Abstract
Background/Aims
This study sought to determine the natural course of Crohn's disease (CD) and identify predictors that could indicate responsiveness to corticosteroid (CS) therapy.
Methods
Patients with active CD who were treated with oral CS at a single institution between August 1994 and February 2008 were retrospectively reviewed. The clinical outcomes at 1 month, 4 months, and 1 year after the treatment, as well as clinical and biochemical parameters at the time of CS initiation, were evaluated.
Results
A total of 96 patients with CD were enrolled. In this study, 37 patients achieved complete remission (38.5%), 49 achieved partial remission (51.0%), and 10 (10.4%) showed no response at 1 month after the initiation of CS treatment. At 4 months and 1 year after treatment, 66 (69.5%) and 47 (56.6%) patients showed prolonged response, 22 (23.2%) and 20 (24.1%) showed steroid dependency, and 7 (7.4%) and 16 (19.3%) showed refractoriness, respectively. Nonstricturing and nonpenetrating behaviors and a lower CD activity index demonstrated clinical significance for mid-term or mid- and long-term steroid responses, respectively.
Conclusions
The short-term response rate to initial oral CS therapy in CD was considerably high, but responsiveness thereafter showed a tendency to decrease with time. Clinical parameters reflecting mild inflammation were associated with responsiveness after CS treatment.
Keywords: Inflammatory bowel diseases, Crohn disease, Adrenal cortex hormones, Steroids
INTRODUCTION
Crohn's disease (CD) is a lifelong illness characterized by recurrent chronic inflammation of the gastrointestinal tract without a known exact etiology. Lesions of CD commonly involve the terminal ileum and proximal colon, but the entire gastrointestinal tract can be affected.1 The goals of treatment for CD are to induce complete remission (CR) of the disease, maintain the remission, and minimize the risk of complications and surgery.
The use of corticosteroids (CS) has been a well-established treatment for active inflammatory bowel disease (IBD) since Truelove and Witts2 reported that oral cortisone could effectively induce remission of ulcerative colitis (UC) in 1955. After the National Cooperative Crohn's Disease Study and the European Cooperative Crohn's Disease Study showed that CS could also effectively induce remission in patients with CD, CS have been widely used to treat the disease for the past 50 years.3,4
While many investigators have reported the usefulness of CS in treating CD,5-8 systemically available CS are associated with various side effects.9 It is still difficult to decide when to start or discontinue CS because of drug dependence that may develop during long-term use of CS and repeated surgery risk when there is unresponsiveness to the treatment with CS.10 Furthermore, CS are known to be ineffective in maintaining remission of CD.11
So far, there have been few papers that have described the natural course of CD in patients after administration of first course CS. Furthermore, most of the published researches were conducted in Western countries. However, the natural course of CD appears to differ somewhat in Asians compared to Western populations. Asian patients tend to experience a milder clinical course, lower relapse rate, and lower surgery rate.12-16 The aim of this study was to determine the natural course of CD in patients who were first treated with oral CS and to find predictive factors that can indicate their responsiveness to such steroid therapy.
MATERIALS AND METHODS
1. Patients
All active CD patients who were diagnosed and treated with oral CS between August 1994 and February 2008 at Severance Hospital in Seoul, Korea were enrolled, and their medical records were retrospectively reviewed. The data for all 239 patients with CD treated with CS were retrieved from our internal database system and from scanned electronic medical records. Finally, 96 patients who had received a first course of oral CS therapy were enrolled. Patients were excluded from the study according to the following criteria: 1) patients who had started CS therapy from other clinics (34 patients); 2) those whose precise starting point of CS therapy could not be determined (64 patients); 3) those who had started on CS intravenously (23 patients); 4) those who had no available medical records of the treatment progress (19 patients); and 5) those who had stopped CS treatment within the first month due to any reason (three patients).
2. Definitions of clinical outcomes
The clinical outcomes of the patients were assessed at 1 month, 4 months, and 1 year after the initiation of CS therapy. Short-term responses (1 month after therapy) were classified into three groups: CR, partial remission (PR), and no response (NR).10,17-19 While CR was defined as the complete resolution of active symptoms (absence of blood, pus or mucus in stool, abdominal pain, fever, and decreased stool frequency [≤2/day]), PR was defined as the clinical improvement of symptoms, specifically, the absence of systemic symptoms such as fever or weight loss and decreased frequencies of bowel movements (≤4/day), abdominal pain, and blood, pus, or mucus in stool.10,18 NR was defined as the absence of clinical improvements. Similarly, mid-term response (at 4 months) and long-term response (at 1 year) were categorized into prolonged response, steroid dependency, and steroid refractoriness. Prolonged response at 4 months was defined as no evidence of clinical recurrence of CD during a scheduled tapering or after discontinuation of CS. Steroid dependency at 4 months was indicated when the patient was unable to taper the dosage for more than 2 weeks or if it was necessary to increase the dosage. If the patient showed no clinical improvement at 4 months after CS therapy, he or she was classified into the steroid refractory group. Prolonged response at 1 year was defined as maintenance of CR or PR without CS, while steroid dependency after 1 year indicated the maintenance of CR or PR using CS continuously or occasionally or the inability to discontinue the drug. Finally, steroid refractoriness at 1 year was defined either as the absence of clinical improvement despite dose increment or the necessity to resort to other treatment modalities such as surgery or infliximab.
To analyze predictive factors of clinical outcomes after CS treatment, patients exhibiting CR or PR at 1 month after treatment and prolonged response after 4 months and 1 year were categorized into the steroid responder group. Patients exhibiting NR at 1 month after CS use, steroid dependency, and steroid refractoriness after 4 months and 1 year were categorized into the steroid nonresponder group.10,20
3. Clinical and biochemical parameters
The clinical parameters of the patients were assessed by retrospectively reviewing their medical records. The collected data included age, sex, duration of the disease, location of CD lesions, and behavior of the illness according to the Vienna classification.21 Medication histories such as initial and total dose of oral CS, length of CS use, side effects of CS, and concomitant medications (i.e., antibiotics, immunomodulators, or 5-amino salicylic acids [5-ASA]) were also obtained for the analysis. Biochemical parameters at the time of initial treatment including C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), hemoglobin level, and albumin level were assessed. At each visit, every patient completed the center's standardized CD questionnaire, which was comprised of questions used to gauge the CD activity index (CDAI), and we re-calculated each patient's CDAI at the time of initial CS treatment using the questionnaire.
This study was approved by the Severance Hospital Institutional Review Board, and the Strengthening the Reporting of Observational Studies in Epidemiology Statement was used as a reference point for key items related to study design and reporting.
4. Statistics and analysis
Continuous variables were compared using Mann-Whitney U test and are presented in this paper as median (range). Categorical variables were compared by Fisher's exact test. Logistic regression analysis was performed to identify predictive variables for clinical outcomes. The p-values less than 0.05 were considered statistically significant. All the statistical analyses were performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Clinical characteristics of patients
A total of 96 patients were enrolled from 239 patients with CS-treated CD. There were 75 males and 21 females whose median age at diagnosis was 23 years (range, 12 to 64 years), and the median disease duration was 4 months (range, 0 to 156 months). The most common location of CD was the ileocolon (63.5%) according to the Vienna classification, while 18 patients (18.8%) had terminal ileum involvement, 10 (10.4%) had colon lesions, and seven had upper gastrointestinal tract involvement (7.3%). Nonstricturing, nonpenetrating type (55.2%) was the most common CD type; the rest of the cases were either penetrating type (31.3%) or stricturing type (13.5%). The median steroid dose was 0.58 mg/kg (range, 0.31 to 0.95 mg/kg), and the median length of initial steroid use was 10 weeks (range, 4 to 40 weeks). In this study, 95 cases (99.0%) were treated with 5-ASA as a concomitant medication, 21 cases also used antibiotics (21.9%), and three cases used immunomodulators (3.1%). During the treatment period, five cases showed complications from CS, and three of them discontinued the medication due to those side effects. The median CDAI before starting CS treatment was 347.5 (range, 161.4 to 559.8) (Table 1).
Table 1.
Baseline Patient Characteristics (n=96)
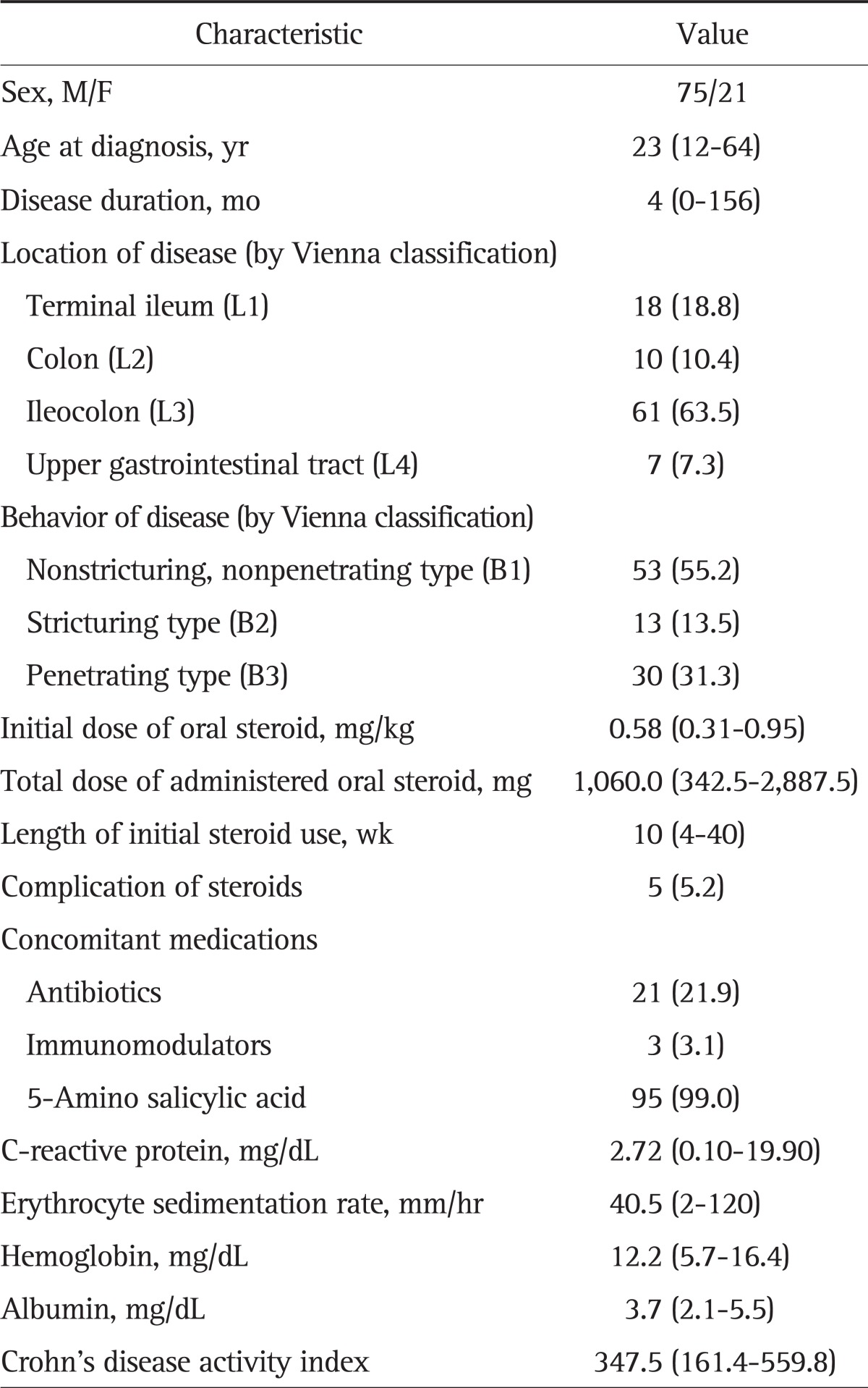
Data are presented as median (range) or number (%).
2. Response rate to CS treatment
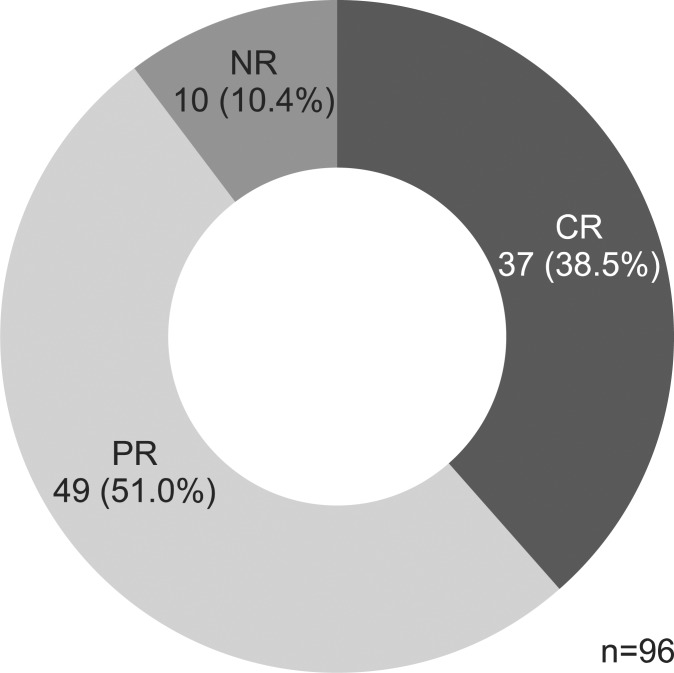
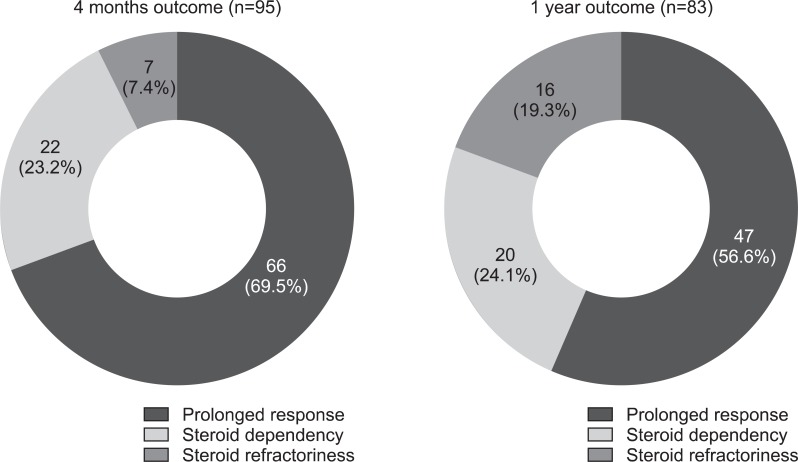
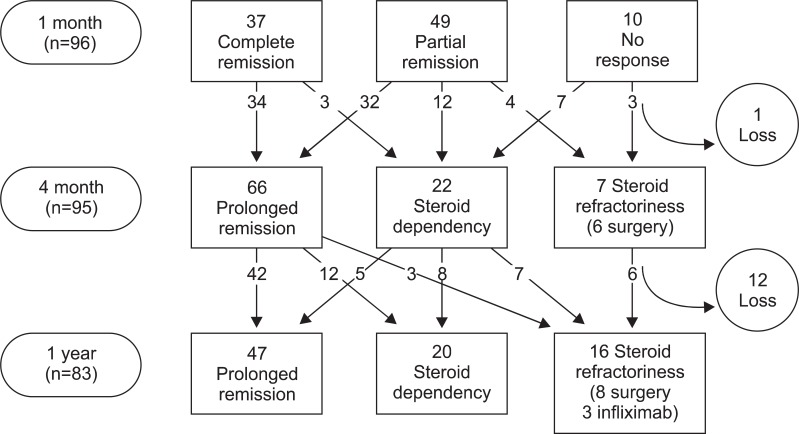
There were 37 CR (38.5%) and 49 PR cases (51.0%), but 10 patients (10.4%) showed NR to oral CS therapy after one month of treatment (Fig. 1). After 4 months, 66 (69.5%) showed prolonged response, 22 (23.2%) showed steroid dependency, and seven (7.4%) showed refractoriness (six underwent surgery, one received immune modulator). After 1 year, 47 patients (56.6%) showed prolonged response, 20 (24.1%) showed steroid dependency, and 16 showed refractoriness (19.3%) (Fig. 2). Of the 16 patients who showed refractoriness, three received infliximab therapy, five underwent immunomodulator treatment, and eight underwent surgery (Fig. 3).
Fig. 1.
Outcomes of steroid use after 1 month of treatment.
CR, complete response; PR, partial response; NR, no response.
Fig. 2.
Outcomes of steroid use after 4 months and 1 year of treatment.
Fig. 3.
Flow chart of the clinical outcomes of patients with active Crohn's disease treated with a first course of oral corticosteroids.
3. Clinical predictive factors
Clinical characteristics of CS responders and nonresponders at one month, 4 months, and 1 year after treatment were compared by univariate analysis. There were no significant differences in age, sex, or location of disease at the time of CS initiation between responders and nonresponders. Moreover, we found no statistically significant differences in the initial dose of CS, the duration of its use, and the use of concomitant medications. However, while nonstricturing, nonpenetrating type CD (41 cases, 62.1%) was most commonly seen in responders at 4 months, penetrating type CD (15 cases, 51.7%) comprised the majority of cases in the nonresponders group, and this was statistically significant (p=0.013). The median CDAIs at the time of CS initiation of the 1-month, 4-month, and 1-year responders were 337.0 (range, 161.4 to 474.2), 311.8 (range, 161.4 to 451.9), and 321.1 (range, 161.4 to 456.6), and those of nonresponders were 386.5 (range, 347.6 to 559.8), 387.8 (range, 293.7 to 559.8), and 380.6 (range, 208.5 to 471.9), respectively. The CDAI at the time of initial CS treatment was significantly higher in nonresponders at all time points (p=0.008, p<0.001, and p=0.009, respectively). Also, the total dose of steroid was higher in the nonresponder group at all time points, and this difference was statistically significant at 1 month after treatment (p=0.032/0.070/0.078, 1 month/4 months/1 year after treatment, respectively) (Table 2).
Table 2.
Comparison of Clinical Factors between Steroid Responders and Nonresponders
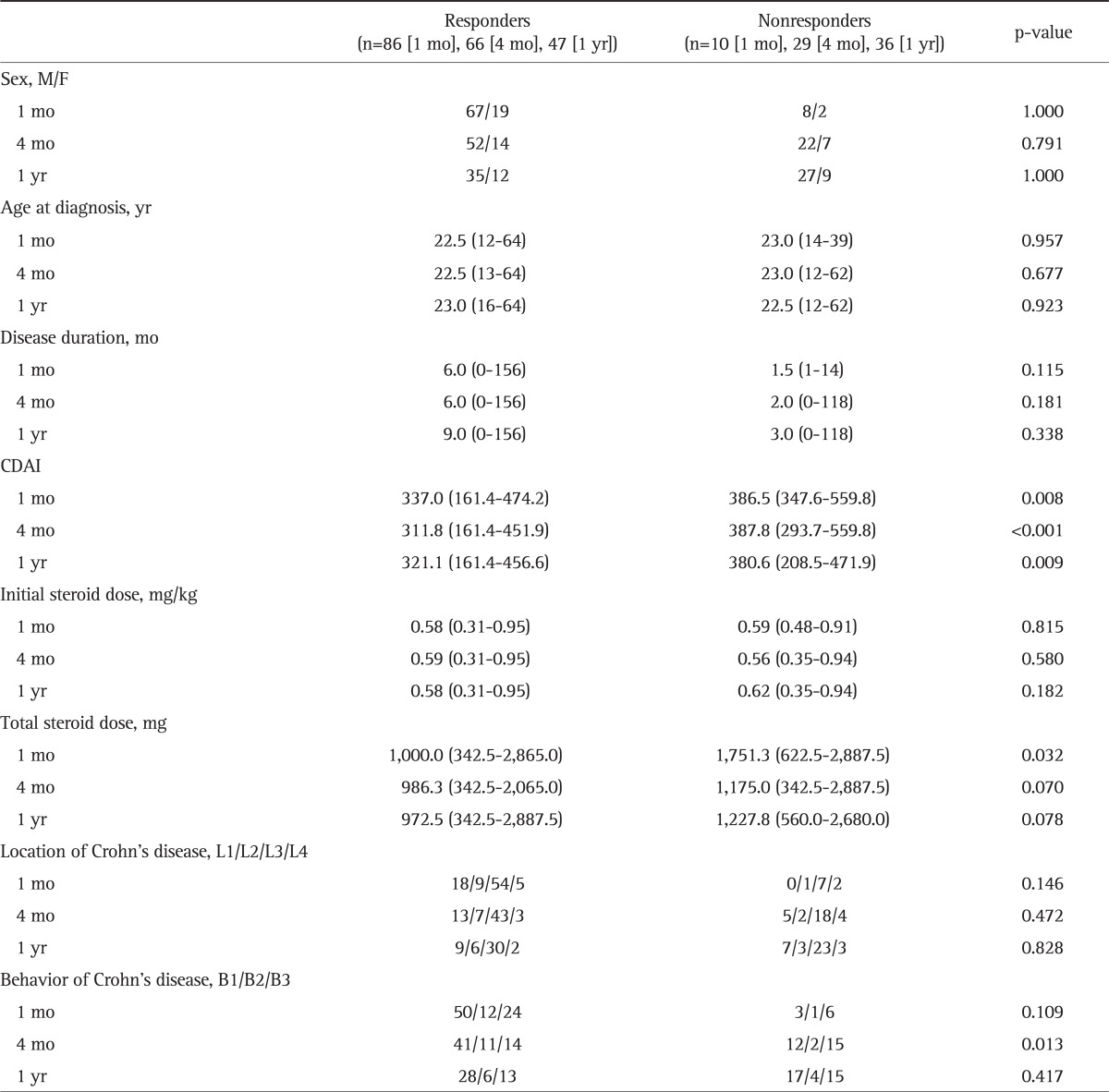
Data are presented as number or median (range).
CADI, Crohn's disease activity index.
CRP at the time of initial CS treatments showed a statistically meaningful difference between the responders and nonresponders at 1 month and 4 months (p=0.015 and p=0.009, respectively). The median CRP level at 1 year in nonresponders was numerically higher than that of responders, but the difference was not statistically significant. The median values of ESR at the time of initial CS treatments in responders at 1 month and 4 months were 39.5 mm/hr (range, 2 to 120 mm/hr) and 38.0 mm/hr (range, 2 to 120 mm/hr), respectively, which were significantly lower than 61.5 mm/hr (range, 12 to 120) and 50.0 mm/hr (range, 4 to 120) in nonresponders, respectively (p=0.043 and p=0.044, respectively). Responders at all time points tended to have numerically higher serum albumin levels at the time of CS treatment compared to nonresponders, but the differences were not statistically significant (Table 3).
Table 3.
Comparison of Biochemical Factors between Steroid Responders and Nonresponders
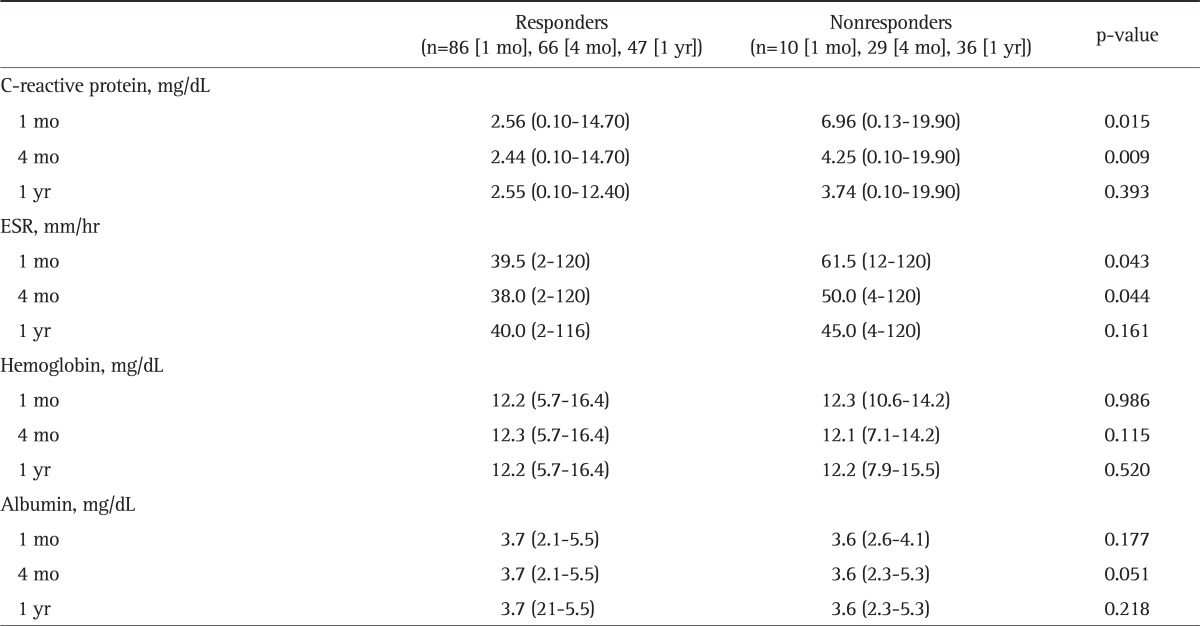
Data are presented as median (range).
ESR, erythrocyte sedimentation rate.
Multivariate analysis was performed for factors including age at diagnosis, location of lesions, and disease behavior at the time of CS treatment, which are known to be correlated with steroid dependency in CD.22 CDAI and biochemical markers such as CRP, ESR, and serum albumin level at the time of initial treatment were also used for the analysis. Nonstricturing, nonpenetrating behavior (B1), and lower CDAI were shown to be predictors for responders at four months after the treatment (p=0.025 and p=0.002, respectively), and CDAI was a statistically significant predictor of treatment response after 1 year of CS therapy (p=0.029) (Table 4).
Table 4.
Multivariate Analysis of Clinical and Biochemical Predictors between Steroid Responders and Nonresponders*
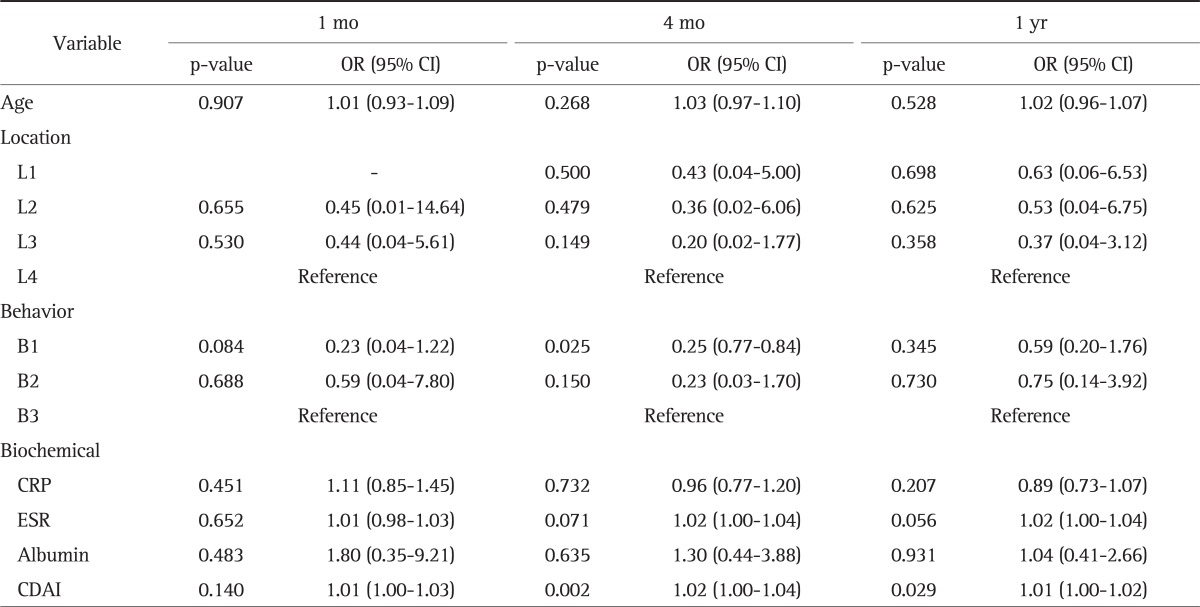
OR, odds ratio; CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; CADI, Crohn's disease activity index.
*The reference is steroid nonresponders.
DISCUSSION
CS therapy is a well-established treatment option for active CD. However, despite its usefulness, patients who receive CS therapy show a wide spectrum of treatment responses, from CR to steroid refractoriness.10,11,17,22-25 Furthermore, CS can cause various complications such as musculoskeletal, endocrine, metabolic, gastrointestinal, and skin complications, depending on the dosage and duration of usage.22 One study reported that 55% of CD patients taking prednisolone (starting dose at 40 mg/day) and 33% of CD patients who used budesonide (starting dose at 9 mg/day) experienced one or more side effects strongly associated with CS therapy.9 Furthermore, because 70% to 90% of CD patients eventually undergo surgery, and 33% to 82% of those patients are subjected to repeated surgery,26 possible increases in surgery-related mortality due to long term use of CS should be taken into consideration. Because of such uncertainty related to the use of CS, many clinicians have agonized over the optimal time of initiation and duration of CS treatment.
Several studies have sought to outline the optimal course of CS therapy in CD patients and to find prognostic factors for disease response. CD remission rates with CS therapy are reported to be 70% to 85%, but with maintenance, rates drop down to 32% to 45% 1 year after CS therapy.11,17 In this study, the short-term (1 month) response rate was 89.6%, which is similar to the results of previous studies. Despite strict definition of remission in our study, the short-term CD remission rate after CS therapy in our population was relatively higher compared to the rates in Western population. The long-term (1 year) response rate was also higher in our study group (56.6%) than in previous studies.10,17 Better response rates in our study suggest that there may be an ethnic difference in CD. Generally, the incidence, sex, and clinical phenotypic distribution of Asian CD patients are considered somewhat different from those of Westerners.12-16 Furthermore, recent studies revealed genetic differences between Asian and Western CD populations. NOD2 mutations, associated with increased susceptibility to CD among Caucasians, do not have a significant correlation with CD patients in Asia.27,28 In contrast, the TNFSF15 gene has been found to have a stronger association with CD in Asians than in Western CD patients.29,30 While the exact etiology of IBD still remains unclear, the disease is thought to result from dysregulation of the immune system when individuals with susceptible genes are exposed to environmental factors.1 Considering such genetic differences, treatment responses can also be expected to differ among races.
One distinctive aspect of our study was that treatment response was evaluated after four months of therapy. Because three months are generally required for steroid tapering and discontinuation, we sought to evaluate the treatment response immediately after the completion of CS therapy by examining the response rate after four months. The results of our study showed that steroid response rates decrease gradually after inducing remission.
Although CS is an established treatment option for inducing remission in active CD, there is no consensus on an optimal dose or route of administration. Previous studies have reported an appropriate oral prednisolone dose to start at 0.8 to 1.0 mg/kg when the goal of treatment is remission of the disease.17,22,23,31 However, in this study, patients showed relatively high remission rates at a median CS dose of 0.58 mg/kg. Although the potential causes of the effects at low doses are difficult to assess because of the retrospective design of our study, these findings suggest that oral administration of 0.58 mg/kg/day of prednisolone may be a sufficient therapeutic dose for Asian CD patients. These observations warrant future investigation.
In order to predict the response rate to CS therapy, clinical and biochemical factors of the responder and nonresponder groups were analyzed. The CDAI of the nonresponder group was significantly higher than that of the responder group, and this result was repeatedly confirmed at 1 month, 4 months, and 1 year after treatment. Also, CRP and ESR tended to be higher in the nonresponder group. It is well known that CRP is an effective means of estimating CD activity32 and that ESR can be a predictive factor of recurrence in inactive CD patients.33 High levels of these parameters and a high CDAI score can represent severe inflammation. Therefore, the more severe the inflammation in CD is, the lower the expected response to CS therapy.
The median serum albumin level was higher in the responder group than in the nonresponder group at all times. In IBD, decreased serum albumin level is explained by several different mechanisms, but its predominant cause is thought to be albumin loss through highly permeable mucosal membranes of the intestine. Furthermore, lower levels of albumin are associated with increased disease severity and degree of inflammation.34 As a consequence, lower serum albumin levels in the nonresponder group in our study may indicate increased inflammation in CD, which is consistent with the results from another Korean study.35
Multivariate analysis showed that CDAI and nonstricturing, nonpenetrating type disease behavior (B1) could be predictors of treatment outcomes. However, larger scale prospective studies are necessary in order for these predictors to be considered for clinical application.
This study has several limitations related to the small sample size and retrospective study design. For example, smoking history, which was known to have adverse effects on disease course, was not evaluated. However, the current study also has many strong points. In addition to the homogenous sample population, patients who received their first CS therapy were studied here. Moreover, clinical response in CD patients treated with oral CS therapy has not been widely studied before.
In conclusion, our study demonstrated that high rates of short-term response to initial oral CS therapy in CD at relatively low doses of CS. Additionally, response rates showed a strong tendency to decrease with time. By analyzing different responses at various time periods after CS therapy, our study determined that CDAI and nonstricturing, nonpenetrating behavior as predictive factors that can be used to identify steroid responders. Further prospective studies are warranted to confirm independent predictive factors.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325:1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 2.Truelove SC, Witts LJ. Cortisone in ulcerative colitis: preliminary report on a therapeutic trial. Br Med J. 1954;2:375–378. doi: 10.1136/bmj.2.4884.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers RW, Switz DM, Sessions JT, Jr, et al. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979;77(4 Pt 2):847–869. [PubMed] [Google Scholar]
- 4.Malchow H, Ewe K, Brandes JW, et al. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984;86:249–266. [PubMed] [Google Scholar]
- 5.Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994;331:836–841. doi: 10.1056/NEJM199409293311303. [DOI] [PubMed] [Google Scholar]
- 6.Caesar I, Gross V, Roth M, et al. Treatment of active and postactive ileal and colonic Crohn's disease with oral pH-modified-release budesonide. German Budesonide Study Group. Hepatogastroenterology. 1997;44:445–451. [PubMed] [Google Scholar]
- 7.Campieri M, Ferguson A, Doe W, Persson T, Nilsson LG The Global Budesonide Study Group. Oral budesonide is as effective as oral prednisolone in active Crohn's disease. Gut. 1997;41:209–214. doi: 10.1136/gut.41.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomsen OO, Cortot A, Jewell D, et al. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide-Mesalamine Study Group. N Engl J Med. 1998;339:370–374. doi: 10.1056/NEJM199808063390603. [DOI] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Löfberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med. 1994;331:842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- 10.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 11.Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2003;(4):CD000301. doi: 10.1002/14651858.CD000301. [DOI] [PubMed] [Google Scholar]
- 12.Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [DOI] [PubMed] [Google Scholar]
- 13.Ye BD, Yang SK, Cho YK, et al. Clinical features and long-term prognosis of Crohn's disease in Korea. Scand J Gastroenterol. 2010;45:1178–1185. doi: 10.3109/00365521.2010.497936. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Xia B, Li J, et al. Retrospective survey of 452 patients with inflammatory bowel disease in Wuhan city, central China. Inflamm Bowel Dis. 2006;12:212–217. doi: 10.1097/01.MIB.0000201098.26450.ae. [DOI] [PubMed] [Google Scholar]
- 15.Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn's disease in the Chinese population. Inflamm Bowel Dis. 2004;10:646–651. doi: 10.1097/00054725-200409000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Iida M, Yao T, Okada M. Long-term follow-up study of Crohn's disease in Japan. The Research Committee of Inflammatory Bowel Disease in Japan. J Gastroenterol. 1995;30(Suppl 8):17–19. [PubMed] [Google Scholar]
- 17.Papi C, Festa V, Leandro G, et al. Long-term outcome of Crohn's disease following corticosteroid-induced remission. Am J Gastroenterol. 2007;102:814–819. doi: 10.1111/j.1572-0241.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 18.Munkholm P, Langholz E, Davidsen M, Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994;35:360–362. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stange EF, Travis SP, Vermeire S, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. Gut. 2006;55(Suppl 1):i1–i15. doi: 10.1136/gut.2005.081950a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512–530. doi: 10.1053/gast.2002.31072. [DOI] [PubMed] [Google Scholar]
- 21.Gasche C, Scholmerich J, Brynskov J, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Yang YX, Lichtenstein GR. Corticosteroids in Crohn's disease. Am J Gastroenterol. 2002;97:803–823. doi: 10.1111/j.1572-0241.2002.05596.x. [DOI] [PubMed] [Google Scholar]
- 23.Irving PM, Gearry RB, Sparrow MP, Gibson PR. Review article: appropriate use of corticosteroids in Crohn's disease. Aliment Pharmacol Ther. 2007;26:313–329. doi: 10.1111/j.1365-2036.2007.03379.x. [DOI] [PubMed] [Google Scholar]
- 24.Michetti P, Mottet C, Juillerat P, et al. Severe and steroid-resistant Crohn's disease. Digestion. 2007;76:99–108. doi: 10.1159/000111023. [DOI] [PubMed] [Google Scholar]
- 25.Navarro FA, Hanauer SB, Kirschner BS. Effect of long-term low-dose prednisone on height velocity and disease activity in pediatric and adolescent patients with Crohn disease. J Pediatr Gastroenterol Nutr. 2007;45:312–318. doi: 10.1097/MPG.0b013e31805b82c6. [DOI] [PubMed] [Google Scholar]
- 26.Krupnick AS, Morris JB. The long-term results of resection and multiple resections in Crohn's disease. Semin Gastrointest Dis. 2000;11:41–51. [PubMed] [Google Scholar]
- 27.Inoue N, Tamura K, Kinouchi Y, et al. Lack of common NOD2 variants in Japanese patients with Crohn's disease. Gastroenterology. 2002;123:86–91. doi: 10.1053/gast.2002.34155. [DOI] [PubMed] [Google Scholar]
- 28.Croucher PJ, Mascheretti S, Hampe J, et al. Haplotype structure and association to Crohn's disease of CARD15 mutations in two ethnically divergent populations. Eur J Hum Genet. 2003;11:6–16. doi: 10.1038/sj.ejhg.5200897. [DOI] [PubMed] [Google Scholar]
- 29.Yang SK, Lim J, Chang HS, et al. Association of TNFSF15 with Crohn's disease in Koreans. Am J Gastroenterol. 2008;103:1437–1442. doi: 10.1111/j.1572-0241.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki K, McGovern D, Ragoussis J, et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn's disease. Hum Mol Genet. 2005;14:3499–3506. doi: 10.1093/hmg/ddi379. [DOI] [PubMed] [Google Scholar]
- 31.Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;(2):CD006792. doi: 10.1002/14651858.CD006792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boirivant M, Leoni M, Tariciotti D, Fais S, Squarcia O, Pallone F. The clinical significance of serum C reactive protein levels in Crohn's disease. Results of a prospective longitudinal study. J Clin Gastroenterol. 1988;10:401–405. doi: 10.1097/00004836-198808000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Brignola C, Campieri M, Bazzocchi G, Farruggia P, Tragnone A, Lanfranchi GA. A laboratory index for predicting relapse in asymptomatic patients with Crohn's disease. Gastroenterology. 1986;91:1490–1494. doi: 10.1016/0016-5085(86)90206-4. [DOI] [PubMed] [Google Scholar]
- 34.Jarnum S, Jensen KB. Fecal radioiodide excretion following intravenous injection of 131-I-albumin and 125-I-immunoglobulin G in chronic inflammatory bowel disease. An aid to topographic diagnosis. Gastroenterology. 1975;68:1433–1444. [PubMed] [Google Scholar]
- 35.Hyun JG, Kim JJ, Kim YH, et al. Analysis of clinical, biochemical and pathologic factors according to the response to initial steroid therapy in active Crohn's disease. Korean J Gastrointest Endosc. 2001;22:406–410. [Google Scholar]