Abstract
Background/Aims
The objective of this study was to evaluate a monoclonal antibody-based test to detect Helicobacter pylori-specific antigen in gastric aspirates from humans.
Methods
Sixty-one volunteers were enrolled in the study. All of the subjects underwent a 13C-urea breath test (UBT) before esophagogastroduodenoscopy. Gastric aspirates were analyzed for pH and ammonia and used for polymerase chain reaction (PCR), culture, and monoclonal antibody-based detection of H. pylori. Multiple biopsies of the gastric antrum and body were obtained for a rapid urease test (RUT) and histological evaluation.
Results
Thirty-six subjects were H. pylori-positive and 25 were H. pylori-negative according to the UBT results. Compared with the H. pylori-negative subjects, H. pylori-positive subjects had a higher pH (4.77±1.77 vs 3.49±1.30, p<0.05) and ammonia level (1,130.9±767.4 vs 184.2±126.3, p<0.0001). The sensitivities and specificities of the PCR test, RUT, culture test, and monoclonal antibody-based test were 100% and 72%, 89% and 100%, 47% and 100%, and 78% and 100%, respectively.
Conclusions
The monoclonal antibody-based test for diagnosing H. pylori infection in gastric aspirates has increased sensitivity compared with the culture test and specificity as high as that of the RUT. The test may be useful as an additive test for examining gastric aspirates.
Keywords: Helicobacter pylori, Gastric aspirate, Monoclonal antibody-based test
INTRODUCTION
Helicobacter pylori is a gram-negative spiral-shaped bacterium and is estimated to infect more than half of the world's population, predominantly in developing countries.1,2 H. pylori is the cause of the gastritis-associated gastrointestinal diseases that include gastric ulcer, duodenal ulcer, gastric cancer, and gastric mucosal-associated lymphoid tissue (MALT) lymphoma.2-4 The methods of diagnosing a H. pylori infection are polymerase chain reaction (PCR), H. pylori stool antigen test (HpSA), culture, histology, rapid urease test (RUT) and the urea breath test (UBT).5 A limitation of the UBT is the need for expensive apparatus, especially the gas chromatography isotope ratio mass spectrometer.6 Treatment with proton pump inhibitor (PPI) may jeopardize the result of RUT by changing the environment where H. pylori is present, especially the antrum. H. pylori cannot survive in achlorhydric mucosa and subsequently, the bacterial load decreases. In addition, PPI themselves may have antiurease property.7 Another reason for false-negative RUT is the presence of intestinal metaplasia. Monoclonal antibody-based H. pylori stool antigen test (HpSA) has a high specificity and sensitivity. However, the approach is limited by the impact of bowel movements, whose influence is still not completely clear. While the test is quite specific, it is possible that rare Helicobacter species present in stools (enteropathic Helicobacters) may also be detected.6
Gastric juice reflects the whole stomach. H. pylori is found in gastric juice due to the turnover of gastric mucosa.8 In this study, we evaluated the efficacy of monoclonal antibody-based HpSA to detect H. pylori specific antigen in gastric aspirate.
MATERIALS AND METHODS
Sixty-one subjects were recruited from January to May 2011 at Saint Carollo Hospital. The subjects gave their written consent to the use of esophagogastroduodenoscopy (EGD) and biopsy procedures. The subjects were interviewed, and data collection forms were completed, in which all clinical information was recorded. The subjects were excluded from the study if they had taken antibiotics, PPI or bismuth compounds in the previous 2 weeks, or had undergone treatment for H. pylori. The subjects with renal insufficiency or liver cirrhosis were also excluded.
The subjects were evaluated for H. pylori status by the UBT using film coated 13C-urea tablets. Breath samples were collected at 0 and 20 minutes after administration of a UBT tablet, and δ-13CO2 (UBT value) was measured by infrared spectrometry using a UbiT-IR300 (Otsuka Pharmaceutical, Tokushima, Japan). The cut-off value for the UBT was 2.5% at 20 minutes. When UBT values were <2.5% or ≥2.5%, test results were evaluated as negative and positive, respectively.
The subjects underwent the EGD after the UBT. After the insertion of the endoscope into the stomach, gastric juice was aspirated from fundal pool and discarded. Ten to twenty milliliters of the fundus specimen was collected in a trap through the suction channel after 40 mL distilled water was sprayed in the antrum for rinsing of gastric mucosa. Gastric aspirate pH was measured with a glass electrode pH meter (Perphect LogRmeter model 370; Orion Research, Beverly, MA, USA). Gastric aspirate ammonia concentration was measured as well (Dimension RxL Max; SIEMENS, Munich, Germany).
1. Preparation of DNA and PCR
Gastric aspirate specimens were centrifuged at 3,000 rpm for 15 minutes and each supernatant was discarded. The pellets were washed in sterile phosphate-buffered saline and 200 µL of a suspension of each pellets was added to tubes. The tubes were centrifuged at 13,000 rpm for 15 minutes and the supernatant was poured off. The pellets were dried and incubated with 50 µL of TE buffer (pH 8.0) including RNase (20 µg/mL) at 37℃ for 1 hour. One microliter of HEPY 1/2 primer (Bioneer, Seoul, Korea) directed to the urea and 5 µL of extracted DNA in an AccuPower R PCR PreMix tube (Bioneer, Seoul, Korea) were used for PCR. The amplification consisted of an initial denaturation at 94℃ for 5 minutes, 30 cycles with denaturation at 94℃ for 30 seconds, annealing at 62℃ for 30 seconds, extension at 72℃ for a minute, and a final extension at 72℃ for 5 minutes. After amplification, 10 µL aliquots of PCR products were analyzed by electrophoresis on 2% agarose gels.
2. Culture
Gastric aspirate specimens were centrifuged at 3,000 rpm for 15 minutes and supernatant was discarded. The pellets were washed in sterile phosphate-buffered saline and 200 µL of pellets were plated on Muller-Hinton agar with vancomycin (100 µg/mL), amphotericin B (50 µg/mL), and nalidixic acid (10.7 µg/mL). After inoculation, the dishes were put into a microaerophilic state with a gaseous mixture consisting of 10% carbon dioxide, 6% oxygen, and 84% nitrogen and incubated for 7 days at 37℃. The resulting cultures were identified based on their colonial appearance, gram staining, morphology, and qualitative reactions for urease, catalase, and oxidase activities.
3. RUT and histology
All subjects underwent RUT and histologic examination. Two antral and one corpus biopsy specimens were obtained from all cases. One antral and one corpus biopsy specimens were used for RUT (ASAN Helicobacter Test; Asan Pharmaceutical, Seoul, Korea) and the other antral specimen was used for hematoxylin-eosin and Giemsa staining. Histologic interpretation of gastric biopsies was performed by a qualified pathologist. Active and chronic inflammation was graded according to the Sydney classification system, using zero to four and zero to three semiquantitative scales, respectively.
4. Monoclonal antibody-based test
Gastric aspirate specimens were tested for H. pylori antigen (Asan easy test® H. pylori Ag; Asan Pharmaceutical). This test was developed to detect H. pylori-specific antigen in stools in a monoclonal antibody-based immunochromatographic assay. After dilution of gastric aspirate in 0.2 mol/L phosphate buffer at pH 7.4, gastric aspirate specimens were centrifuged at 3,000 rpm for 15 minutes and each supernatant was discarded. The pellets were washed in sterile phosphate-buffered saline and 200 µL suspension of each pellets was added to the wells of Asan easy test® H. pylori Ag. The test was read after 5 minutes incubation at ambient temperature. Tests were interpreted as negative if there was a line in the control (C) window only, and as positive if there was any evidence of an additional line in the test (T) window.
5. Statistical analysis
The statistical significance of differences in gastric aspirate ammonia levels and pH was determined with independent sample t-test. A p<0.05 indicated statistical significance.
RESULTS
Thirty-six of 61 subjects were H. pylori-positive and 25 subjects were H. pylori-negative according to the UBT results (Table 1). Peptic ulcer disease was present in seven (19%) and none (0%) and gastroesophageal reflux disease was present in four (11%) and four (16%) of the H. pylori-positive and H. pylori-negative subjects, respectively. As expected, peptic ulcer disease was relatively common in H. pylori-positive subjects.
Table 1.
Characteristics of the Subjects According to the Urea Breath Test Results
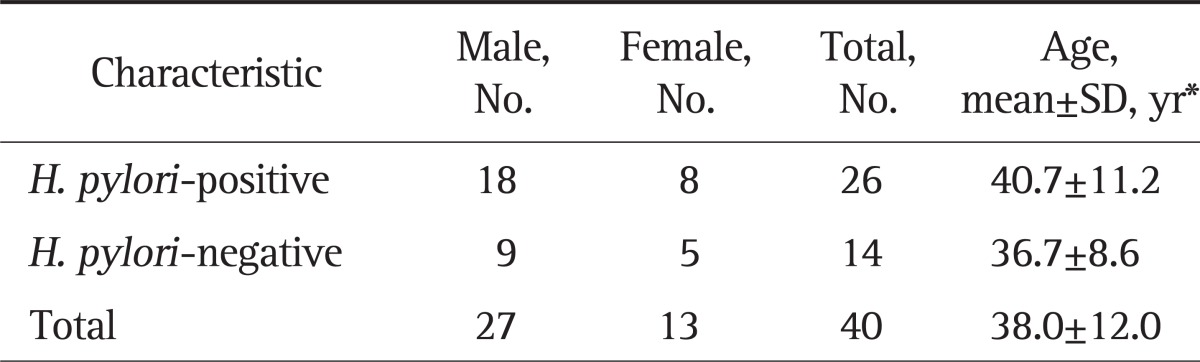
H. pylori, Helicobacter pylori.
*p>0.05.
Gastric aspirate ammonia levels ranged from 167 to 2,666 µmol/L in H. pylori-positive subjects. Gastric aspirate ammonia levels ranged from 80 to 536 µmol/L in H. pylori-negative subjects. Gastric aspirate ammonia levels were significantly higher in H. pylori-positive than H. pylori-negative subjects (Table 2). Gastric aspirate pH ranged from 1.89 to 7.04 in H. pylori-positive subjects. Gastric aspirate pH ranged from 1.99 to 6.28 in H. pylori-negative subjects. Gastric aspirate pH was higher in H. pylori-positive than H. pylori-negative subjects (Table 2). Gastric aspirate pH was correlated with gastric juice ammonia levels (Spearman rank correlation coefficient [rs]=0.419, p<0.01).
Table 2.
Gastric Aspirate pH Values and Ammonia Levels
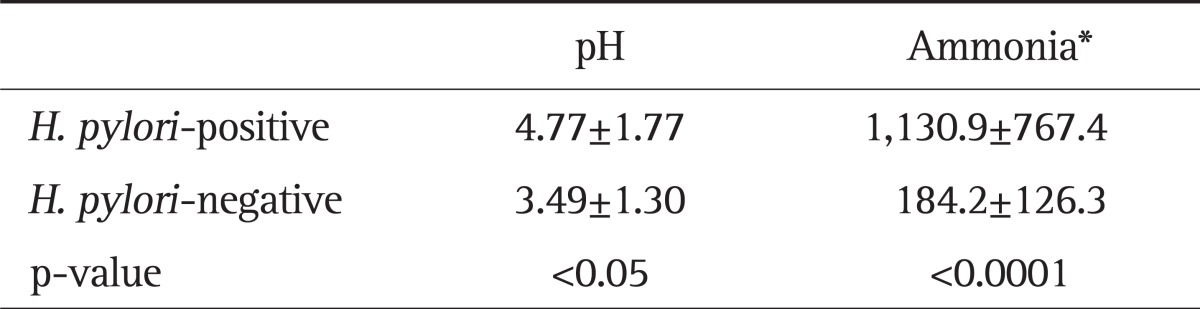
H. pylori, Helicobacter pylori.
*µmol/L, mean±SD.
Histologic interpretation of biopsy in antrum showed difference between H. pylori-positive and H. pylori negative subjects. All of the 36 H. pylori-positive subjects had active inflammation. A majority (89%) of these subjects had grade one to two active inflammation. Active inflammation was present in 16% (4/25) of H. pylori-negative subjects. The number of H. pylori-negative subjects with grade one to two active inflammation was 8%. Chronic inflammation was seen in all H. pylori-positive subjects. Chronic inflammation was seen in 84% (21/25) of H. pylori-negative subjects (Table 3). Active inflammation and chronic inflammation were more common in H. pylori-positive than H. pylori negative subjects (p<0.05).
Table 3.
Active and Chronic Inflammation in the Biopsy Specimens
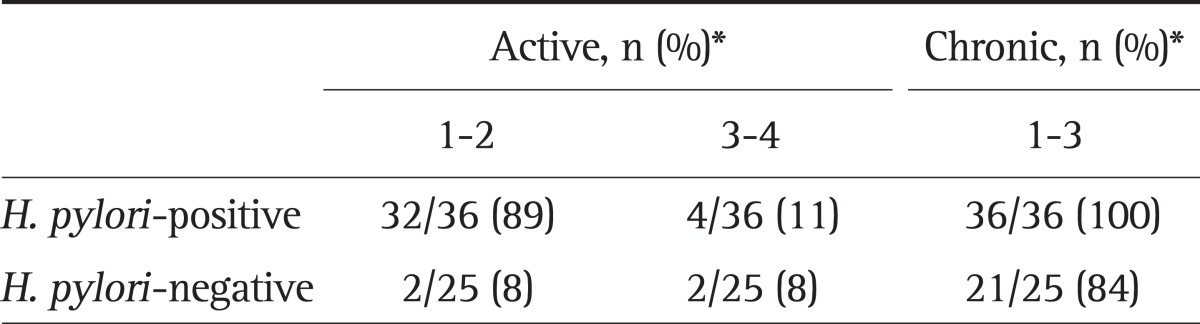
H. pylori, Helicobacter pylori.
*p>0.05.
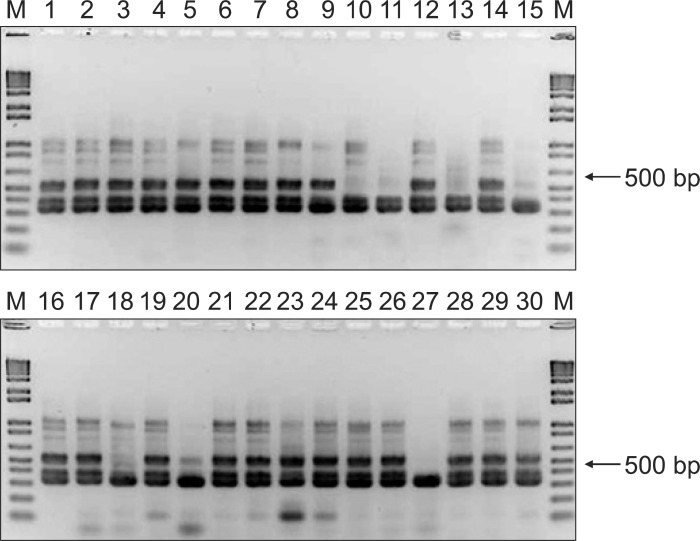
PCR was positive in all 36 H. pylori-positive subjects and RUT was positive in 32. Culture-positive result was obtained in 17 of 36 subjects and the monoclonal antibody-based test was positive in 28 (Fig. 1). The latter test was positive for four subjects who were negative on RUT. This could be due to a patchy distribution of the H. pylori; biopsies obtained from uninfected sites would produce a false-negative RUT result. RUT was positive in eight subjects who tested negative on the monoclonal antibody-based test. If both tests were used to detect H. pylori, the sensitivity and specificity was 100%. The two test approach may be useful to avoid false-negative for the diagnosis of H. pylori during EGD. RUT, culture and monoclonal antibody-based test were negative in all 25 H. pylori-negative subjects. But a false-positive PCR result was obtained in seven H. pylori-negative subjects (Fig. 2). The sensitivity and specificity of PCR, RUT, culture, and monoclonal antibody-based test were 100% and 72%, 89% and 100%, 47% and 100%, 78% and 100%, respectively. The positive predictive value and negative predictive value of PCR, RUT, culture, and monoclonal antibody-based test were 84% and 100%, 100% and 86%, 100% and 57%, and 100% and 76%, respectively (Table 4).
Fig. 1.
The image shows a positive result obtained from an Helicobacter pylori-positive subject (two bands).
Fig. 2.
Polymerase chain reaction in Helicobacter pylori-positive and -negative subjects.
Lane M, 1-kb ladder.
Table 4.
Results of the Diagnostic Tests for Helicobacter pylori Infection Based on the Urea Breath Test Results
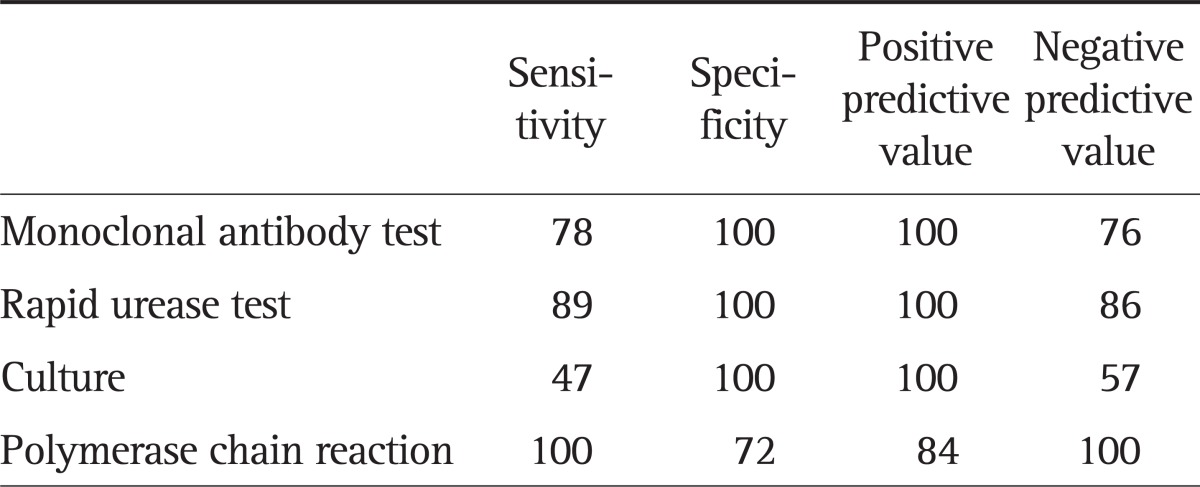
Data are presented in percentage.
DISCUSSION
Indications for H. pylori eradication are all gastric and duodenal ulcer patients who are H. pylori positive whether the ulcer is active or in remission, patients with low grade MALT lymphoma, and patients who have undergone resection of early gastric cancer.9 Therefore, EGD is required for both diagnosis of gastroduodenal disease and documentation of H. pylori infection.
RUT has been widely used because it is simple, cheap, and easy to carry out.10 But, a limitation to RUT is the bacterial load necessary to obtain sufficient sensitivity. A semiquantitative evaluation of the bacteria by histology clearly showed that false-negative urease tests corresponded to the lowest histological scores for H. pylori.11-13 Another limitation is the sampling from only a small part of the stomach (i.e., a few mm2 of a total surface area of 800 cm2), which can lead to possible sampling errors and the subsequent need to do several biopsies.14 Another reason for a false-negative test is the presence of intestinal metaplasia, which also corresponds to an inhospitable environment for H. pylori. Dyes used during chromoendoscopy, such as methylene blue, may result in false-positive RUQ, therefore, the specimens for this test must be taken before spraying the dyes.15 RUT takes 1 to 24 hours to complete. For routine use, most endoscopists read RUT results earlier than recommended, which leads to a marked decrease (20%) in sensitivity.16
Among the noninvasive indirect tests, UBT has the best sensitivity of approximately 95%.17 However, false-negative results may also occur when PPI and antibiotics are used. UBT should be performed to detect H. pylori separately after EGD in patients with gastroduodenal diseases.
HpSA has the advantage of being a direct noninvasive test because it detects H. pylori antigens in an easily-obtained specimen.18,19 The monoclonal antibody-based HpSA has a high specificity and sensitivity. However, the lowest concentration could not be detected after only a 24-hour delay in experimentally spiked specimens.20 Treatment with the mucolytic agent N-acetylcysteine decreases both the specificity and sensitivity.21 While the test is quite specific, it is possible that rare Helicobacter species present in stools may also be detected.
Gastric juice represents a pooled source of events in the entire gastric microenvironment, and it may be valuable for studying H. pylori whose mucosal distribution is patchy and variable. Gastric juice allows the detection of H. pylori by culture, staining, urease test, and PCR because H. pylori is found in gastric juice due to the turnover of gastric mucosa. But the culture sensitivity from gastric juice is much lower.8 In addition, the culture is laborious and requires several days. Gastric juice PCR is highly sensitive and specific.22 However, the possibility of false positives arises when endoscopes or grinding apparati in the lab are not correctly cleaned and PCR is laborious as well.23
The results of this study demonstrated that H. pylori-specific antigen in gastric juice can be detected by a monoclonal antibody-based immunochromatographic assay. The specificity of the monoclonal antibody-based test was higher than PCR and the sensitivity of it was higher than culture as well. Additionally, the monoclonal antibody-based test is cheap, easy to perform, is not time-consuming when compared with culture and PCR. Although the sensitivity of monoclonal antibody-based test in this study was marginally lower than RUT, the specificity was the same. In addition, it did not require biopsy and posed no risk of serious biopsy-related complications such as bleeding.
In conclusion, RUT may not be able to detect the presence of H. pylori due to false negative, whereas gastric juice, being a more global sample, may overcome this limitation because gastric juice reflects the actual microenvironment and the global level of infection in the stomach. Monoclonal antibody-based test from gastric aspirate may be useful for H. pylori detection as an additive test. This is the first study to detect H. pylori by monoclonal antibody-based test in gastric aspirate. Further studies are warranted to clarify the role of monoclonal antibody-based test in gastric aspirate.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Goodwin CS, Mendall MM, Northfield TC. Helicobacter pylori infection. Lancet. 1997;349:265–269. doi: 10.1016/S0140-6736(96)07023-7. [DOI] [PubMed] [Google Scholar]
- 2.Correa P, Piazuelo MB. Evolutionary history of the Helicobacter pylori genome: implications for gastric carcinogenesis. Gut Liver. 2012;6:21–28. doi: 10.5009/gnl.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser MJ. Ecology of Helicobacter pylori in the human stomach. J Clin Invest. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H, Saito Y, Hibi T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue (MALT) lymphoma: updated review of clinical outcomes and the molecular pathogenesis. Gut Liver. 2009;3:81–87. doi: 10.5009/gnl.2009.3.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rautelin H, Lehours P, Mégraud F. Diagnosis of Helicobacter pylori infection. Helicobacter. 2003;8(Suppl 1):13–20. doi: 10.1046/j.1523-5378.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 6.Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuchiya M, Imamura L, Park JB, Kobashi K. Helicobacter pylori urease inhibition by rabeprazole, a proton pump inhibitor. Biol Pharm Bull. 1995;18:1053–1056. doi: 10.1248/bpb.18.1053. [DOI] [PubMed] [Google Scholar]
- 8.Varoli O, Landini MP, LaPlaca M, et al. Presence of Helicobacter pylori in gastric juice. Am J Gastroenterol. 1991;86:249. [PubMed] [Google Scholar]
- 9.Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 10.Prince MI, Osborne JS, Ingoe L, Jones DE, Cobden I, Barton JR. The CLO test in the UK: inappropriate reading and missed results. Eur J Gastroenterol Hepatol. 1999;11:1251–1254. doi: 10.1097/00042737-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Tokunaga Y, Shirahase H, Yamamoto E, et al. Modified rapid urease test for Helicobacter pylori detection in relation to an immunohistochemical stain. J Gastroenterol Hepatol. 2000;15:617–621. doi: 10.1046/j.1440-1746.2000.02213.x. [DOI] [PubMed] [Google Scholar]
- 12.Zaitoun AM. Histology compared with chemical testing for urease for rapid detection of Helicobacter pylori in gastric biopsy specimens. J Clin Pathol. 1993;46:684–685. doi: 10.1136/jcp.46.7.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borromeo M, Lambert JR, Pinkard KJ. Evaluation of "CLO-test" to detect Campylobacter pyloridis in gastric mucosa. J Clin Pathol. 1987;40:462–463. doi: 10.1136/jcp.40.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox AJ. Stomach size and its relation to chronic peptic ulcer. AMA Arch Pathol. 1952;54:407–422. [PubMed] [Google Scholar]
- 15.Hack HM, Parsonnet J, Triadafilopoulos G. Testing negative for Helicobacter pylori after methylene blue spraying of gastric mucosa. Gastrointest Endosc. 1994;40:397–398. doi: 10.1016/s0016-5107(94)70100-8. [DOI] [PubMed] [Google Scholar]
- 16.Laine L, Lewin D, Naritoku W, Estrada R, Cohen H. Prospective comparison of commercially available rapid urease tests for the diagnosis of Helicobacter pylori. Gastrointest Endosc. 1996;44:523–526. doi: 10.1016/s0016-5107(96)70002-0. [DOI] [PubMed] [Google Scholar]
- 17.Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection: a critical review. Aliment Pharmacol Ther. 2004;20:1001–1017. doi: 10.1111/j.1365-2036.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 18.Makristathis A, Barousch W, Pasching E, et al. Two enzyme immunoassays and PCR for detection of Helicobacter pylori in stool specimens from pediatric patients before and after eradication therapy. J Clin Microbiol. 2000;38:3710–3714. doi: 10.1128/jcm.38.10.3710-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agha-Amiri K, Peitz U, Mainz D, Kahl S, Leodolter A, Malfertheiner P. A novel immunoassay based on monoclonal antibodies for the detection of Helicobacter pylori antigens in human stool. Z Gastroenterol. 2001;39:555–560. doi: 10.1055/s-2001-16629. [DOI] [PubMed] [Google Scholar]
- 20.Monteiro L, Gras N, Vidal R, Cabrita J, Megraud F. Detection of Helicobacter pylori DNA in human feces by PCR: DNA stability and removal of inhibitors. J Microbiol Methods. 2001;45:89–94. doi: 10.1016/s0167-7012(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 21.Demirtürk L, Yazgan Y, Tarcin O, et al. Does N-acetyl cystein affect the sensitivity and specificity of Helicobacter pylori stool antigen test? Helicobacter. 2003;8:120–123. doi: 10.1046/j.1523-5378.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 22.Yakoob J, Rasool S, Abbas Z, et al. Gastric juice for the diagnosis of H. pylori infection in patients on proton pump inhibitors. World J Gastroenterol. 2008;14:1539–1543. doi: 10.3748/wjg.14.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roosendaal R, Kuipers EJ, van den Brule AJ, et al. Importance of the fiberoptic endoscope cleaning procedure for detection of Helicobacter pylori in gastric biopsy specimens by PCR. J Clin Microbiol. 1994;32:1123–1126. doi: 10.1128/jcm.32.4.1123-1126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]