Abstract
Background/Aims
The symptoms of inflammatory bowel disease (IBD) fluctuate considerably over time. However, it has not been determined whether these symptoms are affected by the menstrual cycle in female IBD patients. This study analyzed the effects of the menstrual cycle on IBD symptom variation.
Methods
This was a prospective study of 91 study subjects (47 IBD patients and 44 healthy controls) who reported daily symptoms and signs throughout their menstrual cycles.
Results
IBD patients had significantly more frequent gastrointestinal symptoms, such as nausea (30% vs 7%, p=0.006), flatulence (53% vs 22%, p=0.003), and abdominal pain as compared to controls (68% vs 38%, p=0.006). The IBD patients also experienced more frequent systemic premenstrual symptoms than the controls (79% vs 50%, p=0.003). More severe abdominal pain (p=0.002) and lower mean general condition scores (p=0.001) were noted during the menstrual phase as compared to the pre- or post-menstrual phase in both groups. IBD patients experienced more frequent premenstrual gastrointestinal symptoms than controls, but their IBD symptoms did not change significantly during the menstrual cycle.
Conclusions
Knowledge of the cyclic alterations in gastrointestinal and systemic symptoms may be helpful in determining the true exacerbation of disease in female IBD patients.
Keywords: Inflammatory bowel diseases, Menstrual cycle, Women
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic recurrent inflammatory condition of the gut with unknown etiology. Patients with IBD have various presentations such as frequent loose stools with abdominal pain, with or without gross, bloody stools, tenesmus, constipation, fatigue, and weight loss. These symptoms fluctuate considerably over time. Therefore, treatment of IBD consists mainly of interpreting and relieving signs and symptoms.
Many women experience changing gastrointestinal (GI) symptoms caused by hormonal changes related to their menstrual cycle. In clinical practice, many female patients with IBD also report changes in their bowel patterns in relation to their menstrual cycle. Previous studies have revealed that GI symptoms become more prominent in IBD patients during menstrual periods, probably due to changing levels of prostaglandins.1-4 The exact mechanisms underlying the fluctuation in symptoms during the menstrual cycle in relation to ovarian hormones currently remain unclear. Chang et al.5 reported that 40% of female IBD patients experienced fluctuations in symptoms during menstruation. Moreover, IBD patients are more likely to report changes in bowel habits, with the recurrence of diarrhea being more common than constipation.6 It remains to be determined, however, whether symptoms specific to IBD patients such as nocturnal diarrhea, hematochezia, or fecal incontinence also fluctuate during the menstrual cycle.
To date, only a few studies have investigated the relationship between the menstrual cycle and bowel symptom changes in IBD, most of which have limitations such as retrospective study design or lack of IBD-specific symptom evaluation. This is the first prospective study to report on this relationship in patients with IBD. Herein, we evaluated changes and patterns of bowel symptoms during menstrual cycles as reported in a daily diary. This reporting method was employed in order to minimize the possible gap between symptoms reported subjectively by patients and those objectively assessed by doctors.7,8 We also investigated whether IBD-specific symptoms along with nonspecific GI symptoms fluctuate during different phases of the menstrual cycle.
MATERIALS AND METHODS
1. Subjects
A total of 47 IBD patients (27 with ulcerative colitis [UC], 13 with Crohn's disease [CD], and seven with intestinal Beçhet's disease [BD], who were regularly followed at the GI outpatient clinic at Severance Hospital, Yonsei University College of Medicine, and 44 healthy women as controls participated in the study. Documented IBD patients were recruited for the study during outpatient clinic visits and each was asked to complete a daily symptom diary for one menstrual cycle. IBD diagnosis and disease activity were defined by clinical, radiographic, endoscopic, and histological changes.9-12 Only patients who were in clinical remission status or had mild disease severity during the study dates were included. Patients with moderate or severe disease activity were excluded in order to avoid overlooking relatively small menstrual symptom variations. Patients with combined malignant diseases, kidney diseases, cardiovascular diseases, psychiatric illness, or a history of abdominal surgery were also excluded.
2. Data collection
A common questionnaire was given to each subject. The contents addressed smoking, alcohol consumption, pregnancy history, menstrual history, and medications used.
Participants were also asked about GI symptoms and patterns according to the phase of the menstrual cycle. We defined the premenstrual period as '7 days' before the start of menses. The systemic premenstrual syndrome (PMS) and the GI symptoms were reported by the respondents. Systemic PMS was defined as a series of symptoms including headache, anxiety, depression, mastalgia, urinary frequency, and edema before starting menstruation. GI symptoms associated with PMS included diarrhea, constipation, nausea, abdominal pain, flatulence, straining, and tenesmus.13 Finally, a separate questionnaire was given to each IBD patient and control for which they were required to report daily symptoms and signs throughout the menstrual cycle: number of stools (per day), number of loose stools (per day), abdominal pain score (based on a scale of 0 to 3, where: 0, none; 1, mild; 2, moderate; 3, severe), general condition score (based on a scale of 0 to 5, where: 0, perfect; 1, very good; 2, good; 3, average; 4, poor; 5, terrible), nocturnal diarrhea (number, %), hematochezia (number, %), fecal incontinence (number, %), and the need for anti-diarrheal agents (number, %). Since the length of a menstrual cycle varies from month to month and from women to women, we assumed the cycle length to be 28 days, each cycle including a premenstrual (7 days before the start of menses), menstrual, and postmenstrual (7 days after the end of menses) phase. The completed questionnaire was collected when the patients visited the outpatient clinic for a routine follow-up. The questionnaire and study protocol were approved by the Severance Hospital Institutional Review Board. This study protocol was also designed in accordance with the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants or their guardians before they were enrolled in the study.
3. Sample size and statistical analysis
Sample sizes of 47 in the disease group and 44 in the control group achieved a power of 87% for detecting a difference in PMS proportion of 0.3. The PMS proportion was assumed to be 0.8 in the disease group and 0.5 in the control group. A two-sided independent t-test with pooled variance was used, and the significance level of the test was set at 0.05. Data are expressed as means±standard deviations (SDs) or frequencies and percentages. The two-sided independent t-test and chi-square test were used to test for differences in the mean values of continuous measures and frequency rates between the two groups, respectively. We used generalized estimating equations (GEE) to analyze day-to-day menstrual symptom data between the two groups. This approach allowed us to consider individual correlations in the data by way of a working covariance matrix. Daily data, such as the number of stools and the number of loose stools, were assumed to follow the Poisson distribution. Because the distribution of the daily scaled data including abdominal pain, general condition, and abdominal tenderness were positively skewed, these variables were log transformed in the GEE analysis. However, they are expressed as nontransformed scales with mean±SDs for the sake of clarity. The GEE model included main effect terms for group and menstrual phases and an interaction term between the groups and menstrual phase. The interaction was tested in order to detect whether there was a differential group effect following the menstrual phase. Z-score statistics based on GEE empirical standard errors were used to test the main effect and interaction terms. All tests were judged to be significant at a 5% significance level.
RESULTS
1. Baseline characteristics of the IBD patient and control groups
Table 1 shows the baseline characteristics of the disease and control groups. There were 47 IBD patients and 44 controls. The mean age was 35±8 years for the IBD group and 33±3 years for the control group. There were no significant differences in height, weight, or BMI between the two groups. There were more parous women (36% vs 25%) and more current smokers (4% vs 0%) in the disease group. The mean length of the menstrual cycle (32.17±3.53 days vs 32.20±6.20 days) and the mean menstrual period duration (5.22±1.18 days vs 5.22±1.20 days) did not differ between the two groups. Among the patients, 62% were on 5-aminosalicylic acids or sulfasalazine, 30% were on azathioprine/6-mercaptopurine, 8% were on systemic steroids, and 4% were on infliximab therapy. Thirty-eight patients (80%) were in clinical remission; the rest showed mild disease severity at the time of study. Premenstrual symptoms were significantly more frequent in the IBD patients than in the controls (79% vs 50%, p=0.003), as shown in Table 2.
Table 1.
Baseline Characteristics of Women with Inflammatory Bowel Disease and Controls
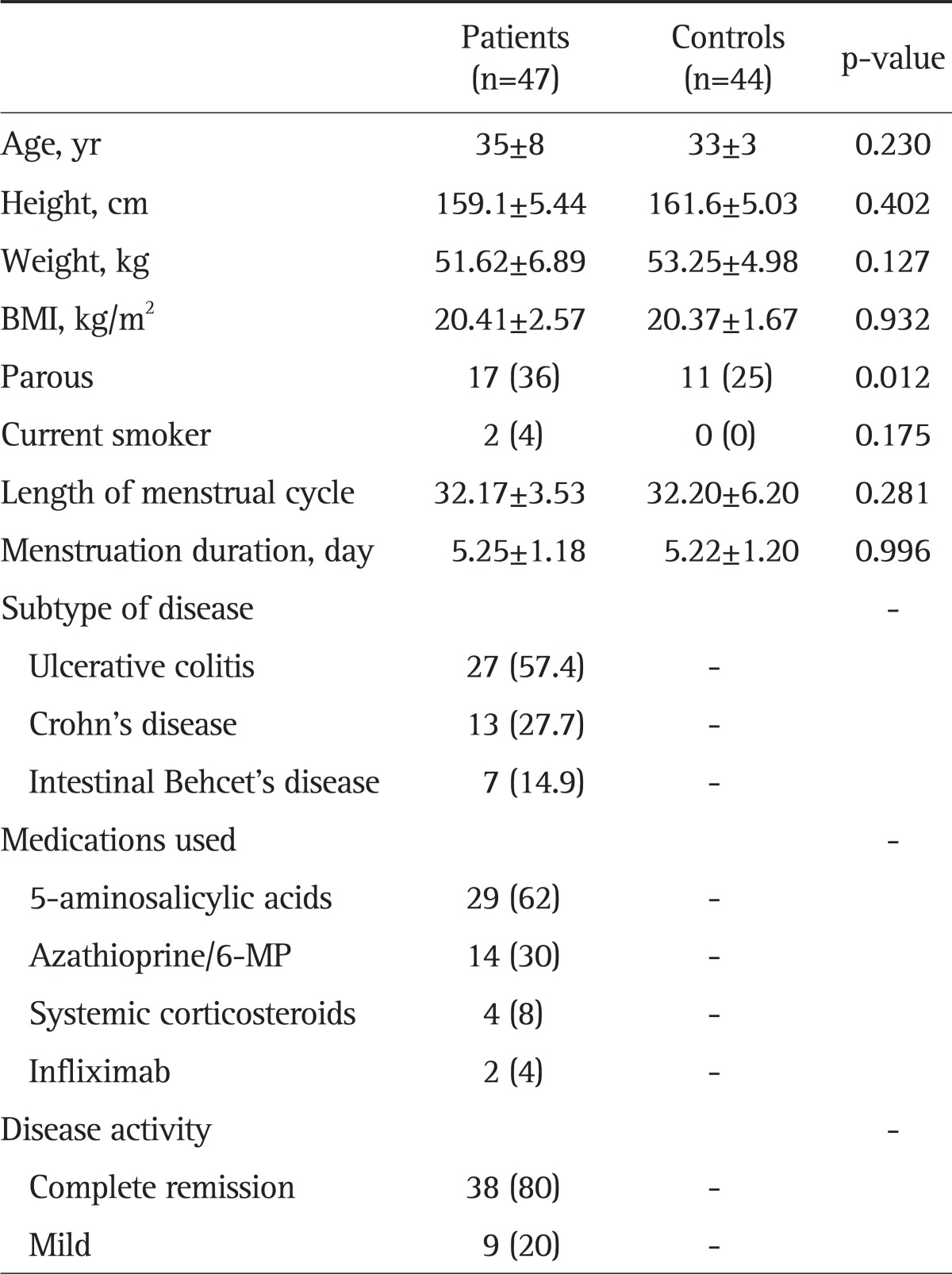
Data are presented as mean±SD or number (%).
Table 2.
Comparison of Symptoms between Women with Inflammatory Bowel Disease and Controls
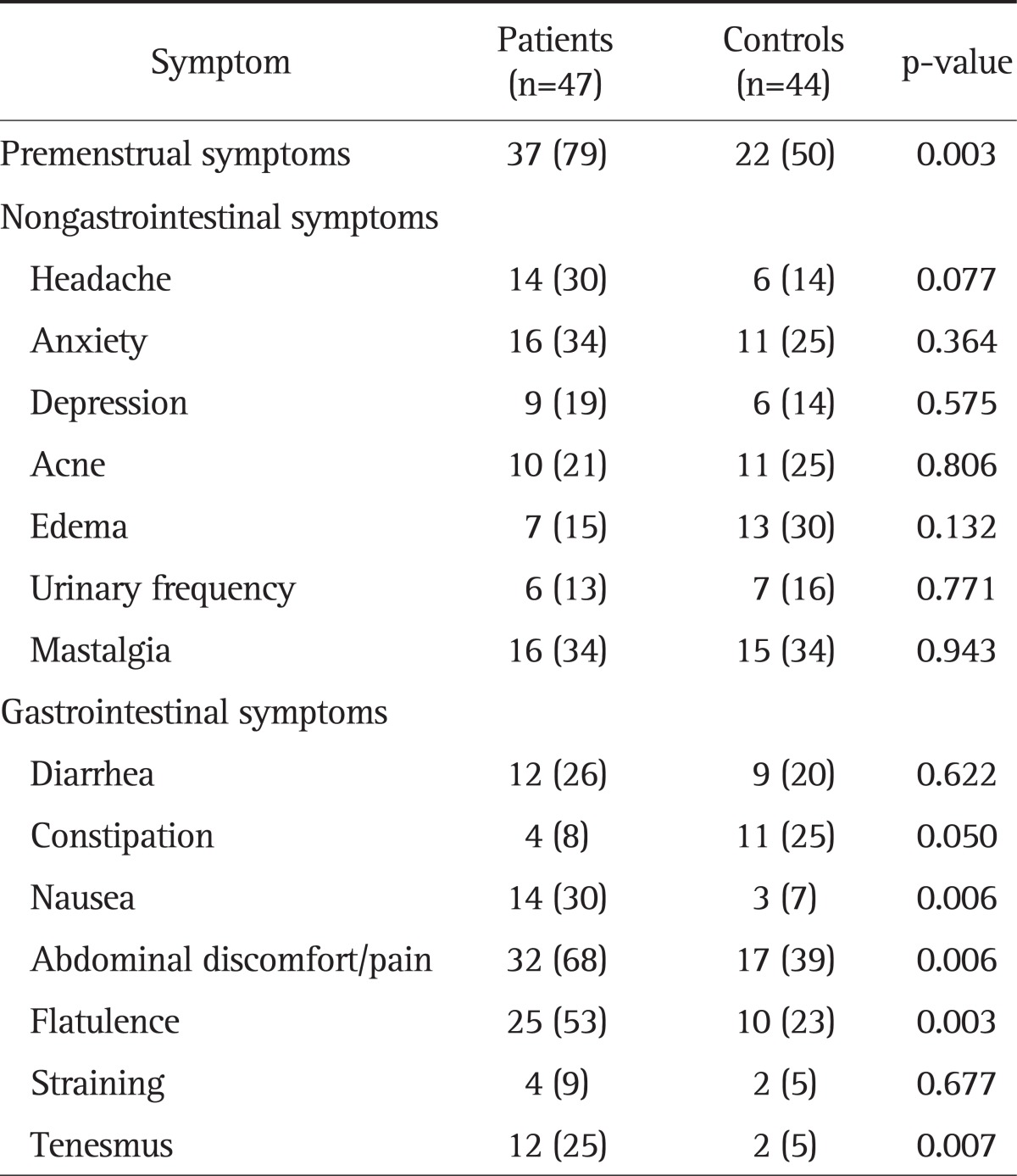
Data are presented as number (%).
We compared the subjective symptoms reported by each subject during menstruation. This questionnaire was not necessarily completed during the time of menses. For GI symptoms, nausea (30% vs 7%, p=0.006), abdominal discomfort/pain (68% vs 38%, p=0.006), flatulence (53% vs 22%, p=0.003), and tenesmus (25% vs 4%, p=0.007) were reported at a significantly higher frequency in the patient group. Diarrhea, constipation and straining were not significantly different between the two groups. The systemic premenstrual symptoms that were evaluated included headache, anxiety, depression, acne, edema, urinary frequency, and mastalgia. There were no significant differences in these symptoms as a whole between the two groups. While the IBD group reported more headaches (30% vs 14%), anxiety (34% vs 25%), depression (19% vs 14%) and mastalgia (34% vs. 34%), the control group reported more acne (21% vs. 25%), edema (15% vs. 29%), and urinary frequency (13% vs. 16%).
2. Relationships between menstrual cycle and IBD symptoms
We evaluated the relationships between menstrual cycle and GI symptoms. Table 3 compares the symptoms among the three phases of the menstrual cycle: premenstrual, menstrual, and postmenstrual. The patient group experienced a significantly higher number of stools (p<0.001), higher number of loose stools (p<0.001), and more severe mean abdominal pain (p=0.001) than the controls in all phases. We then compared the fluctuation in symptoms according to menstrual phase. Both patients and controls reported more symptoms during the menstrual phase than the premenstrual or postmenstrual phase.
Table 3.
Mean Gastrointestinal Symptom Severity during Phases of the Menstrual Cycle in Inflammatory Bowel Disease Patients and Healthy Controls
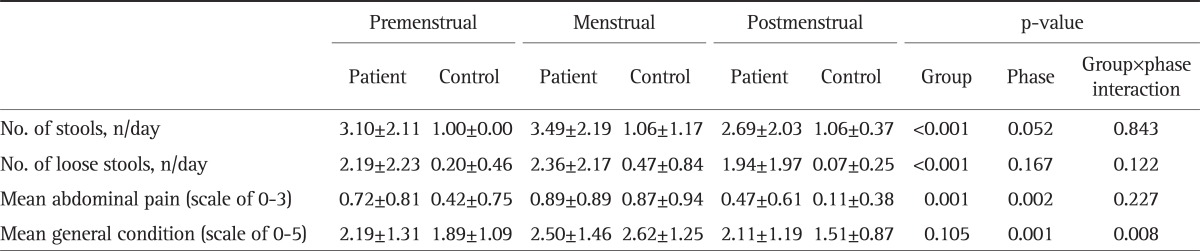
Data are presented as mean±SD.
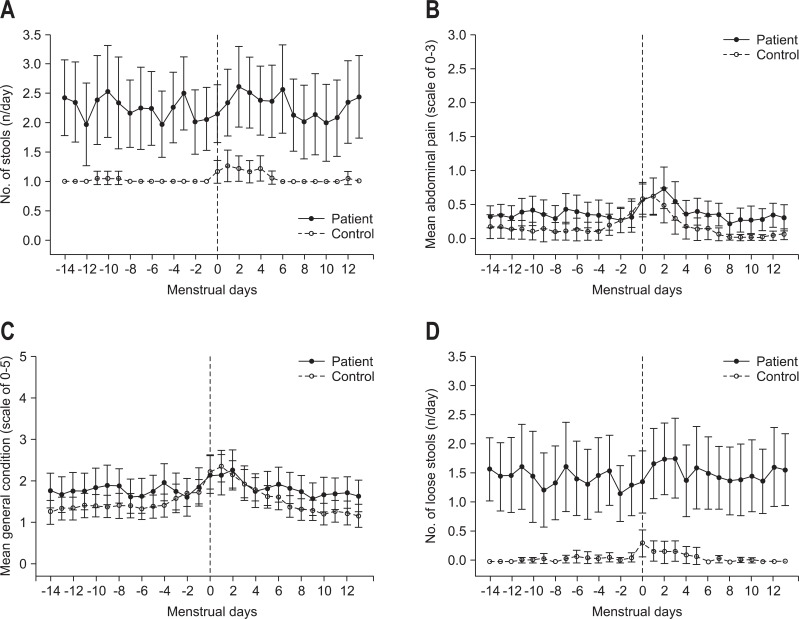
Both patients and controls reported significantly more severe abdominal pain (p=0.002) and poorer general condition (p=0.001) scores during the menstrual phase than during the pre- or postmenstrual phases. However, the number of stools and loose stools did not change significantly during the different phases of menstruation. Fig. 1 shows the comparison of daily trends in regards to GI symptoms (number of stools, number of loose stools, mean abdominal pain score, and mean general condition score).
Fig. 1.
Gastrointestinal symptom scales throughout the menstrual cycle for women with inflammatory bowel disease and women in the control group. (A) Number of stools. (B) Mean abdominal pain. (C) Mean general condition. (D) Number of loose stools.
The IBD-specific symptoms such as nocturnal diarrhea, hematochezia, fecal incontinence, and the need for anti-diarrheal agents were mainly limited to the patient group. We therefore evaluated whether these IBD symptoms were aggravated during the menstrual phase as shown in Table 4. Nocturnal diarrhea and hematochezia were most commonly reported during the menstrual phase, but fecal incontinence and the need for anti-diarrheal agents were more common during the premenstrual phase. However, there was no significant difference in these symptoms between the three phases, and they did not worsen at the time of menses.
Table 4.
Mean Specific Inflammatory Bowel Disease (IBD)-Related Symptom Frequency of IBD Patients According to Phases of the Menstrual Cycle
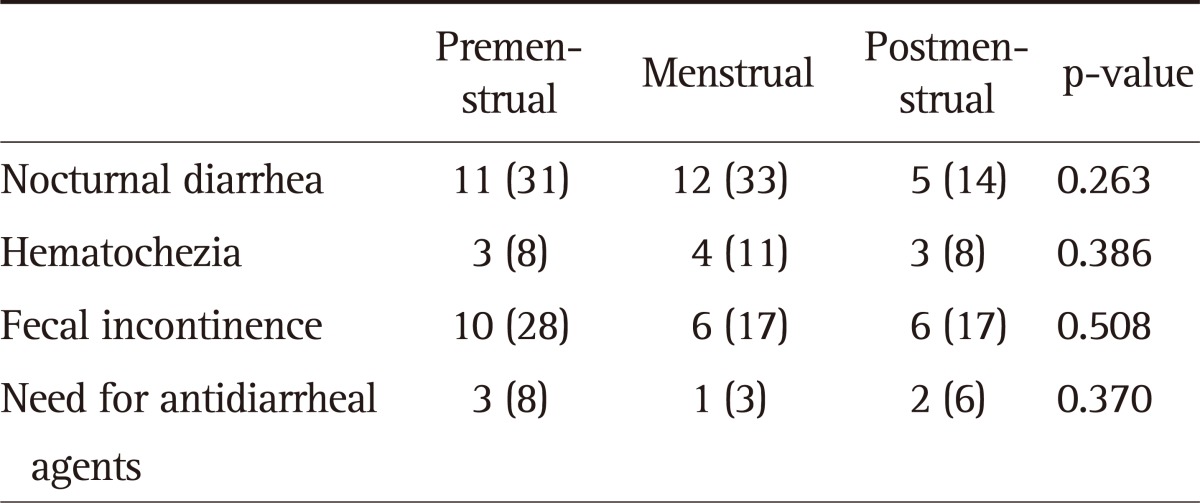
Data are presented as number (%).
DISCUSSION
We investigated whether IBD-specific symptoms along with nonspecific GI symptoms fluctuated during different phases of the menstrual cycle in IBD patients compared to healthy controls. The strengths of our study include its prospective design in which subjects were asked to report their symptoms and bowel habits daily. This design minimizes possible recall bias and reduces over- or underestimation of symptoms. Moreover, the statistical analysis used in this study allowed us to precisely compare trends in individual results both phase-wise and group-wise.
In this study, we found that the IBD patients experienced more frequent premenstrual GI symptoms than the controls, but systemic premenstrual symptoms did not differ significantly between the two groups. We also found that the bowel habits of both groups changed during the different phases of the menstrual cycle, although significant differences were not found. More severe mean abdominal pain and aggravated mean general condition scores were observed in both groups during the menstrual phase, compared to other phases.
Finally, the patient group experienced a significantly higher number of stools, a higher number of loose stools, and more severe mean abdominal pain throughout the menstrual cycle. However, specific IBD-related symptoms such as nocturnal diarrhea, hematochezia, fecal incontinence, and the need for antidiarrheal agents did not change according to menstrual phase in the IBD patients. The menstrual cycle is often overlooked, although it can be a confounding variable in disease activity in women with IBD. As the severity of IBD is evaluated using symptom-based criteria, physicians should be aware that worsening symptoms may not be due to disease exacerbation, but may rather be due to menstruation. This implies that menstrual cycles should be considered by physicians in clinical settings.
Premenstrual syndrome (PMS) is characterized as the cyclic recurrence of symptoms during the luteal phase of the menstrual cycle. The PMS diagnostic criteria were developed by the University of California at San Diego and the National Institute of Mental Health, and emphasize the periodicity and severity of symptoms. The etiology of PMS is multifactorial, and it is related to ovulating hormones secreted during the menstrual cycle. Previous studies have reported on the symptomatology of IBD or irritable bowel syndrome (IBS) in association with the menstrual cycle, although the mechanisms underlying the effect of these hormones on symptoms have not yet been elucidated. It was previously reported that 67% of healthy women experienced irritability, 35% depression, 19% diarrhea, and 13% constipation during the premenstrual phase.13-15 Heitkemper et al.1 evaluated symptoms across the menstrual cycle in women with IBS. The ovulatory cycle was confirmed by measuring luteinizing hormone surge and progesterone levels in each subject. They compared the frequency of bowel symptoms such as diarrhea, constipation, and alternations in symptoms across the menstrual cycle and concluded that the IBS patients reported more GI and somatic symptoms than the controls.1 Unfortunately, the phenomenon of symptom fluctuation during the menstrual cycle in relation to ovarian hormones was not clearly explained. A study by Kane et al.2 found that PMS and menstrual symptoms are reported more frequently by patients with IBS and IBD than by controls, and IBS and IBD patients are more likely to report a "cyclical" pattern to their bowel habits.2 However, their study was a retrospective study based on telephone interviews, so the subjects may have over reported their symptoms. Parlak et al.16 conducted a similar study assessing the pattern of GI and psychosomatic symptoms during the menstrual cycle in women with IBD. They demonstrated that there is a cyclic pattern in defecation during the menstrual phase.16 Their results are in accordance with those of our study in that the occurrence of GI symptoms were higher in IBD patients than in controls. However, in study of Parlak et al.,16 there was no mention of specific IBD symptoms being manifested more frequently in patients. Thus, our study is the first to use prospective diary data to assess the effects of the menstrual cycle on IBD symptoms.
The mechanism underlying changes in GI symptoms during the menstrual cycle has been linked to gonadal hormones such as prostaglandins. Dysmenorrhea is linked to an excess or imbalance of prostaglandins and arachidonic acid metabolites. Production of the prostaglandin subtype PGE2 in the intestines leads to increased contraction of the colonic smooth muscle. The higher frequency of defecation during the menstrual phase may be caused by an excessive prostaglandin release from the uterine cavity.17
Gonadal hormones also influence pain sensitivity via a variety of mediators including serotonin (5-hydroxytryptamine, 5-HT), which is ubiquitous in the GI tract.18 Borman et al.19 proposed that serotonin receptors contribute to the putative 5-HT-induced colonic smooth muscle hypersensitivity associated with IBS. Estrogen is associated with 5-HT receptors via estrogenic pathways, which influence tryptophan hydroxylase, the synthetic enzyme for 5-HT. Estrogen is also known to enhance serotonergic postsynaptic responsiveness in the central nervous system. Therefore, estrogen-serotonin interactions lead to increased vulnerability to fluctuating bowel symptoms and mood changes during the menstrual cycle. GI symptoms such as abdominal pain appear before menstruation when there is a rapid decline in estrogen production. This is because estrogen alleviates cramps by reducing the manifestation of serotonin receptors.20,21 A recent study revealed that estrogen and progesterone could relieve colon contractility in Sprague-Dawley rats.22 This phenomenon was evident in our results, which showed that both patients and controls experienced more severe abdominal pain during the premenstrual and menstrual phases than in the postmenstrual phase. However, there is no evidence that ovarian hormones influence the disease activity of IBD, as IBD specific symptoms did not fluctuate significantly during the menstrual cycle in our study. Hormonal analysis with respect to the menstrual cycle needs further research and investigation.
One limitation of our study is that the symptoms we observed were mainly related to UC, but not to CD and BD. Furthermore, the results were obtained from the subjective symptom evaluations of each patient, although menstrual periods are affected by multiple factors such as stress, exercise, food intake, and medication. The concomitant use of medications or immunosuppressive agents taken by patients with IBD could overshadow the symptoms and thus act as a confounding factor. The issue of intra-individual variability is another concern when assessing the consistency of symptoms. It may be necessary to use long-term follow-up questionnaires to examine the manifestations of repeating GI and systemic symptoms on an individual basis.
In conclusion, our study highlights the fact that IBD patients are more likely to report PMS and GI symptoms than healthy women, without exacerbation of disease specific symptoms. Physicians should consider a gender-based approach in clinical practice, and acknowledge that gender-specific issues such as menstruation can influence symptoms and signs throughout the menstrual cycle. Lastly, knowledge of cyclic symptom alterations may be helpful in determining the true exacerbation of IBD.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Heitkemper MM, Chang L. Do fluctuations in ovarian hormones affect gastrointestinal symptoms in women with irritable bowel syndrome? Gend Med. 2009;6(Suppl 2):152–167. doi: 10.1016/j.genm.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93:1867–1872. doi: 10.1111/j.1572-0241.1998.540_i.x. [DOI] [PubMed] [Google Scholar]
- 3.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okoro NI, Kane SV. Gender-related issues in the female inflammatory bowel disease patient. Expert Rev Gastroenterol Hepatol. 2009;3:145–154. doi: 10.1586/egh.09.1. [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 6.Knight LC, Parkman HP, Brown KL, et al. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol. 1997;92:968–975. [PubMed] [Google Scholar]
- 7.Bolge SC, Waters H, Piech CT. Self-reported frequency and severity of disease flares, disease perception, and flare treatments in patients with ulcerative colitis: results of a national internet-based survey. Clin Ther. 2010;32:238–245. doi: 10.1016/j.clinthera.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Ebert EC. Gastrointestinal manifestations of Behcet's disease. Dig Dis Sci. 2009;54:201–207. doi: 10.1007/s10620-008-0337-4. [DOI] [PubMed] [Google Scholar]
- 9.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 10.Cheon JH, Han DS, Park JY, et al. Development, validation, and responsiveness of a novel disease activity index for intestinal Behcet's disease. Inflamm Bowel Dis. 2011;17:605–613. doi: 10.1002/ibd.21313. [DOI] [PubMed] [Google Scholar]
- 11.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 12.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timonen S, Procopé BJ. The premenstrual syndrome: frequency and association of symptoms. Ann Chir Gynaecol Fenn. 1973;62:108–116. [PubMed] [Google Scholar]
- 14.Dickerson LM, Mazyck PJ, Hunter MH. Premenstrual syndrome. Am Fam Physician. 2003;67:1743–1752. [PubMed] [Google Scholar]
- 15.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 15, April 2000. Premenstrual syndrome. Obstet Gynecol. 2000;95:1–9. [Google Scholar]
- 16.Parlak E, Dağli U, Alkim C, et al. Pattern of gastrointestinal and psychosomatic symptoms across the menstrual cycle in women with inflammatory bowel disease. Turk J Gastroenterol. 2003;14:250–256. [PubMed] [Google Scholar]
- 17.Sales KJ, Milne SA, Williams AR, Anderson RA, Jabbour HN. Expression, localization, and signaling of prostaglandin F2 alpha receptor in human endometrial adenocarcinoma: regulation of proliferation by activation of the epidermal growth factor receptor and mitogen-activated protein kinase signaling pathways. J Clin Endocrinol Metab. 2004;89:986–993. doi: 10.1210/jc.2003-031434. [DOI] [PubMed] [Google Scholar]
- 18.De Ponti F. Pharmacology of serotonin: what a clinician should know. Gut. 2004;53:1520–1535. doi: 10.1136/gut.2003.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borman RA, Tilford NS, Harmer DW, et al. 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol. 2002;135:1144–1151. doi: 10.1038/sj.bjp.0704571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mark CJ, Tatchum-Talom R, Martin DS, Eyster KM. Effects of estrogens and selective estrogen receptor modulators on vascular reactivity in the perfused mesenteric vascular bed. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1969–R1975. doi: 10.1152/ajpregu.00260.2007. [DOI] [PubMed] [Google Scholar]
- 21.Houghton LA, Brown H, Atkinson W, et al. 5-hydroxytryptamine signalling in irritable bowel syndrome with diarrhoea: effects of gender and menstrual status. Aliment Pharmacol Ther. 2009;30:919–929. doi: 10.1111/j.1365-2036.2009.04121.x. [DOI] [PubMed] [Google Scholar]
- 22.Li TJ, Yu BP, Dong WG, Luo HS, Xu L, Li MQ. Ovarian hormone modulates 5-hydroxytryptamine 3 receptors mRNA expression in rat colon with restraint stress-induced bowel dysfunction. World J Gastroenterol. 2004;10:2723–2726. doi: 10.3748/wjg.v10.i18.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]