flange, cervical type (case 4). Note the funnel-shaped proximal flange (arrowheads).
DISCUSSION
In cases of gastrointestinal leakages after surgery, the goal of treatment is the sufficient drainage of the leakage and stopping further contamination by the gastrointestinal contents. The most effective treatment is controversial and mainly depends on whether the leakage is well-drained and whether the patient develops any septic signs.6
Although the placement of a covered stent cannot immediately achieve the perfect sealing of a surgical leak, it can induce spontaneous healing by decreasing the amount of leakage and can improve the patient's nutritional state by enabling earlier enteral feeding. Thus, if the leakage is well-drained, the insertion of a covered stent can be the treatment of choice. In the four patients described in this case series, perfect sealing was not immediately achieved because drainage persisted for several days to weeks despite the stent insertion. However, the amount of drainage gradually decreased after the insertion, enteral feeding was started earlier, and complete healing was finally achieved.
A stent for a gastrointestinal leak should be equipped with a membrane, because the stent should cover the opening of the leak and could be retrieved after complete sealing. Covered stents that are used for sealing are prone to migrate because there is no significant narrowing in most cases. In one study, the migration rates of the self-expanding plastic stents to treat esophageal leaks were as high as 30%.7 The use of endoclips may prevent migration,8 but additional data are required. Moreover, it is not always feasible to firmly apply endoclips to the bowel walls.
Technique of Shim et al.5 was designed to prevent migration. Threads provide temporary fixation to an earlobe, and the proximal flange is partially uncovered.5 These must have been effective in case 2, because the second stent (which was used in conjunction with Shim's technique) did not migrate, but the first stent, for which a conventional technique was used, did migrate. However, a partially uncovered proximal flange may be an obstacle when retrieving the stent, because the bare portion may be firmly embedded into the mucosa. In fact, minor bleeding occurred during the retrieval of the stent in case 2. Thus, we used an anti-migration stent with a decreased width in the bare portion in case 3 and a fully covered stent with a fixation to an earlobe in case 4.
How long the stent can be safely maintained in patients with benign disorders without causing complications has been debated. Song et al.4 suggested a maximum of 8 weeks on the basis of the low rates of stent-induced strictures. In each of the four cases, the stents were removed within 2 to 7 weeks after the insertion. In cases involving gastrointestinal leaks, there are few data regarding how to verify the complete healing of surgical leaks and to estimate the time of the stent removal. We could decide the optimal removal time mainly by the minimal amount of drainage (<25 mL/day), radiocontrast studies, and the endoscopic findings. In summary, the temporary placement of a covered stent can be the treatment of choice for various gastrointestinal leaks after surgery, particularly when the defect cannot be sealed by conservative care and the leakage is well-drained.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201:253–262. doi: 10.1016/j.jamcollsurg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Tilanus HW, Bossuyt P, Schattenkerk ME, Obertop H. Treatment of oesophageal perforation: a multivariate analysis. Br J Surg. 1991;78:582–585. doi: 10.1002/bjs.1800780519. [DOI] [PubMed] [Google Scholar]
- 3.Doniec JM, Schniewind B, Kahlke V, Kremer B, Grimm H. Therapy of anastomotic leaks by means of covered self-expanding metallic stents after esophagogastrectomy. Endoscopy. 2003;35:652–658. doi: 10.1055/s-2003-41509. [DOI] [PubMed] [Google Scholar]
- 4.Song HY, Jung HY, Park SI, et al. Covered retrievable expandable nitinol stents in patients with benign esophageal strictures: initial experience. Radiology. 2000;217:551–557. doi: 10.1148/radiology.217.2.r00nv03551. [DOI] [PubMed] [Google Scholar]
- 5.Shim CS, Cho YD, Moon JH, et al. Fixation of a modified covered esophageal stent: its clinical usefulness for preventing stent migration. Endoscopy. 2001;33:843–848. doi: 10.1055/s-2001-17326. [DOI] [PubMed] [Google Scholar]
- 6.Kauer WK, Stein HJ, Dittler HJ, Siewert JR. Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc. 2008;22:50–53. doi: 10.1007/s00464-007-9504-5. [DOI] [PubMed] [Google Scholar]
- 7.Gelbmann CM, Ratiu NL, Rath HC, et al. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy. 2004;36:695–699. doi: 10.1055/s-2004-825656. [DOI] [PubMed] [Google Scholar]
- 8.Park JI, Park JJ, Oh JY, et al. A case of endoscopic stenting for anastomotic leakage after total gastrectomy. Korean J Gastrointest Endosc. 2009;39:369–373. [Google Scholar]