Abstract
Background/Aims
The Central California Valley has a diverse population with significant proportions of Hispanics and Asians. This cross-sectional study was conducted to evaluate the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) in healthy blood donors in the Valley.
Methods
A total of 217,738 voluntary blood donors were identified between 2006 and 2010 (36,795 first-time donors; 180,943 repeat donors).
Results
Among the first-time donors, the HBV and HCV prevalence was 0.28% and 0.52%, respectively. Higher HBV prevalence seen in Asians (3%) followed by Caucasians (0.05%), African Americans (0.15%), and Hispanics (0.05%). Hmong had a HBV prevalence of 7.63% with a peak prevalence of 8.76% among the 16- to 35-year-old age group. Highest HCV prevalence in Native Americans (2.8) followed by Caucasians (0.59%), Hispanics (0.45%), African Americans (0.38%), and Asians (0.2%).
Conclusions
Ethnic disparities persist with regard to the prevalence of HBV and HCV in the Central California Valley. The reported prevalence may be an underestimate because our study enrolled healthy volunteer blood donors only. The development of aggressive public health measures to evaluate the true prevalence of HBV and HCV and to identify those in need of HBV and HCV prevention measures and therapy is critically important.
Keywords: Chronic hepatitis C, Chronic hepatitis B, Central Valley of California, Hmong, Blood donors
INTRODUCTION
Despite significant progress made over the last few decades in the prevention and management of chronic hepatitis B virus HBV) and hepatitis C virus (HCV) infections, approximately 240 million people are still chronically infected with HBV and 130 to 170 million with HCV worldwide.1,2 Chronic hepatitis B and C are responsible for 60% to 70% of hepatocellular carcinoma (HCC) worldwide.3 HCV-related mortality has increased annually between 1999 and 2007 in the United States (US) and has surpassed human immune deficiency virus (HIV) related mortality. Most of the deaths from HBV and HCV occurred in middle-aged persons.4 In Asian and Western Pacific countries where HBV is endemic, estimated prevalence of chronic HBV infection ranges from 2.4% to 16.0%, and liver cancer is a leading cause of mortality.5,6 Americans of Asian and Pacific Islander descent experience the greatest burden of the disease in the US. Although Asian Americans comprise only 5% of the US population, they represent more than half of the estimated 1.4 to 2 million people chronically infected with hepatitis B in this country.7-9 As opposed to the overall prevalence of 0.4%, the prevalence of HBV in Mexican Americans is 2.9%.10
Approximately, 4.1 million individuals are presently infected with HCV in the US.11,12 The prevalence of HCV varies geographically and across ethnicities.13 Minorities represent the majority of chronic hepatitis C cases in the Los Angeles County Hepatitis Clinic.14 Hispanics have an overall prevalence rate of 2.6% for HCV; have faster liver fibrosis progression rates and are infected at an earlier age.15 HCV infection and Hispanic ethnicity independently increase the risk for HCC-related mortality.16 American Indians and Alaska Natives are disproportionately affected by chronic liver disease; in particular, the incidence of HCV is rising.17 Black men are more likely to be infected with HBV and/or HCV (6%) than Mexican-American (3%) and White (2%) men.18 The National Health and Nutrition Examination Survey (NHANES) from 2001 through 2008 indicate that fewer than half of those infected with HCV may be aware of their infection.19 The viral hepatitis surveillance system in the US is highly fragmented and poorly developed. The Institute of Medicine has recently made recommendations in four areas: surveillance, knowledge and awareness, hepatitis B immunization, and ser-vices.20
The Central Valley of California outranks 20 states in population count in the US, and by the year 2020, it is projected to outrank 33 states.21 The Central Valley has a diverse ethnic population composition, about 30% to 68% Hispanics and 3% to 14% Asians across various counties. Our recent prospective study revealed that approximately one out of every six Hmong immigrants in the Central California Valley is infected with HBV.22 Therefore; the disparities in prevalence of viral hepatitis are expected in the valley as compared to general US population. These ethnic disparities may also contribute to the adverse clinical outcomes of the affected population.15-18,23,24 The goal of our study was to evaluate the prevalence of HBV and HCV in the general population such as healthy blood donors in the Central California Valley.
MATERIALS AND METHODS
We reviewed blood donor laboratory screening records from Central California Blood Center (CCBC) for the period January 2006 through December 2010. CCBC is accredited by American Association of Blood Banks (AABB, the standard setting organization formerly known) and is licensed by the US Food and Drug Administration (FDA). As such, CCBC performs pre-donation donor screening using an FDA-approved, standardized Uniform Donor Health History Questionnaire developed through AABB, which is designed in part to exclude potential donors judged to be at increased risk for exposure to infectious diseases. Examples of such donors include those with a clinical history of infection with HIV, HBV, HCV, or other communicable diseases (e.g., syphilis), those who have participated in certain defined high-risk behaviors (e.g., intravenous [IV] drug use), donors with past travel to foreign countries or foreign born donors in some cases, and those with various other medical conditions.25 A cross-sectional review of HBV and HCV positivity among all donors was investigated for this study.
Routine donor laboratory screening was performed according to standard operating procedures at CCBC that included performing the most current versions of the following tests: hepatitis B surface antigen (HBsAg) (HBsAg EIA 3.0, Genetic Systems™; BIO-RAD Laboratories, Redmond, WA, USA), antibody to hepatitis B core antigen (anti-HBc, ORTHO™ HBc ELISA Test System; Ortho Clinical Diagnostics, Raritan, NJ, USA), antibody to HCV (anti-HCV, ORTHO™ HCV version 3.0 ELISA Test System; Otho Clinical Diagnostics), HCV nucleic acid amplification testing (HCV NAT, Procleix™ HIV-1/HCV Assay; Gen-Probe Inc., San Diego, CA, USA). Follow-up confirmation testing was also performed after obtaining positive results in the initial screening tests (except for anti-HBc and HCV NAT, for which confirmatory testing is either not available [anti-HBc] or not indicated), and included the following tests: HBsAg neutralization (Genetic Systems™ HBsAg Confirmatory Assay 3.0; BIO-RAD Laboratories), and anti-HCV recombinant immunoblot assay (RIBA, Chiron™ RIBA™ HCV 3.0 Strip Immunoblot Assay; Novartis Diagnostics, Emeryville, CA, USA).
Statistical analysis
The data was tabulated using Microsoft Excel (Microsoft, Redmond, WA, USA). Prevalence rates with 95% confidence intervals (CIs) were calculated. Comparison of proportions between the groups was calculated using chi-square test. A p-value of less than 0.05 was considered statistically significant. Statistical programs that were utilized include Microsoft Excel and Stata, version 11.1 (Stata Co., College Station, TX, USA).
The study was approved by the Institutional Review Board at the University of California San Francisco Fresno Medical Education Program.
RESULTS
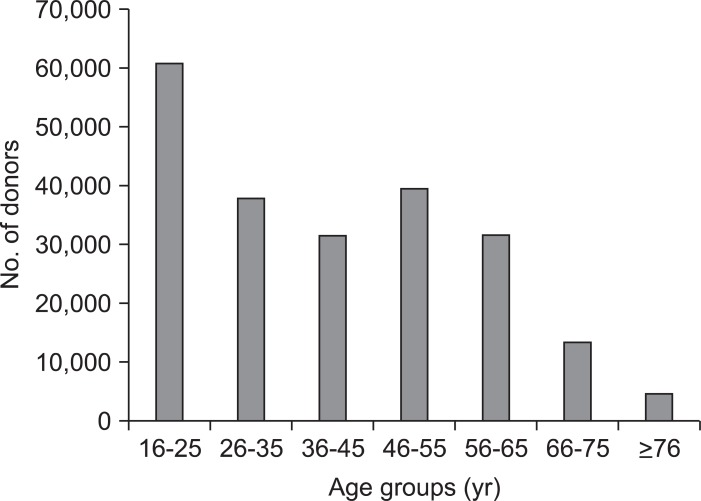
A total of 219,450 voluntary blood donor records were reviewed and analyzed. Records of 1,712 donors were excluded due to the lack of basic demographic information. The study population was made up of 217,738 donors, of which, 36,795 were first-time donors and 180,943 were repeat donors. Males constituted 47% (n=102,248) of the study population while females constituted 53% (n=115,490). Predominant ethnic groups were Caucasians (55.6%) followed by Hispanics (32.1%), Asians (4.1%), African Americans (2%), and Native Americans (0.9%). Ethnicity was entered as 'others' for the remaining 5.3% of the sample. Majority of the donors were between ages 16 and 65 years old with the highest representation (28%) noted among the 16 to 25 years old donor group (Fig. 1).
Fig. 1.
Donor age distributions.
1. HBV prevalence
Table 1 lists the prevalence of HBsAg positivity with 95% CIs across ethnicities in first-time and repeat donors. Overall, HBV prevalence of 0.06% (95% CI, 0.05 to 0.07) was noted with 0.28% (95% CI, 0.22 to 0.33) among first-time donors and 0.01% (95% CI, 0.007 to 0.02) among repeat donors.
Table 1.
Hepatitis B Prevalence among Donors from 2006 to 2010 at the Central California Blood Center
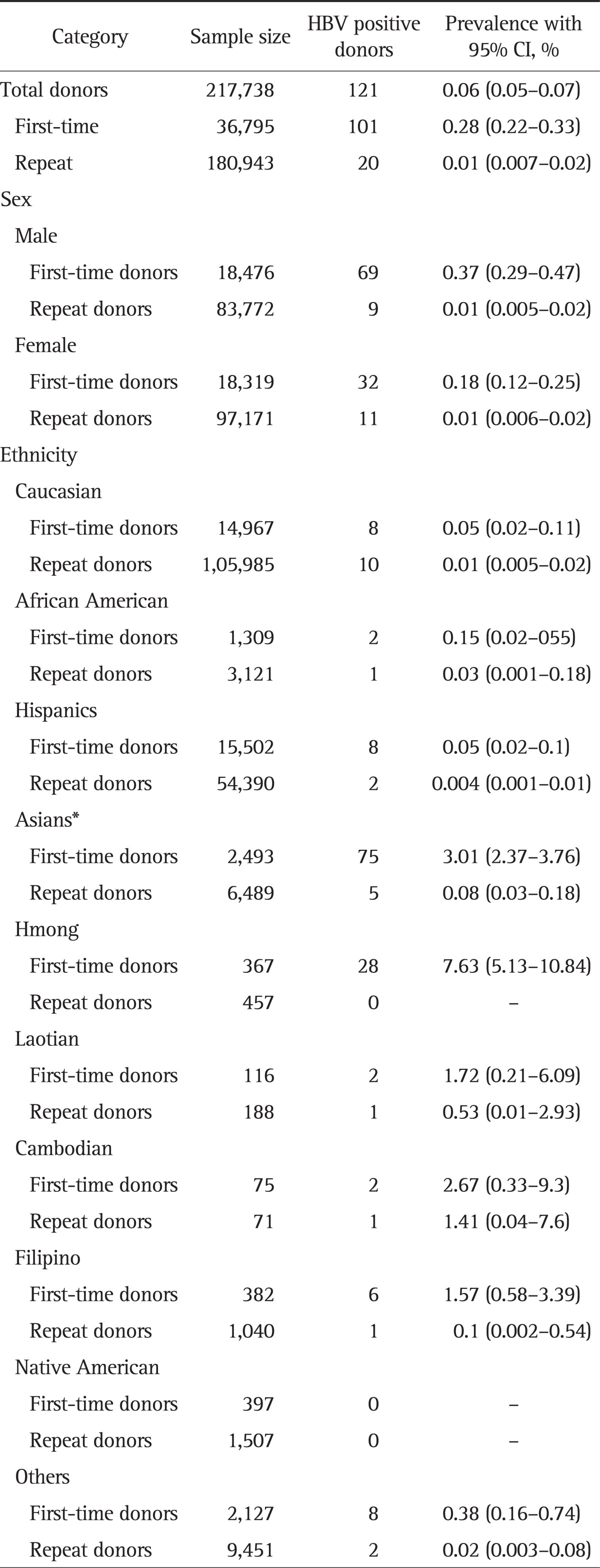
HBV, hepatitis B virus; CI, confidence interval.
*Inclusive of Hmong, Laotian, Cambodian, and Filipino.
1) First-time donors
Males had higher HBV prevalence among first-time donors compared with that in females (0.37% vs 0.18%). Across all ethnicities, Asians including Hmong, Laotian, Cambodian, and Filipino were noted to have the highest HBV prevalence of 3% (95% CI, 2.37 to 3.76) compared to any other ethnic groups (Caucasians 0.05, African Americans 0.15, and Hispanics 0.05) (Table 1). Among Asian subgroups, Hmong population was noted to have highest HBV prevalence of 7.63% (95% CI, 5.1 to 10.8) with peak prevalence of 8.76% among the age group, 16 to 35 years. Other Asian subpopulations studied include Filipinos, Cambodians, and Laotian were also noted to have higher HBV prevalence of 1.57% (95% CI, 0.58 to 3.39), 2.67% (95% CI, 0.33 to 9.3), and 1.72% (95% CI, 0.21 to 6.09), respectively. The wider CIs are likely due to small sample sizes.
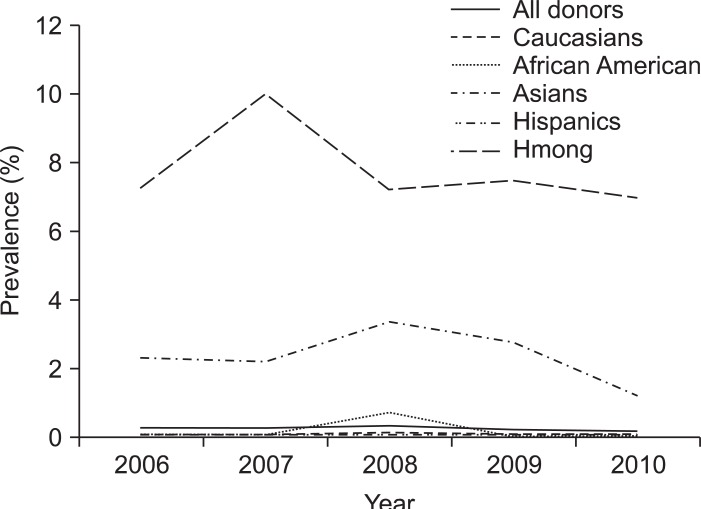
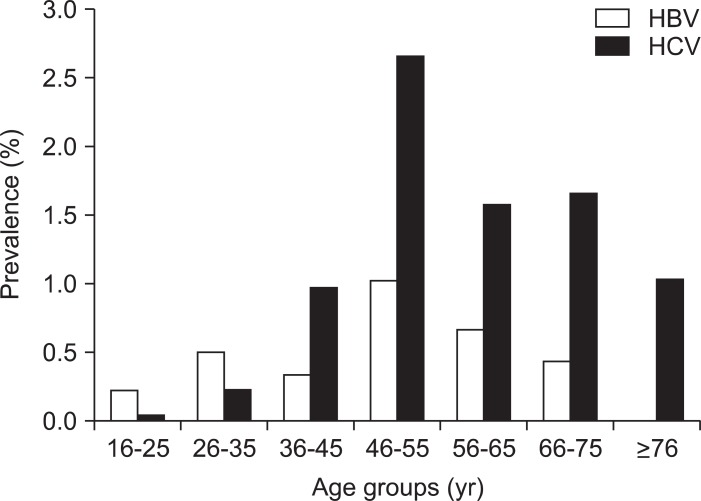
Fig. 2 displays the HBV prevalence trends and comparisons among all donors and various ethnic groups studied yearly from 2006 to 2010. The prevalence of HBV infection remained more or less stable among all donor groups, except among Asians where it has declined since 2008. When we looked across the age groups among all ethnicities, the peak prevalence of 1.03% and 0.7% was noted among age groups 46 to 55 and 56 to 65 years, respectively. The prevalence of 0.23% and 0.51% among younger age groups 16 to 25 and 26 to 35 years was largely contributed by the Asian and Hmong populations who are observed to have prevalence of 2.92% and 8.76%, respectively (Figs. 2 and 3).
Fig. 2.
Hepatitis B virus prevalence trends among first-time donors from 2006 to 2010.
Fig. 3.
Hepatitis B and C prevalence trends across different age groups among first-time donors.
HBV, hepatitis B virus; HCV, hepatitis C virus.
2) Repeat donors
Repeat donor HBV prevalence rates were lower compared with first-time donors in all ethnic groups with Asians including Hmong, Laotian, Cambodian, and Filipino reporting 0.08% followed by African Americans (0.03%), Caucasians (0.01%), and Hispanics (0.004%) (Table 1).
The proportions of HBV positivity when compared across various ethnicities among the first-time and repeat donors were found to be statistically significant with p-values of less than 0.0001 and 0.0006, respectively. Across gender, p-value was significant only among the first-time donors (0.0013) whereas, it was 0.993 among the repeat donors.
2. HCV prevalence
Table 2 lists the prevalence of hepatitis C with 95% CIs across ethnicities in first-time and repeat donors. Overall, HCV prevalence of 0.11% (95% CI, 0.09 to 0.12) was noted with 0.52% (95% CI, 0.45 to 0.6) among first-time donors and 0.03% (95% CI, 0.02 to 0.033) among repeat donors. Males had higher HCV prevalence among first-time donors and repeat donors, compared with that in females (0.62% vs 0.42% and 0.03% vs 0.02%).
Table 2.
Hepatitis C Prevalence among Donors from 2006 to 2010 at the Central California Blood Center
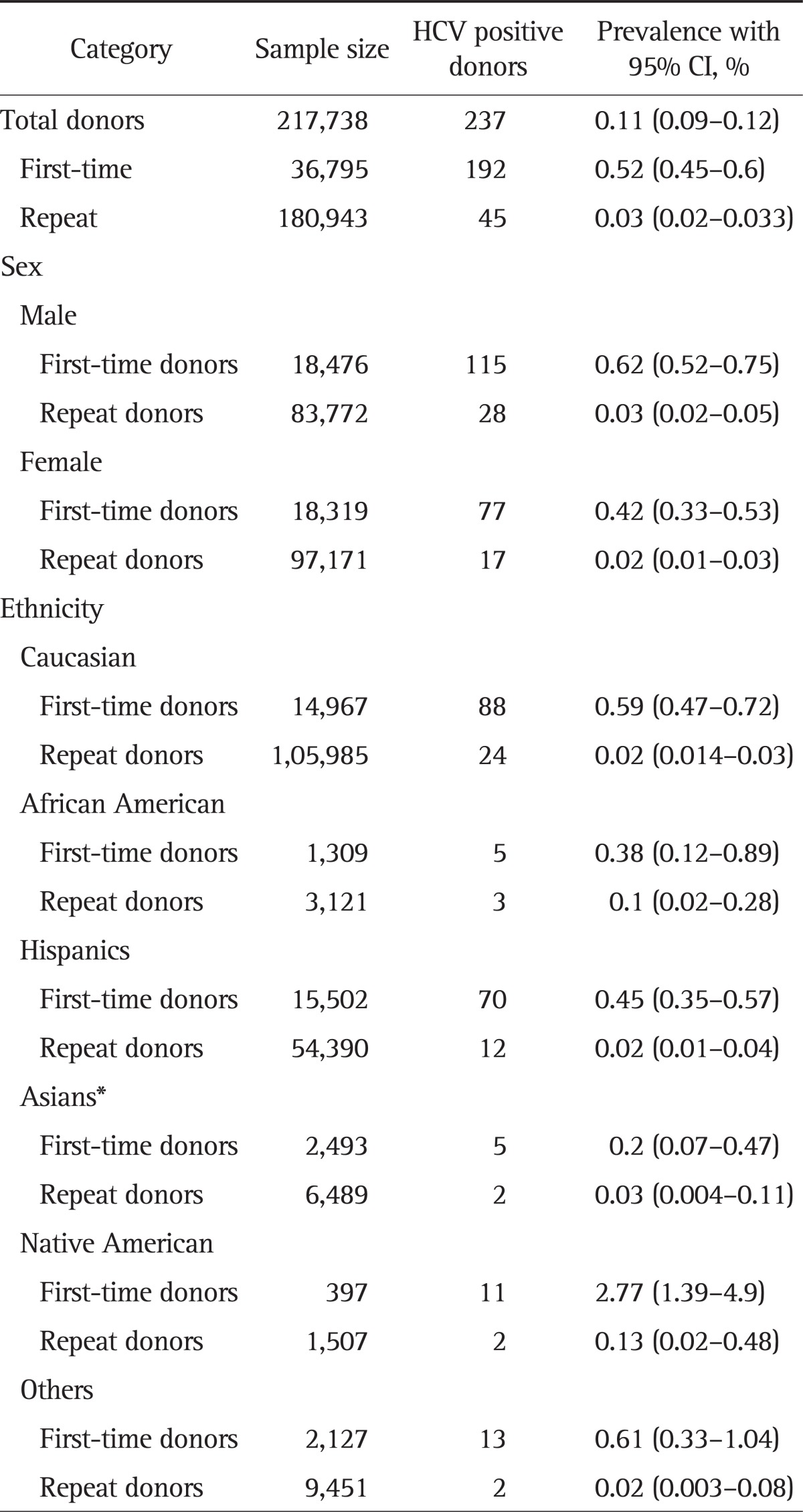
HCV, hepatitis C virus; CI, confidence interval.
*Inclusive of Hmong, Laotian, Cambodian, and Filipino.
1) First-time donors
Across ethnicities, Native Americans were noted to have the highest HCV prevalence of 2.77% (95% CI, 1.39 to 4.9) compared to any other ethnic groups (Caucasians 0.59%, African Americans 0.38%, Hispanics 0.45%, and Asians 0.2%) (Table 2). HCV prevalence rates have declined among first time donors from 0.92% in 2006 to 0.32% in 2010, whereas it remained constant around 0.02% to 0.03% among repeat donors. Peak HCV prevalence was noted in 2006 among all ethnic groups compared to subsequent years. Higher prevalence was noted in donors over 35 years of age with peak prevalence of 2.67% among age group 46 to 55 years (Fig. 3).
2) Repeat donors
Repeat donor HCV prevalence rates were lower compared with first-time donors in all ethnic groups with 0.13% (95% CI, 0.02 to 0.48) in Native Americans followed by African Americans (0.1%), Asians (0.03%), Hispanics (0.02%), and Caucasians (0.02%) (Table 2).
The proportions of HCV positivity when compared across the gender and ethnicity among the first-time donors were found to be statistically significant with p-values of 0.03 and less than 0.00001, respectively. Whereas, among repeat donors, statistical significance was not found across gender (p=0.1) and ethnicity (p=0.175).
DISCUSSION
This study is by far the largest of its kind to elucidate the epidemiology of HBV and HCV infection in the heterogeneous voluntary blood donor population in the Central California Valley. The overall prevalence of HBV and HCV reported in our study may be an underestimate of the true prevalence in the valley as the study included voluntary donor population with the risk of certain degree of self-selection. Prevalence estimates in blood donors are typically lower compared to the general population due to the pre-donation screening for behavioral risk factors, thus eliminating the high risk groups.26-28
The HBV prevalence in the general US population estimated by NHANES surveys during 1976 to 1980, 1988 to 1994, and 2005 to 2006 was found to be 0.33%, 0.42%, and 0.30%, respectively.29,30 Earlier studies on blood donor populations re-ported that the prevalence of HBV in first-time donors remained stable since the late 1970s. Bastiaans et al.31 and Glynn et al.,26 reported HBV prevalence of 0.26% (1977 to 1978) and 0.2% (1991 to 1996) respectively among first-time donors at American Red Cross blood service centers across the US. Our study also reports the similar prevalence rates (about 0.2% to 0.3%) of HBV among first-time donors from 2006 to 2010. HBV prevalence was lowest among Caucasians and Hispanics in our study sample and is consistent with that in the general population as reported earlier on NHANES data where prevalence of chronic HBV among non-Hispanic Whites and Mexican Americans was 0.09% and 0.07%, respectively.10 However, certain ethnic groups of the study population were noted to have disproportionately high HBV prevalence. Among Asians and particularly Hmong, HBV prevalence was detected at a frequency greater than it was in other ethnic groups. It is well known that Asians especially Hmong tend to have high HBV prevalence5-9 and their true prevalence in the US is still unknown as they are underrepresented in large population studies like NHANES.11,29 The HBV prevalence in Asians has declined over the years from 2006 to 2010 in our study population. In contrast, the proportion of total HBV positive donors remained relatively constant among all first-time donors. The 0.01% prevalence of HBV among repeat donors can be attributed to the missed cases during previous donations that were possibly in the window period, or might have acquired new infection.
The peak HBV prevalence was noted among older age groups (Fig. 3), but this does not undermine the fact that younger age groups had lower prevalence. In fact, when we looked at the first-time donors across all ethnicities, Asians and especially Hmong form the predominant groups with the highest HBV prevalence of 2.92% and 8.76% among younger donors (16 to 35 years). This implies that those populations might have acquired HBV through vertical and horizontal transmissions. As HBV is endemic to most Asian countries, high prevalence is expected but the concerning fact is that these younger donors were likely second generation Americans born in the US who acquired HBV despite universal HBV immunization programs in place.26 Unfortunately, we do not have information on the birth records of the donors to support our claim. Universal implementation of HBV vaccination since the early 1990s in the US has resulted in decline of new cases of HBV, and the greatest decline is noted among younger age groups (<15 years).32 Nevertheless, it highlights the ethnic disparities and gaps in healthcare delivery with a dire need for more effective interventions from the public health departments in the Central Valley.
African American ethnicity is one of the predominant ethnic groups for high HBV prevalence and in previous NHANES surveys, non-Hispanic Blacks were found to have HBV prevalence significantly higher than non-Hispanic Whites and Mexican Americans.18,30 However, the HBV prevalence among first-time African American donors in our study was low (0.15%) with only two among first-time donors and three among all donors positive for HBV over a period of 5 years. This low prevalence may not be an accurate representation of the general population, which is estimated at 0.8% to 0.9%10 and can be attributed to some extent to increased self-deferral of high risk groups, effective pre-donation screening by the blood bank and relatively lower representation of African Americans in our sample. Also, wide-spread hepatitis B immunization programs may have led to a decline in HBV prevalence by preventing early childhood infection as well as providing immunity for adolescents and adults before they engage in high-risk behaviors.26,33 To confirm our findings, more population based studies are needed in the Central Valley to elucidate the trend of HBV prevalence among the African-American population. Other factors examined in this study include the prevalence across genders, with males having higher prevalence across all ethnicities. Males have greater likelihood of acquiring the infection as they are prone for high risk behaviors.
The prevalence of HCV in the general US population estimated using the data from the NHANES III, NHANES 1999 to 2002 was 1.6% to 1.8%.12 HCV prevalence among first-time blood donors in the US was estimated at 0.36% in early 1990s and was shown to have decreased since then by the subsequent studies. An estimated 0.23% prevalence was noted in a large cross-sectional study done on the data from six US blood donor centers during 2006 to 2007. The highest prevalence was noted among males and older age donor groups.26,34-36 The overall seroprevalence of HCV in the Central Valley blood donor population was found to be 0.11%. Among the first-time donors the overall prevalence was 0.52%, higher than that reported in previous donor studies. Lower HCV prevalence (0.03%) among repeat donors is expected as they tend to have less risk-prone lifestyle.
In NHANES surveys and previous blood donor studies, the prevalence of HCV was higher among non-Hispanic Blacks than among non-Hispanic Whites and Mexican Americans, and higher among men than among women.11,18,36 The peak age group of HCV infection was 35 to 39 years in 1988 to 1994 and that increased to 45 to 49 years in 1999 to 2002. Lowest prevalence was noted in those less than 30 years of age.12 IV drug users and those with history of multiple sex partners had higher HCV prevalence.11 In our study population, males had higher prevalence of HCV than females. Across age groups, the peak HCV prevalence was noted in first-time donors over age 35 years with highest among 46 to 55 year old group (2.7%), indicating that the majority may have been exposed in the 1970s and 1980s when incidence was highest (Fig. 3).11 Even though, a gradual decline in HCV prevalence was observed among first-time donors in the Valley with estimates decreasing from 0.92% in 2006 to 0.32% in 2010, it is still concerning especially when compared with previous donor studies elsewhere in the US.35,36 Those among positive for HCV, Native Americans followed by Caucasians and Hispanics, form the predominant ethnic groups. Interestingly, African Americans overall had low HCV prevalence compared to Caucasians and Hispanics. However, it is widely known from previous studies that African Americans generally have high rates of HCV predominantly with genotype 1, and are at increased risk for HCC and mortality compared to Caucasians.37 This is important as Black Americans are less likely than Whites to respond to treatment with peg-interferon plus ribavirin.38 Therefore, more population based studies are needed to elucidate the true prevalence of HCV among African Americans in the Central California Valley.
Even though Native Americans tend to suffer disproportionate burden of chronic liver disease, the true prevalence of HCV is largely unknown.39,40 In a prospective study on Native Americans at an urban clinic, HCV prevalence was noted to be 8.6% (95% CI, 5.1 to 12.1) with higher prevalence in males compared to females. Most common risk factors identified were IV drug or cocaine use followed by receiving tattoos and sexual partners with HCV.39 In a study of HCV-positive Alaskan Natives, 60.1% had a history of IV drug use while 14% had a history of blood transfusion.41 HCV prevalence of 2.77% (95% CI, 1.39 to 4.9) among Native Americans in our study is a concern even though; it appears to have declined from 5.26% in 2006 to 0.96% in 2010. The reported values may not be the true es-timates because of the small sample size, also evident from the wider CI. Limited data are available on the barriers to receiving healthcare and type of HCV genotype prevalent among them.39 Native Americans, along with other minority populations, are underrepresented and understudied in large population studies like NHANES. Palpably, this underserved ethnic group demand more attention from the public health authorities in identifying the barriers to preventive healthcare.
From our results, the overall reported prevalence of HBV and HCV has declined over the years, possibly due to better preventive measures. However, the risk of cirrhosis and liver cancer continues to remain unchanged from already acquired chronic infections. The HBV and HCV rates are expected to be lower among repeat donors when compared with first-time donors as they have tested negative and successfully got through the predonation screening on their last donation. It is also improbable that they would practice high risk behaviors in subsequent years.
The population studies (like NHANES) estimating the HBV and HCV prevalence could have potentially underreported the true prevalence as they did not include statistically valid representation from Asians, Pacific Islanders, Native Americans, and Alaskan Natives. More importantly, those studies potentially excluded high risk individuals (institutionalized, homeless, incarcerated, etc.) and it is estimated that the prevalence of HBV and HCV in institutionalized, homeless, or incarcerated individuals would be 10-fold higher than the general population.11,29,42 Similar to the previous blood donor studies, one of our major limitations was that the study was done on a highly selective population and expected to have low prevalence of HBV and HCV compared to the general population. Also, we are lacking information on demographics and other risk factors like IV drug users, obesity, alcohol consumption, gravidity, etc. Our study population does not account for those who are chronically exposed to blood and blood products like hemodialysis patients and healthcare workers who are expected to have high HBV and HCV prevalence. African American, Native Americans, and Asian subgroups are underrepresented compared to Hispanics and Caucasians in this study. Increased rates of repeat donors might have influenced the overall prevalence estimates.
In conclusion, our study highlights the persistence of ethnic disparities in the prevalence of HBV and HCV in the Central Valley of California. The study also highlights the higher overall HCV prevalence among first-time blood donors in the Central Valley compared to previous donor studies in the US. The reported prevalence of HBV and HCV in our study may be an underestimate and inferences can only be made regarding the existing prevalence of these infections in the Central Valley's general population.
The dearth of awareness of prevalence, proper screening targets and solid strategies for prevention and medical management likely contribute to on-going transmission of these viral diseases. Therefore, more population based studies are needed to elucidate the actual prevalence and identify risk factors in the Central Valley. Culturally relevant and trusted sources of communication are required to increase and promote awareness for accessibility of appropriate services. Programs and venues for community outreach, identifying the infected persons, harm reduction through vaccination and needle exchange programs, social support and medical management are badly needed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 5.Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38(10 Suppl 3):S158–S168. doi: 10.1097/00004836-200411003-00008. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Screening for chronic hepatitis B among Asian/Pacific Islander populations: New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:505–509. [PubMed] [Google Scholar]
- 7.Rawls RA, Vega KJ. Viral hepatitis in minority America. J Clin Gastroenterol. 2005;39:144–151. [PubMed] [Google Scholar]
- 8.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B Division of Vital Statistics. Deaths: final data for 2007. Natl Vital Stat Rep. 2010;58:1–136. [PubMed] [Google Scholar]
- 9.Kim WR. Epidemiology of hepatitis B in the United States. Hepatology. 2009;49(5 Suppl):S28–S34. doi: 10.1002/hep.22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007;42:513–521. doi: 10.1007/s00535-007-2064-6. [DOI] [PubMed] [Google Scholar]
- 13.Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:41–46. doi: 10.7150/ijms.3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonacini M, Groshen MD, Yu MC, Govindarajan S, Lindsay KL. Chronic hepatitis C in ethnic minority patients evaluated in Los Angeles County. Am J Gastroenterol. 2001;96:2438–2441. doi: 10.1111/j.1572-0241.2001.04050.x. [DOI] [PubMed] [Google Scholar]
- 15.Blessman DJ. Chronic hepatitis C in the Hispanic/Latino population living in the United States: a literature review. Gastroenterol Nurs. 2008;31:17–25. doi: 10.1097/01.SGA.0000310931.64854.5f. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Stepanova M. Hepatitis C virus infection, age, and Hispanic ethnicity increase mortality from liver cancer in the United States. Clin Gastroenterol Hepatol. 2010;8:718–723. doi: 10.1016/j.cgh.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Scott JD, Garland N. Chronic liver disease in Aboriginal North Americans. World J Gastroenterol. 2008;14:4607–4615. doi: 10.3748/wjg.14.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores YN, Yee HF, Jr, Leng M, et al. Risk factors for chronic liver disease in Blacks, Mexican Americans, and Whites in the United States: results from NHANES IV, 1999-2004. Am J Gastroenterol. 2008;103:2231–2238. doi: 10.1111/j.1572-0241.2008.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 21.United States Census Bureau. American community survey [Internet] United States Census Bureau: 2010. [cited 2011 Dec 21]. Available from: http://www.census.gov/acs/www/ [Google Scholar]
- 22.Sheikh MY, Mouanoutoua M, Walvick MD, et al. Prevalence of hepatitis B virus (HBV) infection among Hmong immigrants in the San Joaquin Valley. J Community Health. 2011;36:42–46. doi: 10.1007/s10900-010-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Torres M. Latinos and chronic hepatitis C: a singular population. Clin Gastroenterol Hepatol. 2008;6:484–490. doi: 10.1016/j.cgh.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Torres M, Jeffers LJ, Sheikh MY, et al. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis C. N Engl J Med. 2009;360:257–267. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- 25.Fridey JL, Townsend MJ, Kessler DA, Gregory KR. A question of clarity: redesigning the American Association of Blood Banks blood donor history questionnaire: a chronology and model for donor screening. Transfus Med Rev. 2007;21:181–204. doi: 10.1016/j.tmrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Glynn SA, Kleinman SH, Schreiber GB, et al. Retrovirus Epidemiology Donor Study (REDS) Trends in incidence and prevalence of major transfusion-transmissible viral infections in US blood donors, 1991 to 1996. JAMA. 2000;284:229–235. doi: 10.1001/jama.284.2.229. [DOI] [PubMed] [Google Scholar]
- 27.Busch MP, Young MJ, Samson SM, Mosley JW, Ward JW, Perkins HA The Transfusion Safety Study Group. Risk of human immunodeficiency virus (HIV) transmission by blood transfusions before the implementation of HIV-1 antibody screening. Transfusion. 1991;31:4–11. doi: 10.1046/j.1537-2995.1991.31191096183.x. [DOI] [PubMed] [Google Scholar]
- 28.Zuck TF. Transfusion-transmitted AIDS reassessed. N Engl J Med. 1988;318:511–512. doi: 10.1056/NEJM198802253180809. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. NHANES 2001-2002 public data general release file documentation [Internet] Centers for Disease Control and Prevention; 2004. [cited 2011 Dec 21]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/general_data_release_doc.pdf. [Google Scholar]
- 30.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastiaans MJ, Nath N, Dodd RY, Barker LF. Hepatitis-associated markers in the American Red Cross volunteer blood donor population. IV. A comparison of HBV-associated serologic markers in HBsAg-positive first-time and repeat blood donors. Vox Sang. 1982;42:203–210. doi: 10.1111/j.1423-0410.1982.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 32.Daniels D, Grytdal S, Wasley A Centers for Disease Control and Prevention (CDC) Surveillance for acute viral hepatitis: United States, 2007. MMWR Surveill Summ. 2009;58:1–27. [PubMed] [Google Scholar]
- 33.Coleman PJ, McQuillan GM, Moyer LA, Lambert SB, Margolis HS. Incidence of hepatitis B virus infection in the United States, 1976-1994: estimates from the National Health and Nutrition Examination Surveys. J Infect Dis. 1998;178:954–959. doi: 10.1086/515696. [DOI] [PubMed] [Google Scholar]
- 34.Murphy EL, Bryzman S, Williams AE, et al. Demographic determinants of hepatitis C virus seroprevalence among blood donors. JAMA. 1996;275:995–1000. [PubMed] [Google Scholar]
- 35.Zou S, Notari EP, 4th, Stramer SL, Wahab F, Musavi F, Dodd RY. Patterns of age- and sex-specific prevalence of major blood-borne infections in United States blood donors, 1995 to 2002: American Red Cross blood donor study. Transfusion. 2004;44:1640–1647. doi: 10.1111/j.0041-1132.2004.04153.x. [DOI] [PubMed] [Google Scholar]
- 36.Murphy EL, Fang J, Tu Y, et al. Hepatitis C virus prevalence and clearance among US blood donors, 2006-2007: associations with birth cohort, multiple pregnancies, and body mass index. J Infect Dis. 2010;202:576–584. doi: 10.1086/654882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiley TE, Brown J, Chan J. Hepatitis C infection in African Americans: its natural history and histological progression. Am J Gastroenterol. 2002;97:700–706. doi: 10.1111/j.1572-0241.2002.05555.x. [DOI] [PubMed] [Google Scholar]
- 38.Muir AJ, Bornstein JD, Killenberg PG Atlantic Coast Hepatitis Treatment Group. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 39.Neumeister AS, Pilcher LE, Erickson JM, et al. Hepatitis-C prevalence in an urban native-American clinic: a prospective screening study. J Natl Med Assoc. 2007;99:389–392. [PMC free article] [PubMed] [Google Scholar]
- 40.Singh GK, Hoyert DL. Social epidemiology of chronic liver disease and cirrhosis mortality in the United States, 1935-1997: trends and differentials by ethnicity, socioeconomic status, and alcohol consumption. Hum Biol. 2000;72:801–820. [PubMed] [Google Scholar]
- 41.McMahon BJ, Hennessy TW, Christensen C, et al. Epidemiology and risk factors for hepatitis C in Alaska natives. Hepatology. 2004;39:325–332. doi: 10.1002/hep.20046. [DOI] [PubMed] [Google Scholar]
- 42.Solomon L, Flynn C, Muck K, Vertefeuille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81:25–37. doi: 10.1093/jurban/jth085. [DOI] [PMC free article] [PubMed] [Google Scholar]