Abstract
The mucosa of the gastrointestinal (GI) tract exhibits hydrophobic, nonwettable properties that protect the underlying epithelium from gastric acid and other luminal toxins. These biophysical characteristics appear to be attributable to the presence of an extracellular lining of surfactant-like phospholipids on the luminal aspects of the mucus gel layer. Phosphatidylcholine (PC) represents the most abundant and surface-active form of gastric phospholipids. PC protected experimental rats from a number of ulcerogenic agents and/or conditions including nonsteroidal anti-inflammatory drugs (NSAIDs), which are chemically associated with PC. Moreover, preassociating a number of the NSAIDs with exogenous PC prevented a decrease in the hydrophobic characteristics of the mucus gel layer and protected rats against the injurious GI side effects of NSAIDs while enhancing and/or maintaining their therapeutic activity. Bile plays an important role in the ability of NSAIDs to induce small intestinal injury. NSAIDs are rapidly absorbed from the GI tract and, in many cases, undergo enterohepatic circulation. Thus, NSAIDs with extensive enterohepatic cycling are more toxic to the GI tract and are capable of attenuating the surface hydrophobic properties of the mucosa of the lower GI tract. Biliary PC plays an essential role in the detoxification of bile salt micelles. NSAIDs that are secreted into the bile injure the intestinal mucosa via their ability to chemically associate with PC, which forms toxic mixed micelles and limits the concentration of biliary PC available to interact with and detoxify bile salts. We have worked to develop a family of PC-associated NSAIDs that appear to have improved GI safety profiles with equivalent or better therapeutic efficacy in both rodent model systems and pilot clinical trials.
Keywords: Phosphatidylcholines, Non-steroidal anti-inflammatory agents, Phospholipids, Bile
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for their anti-inflammatory, analgesic, and antipyretic effects. The limitation of NSAID usage is related to their major side effect on the gastrointestinal (GI) tract which can result in bleeding, intestinal perforation and significant morbidity and mortality. Over 30% to 60% of aspirin or NSAID users have a GI intolerance.1 GI side effects of NSAIDs are partly due to the ability of NSAIDs to inhibit cyclooxygenase-I (COX-1) that is constitutively expressed in the GI mucosa and is important for the production of "cytoprotective" prostaglandins which are necessary for normal homeostasis. NSAIDs known as coxibs were developed to selectively inhibit COX-2 that is associated with inflammatory processes, and spare the protective COX-1.1,2 However, the utilization of coxibs as GI-safer NSAIDs has been curtailed due to linkage of their use to life-threatening cardiovascular disease.2 Although it is clear that the GI side effects of NSAIDs are in part attributable to their ability to inhibit the biosynthesis of gastroprotective prostaglandins, a significant amount of evidence exists that NSAIDs induce GI ulcers and complications such as bleeding, perforation and stricture by a prostaglandin-independent mechanism.1,3
NSAIDs are amphiphilic molecules that are capable of strong association with phospholipids.3-11 NSAIDs disrupt extracellular layers, possibly a phospholipid monolayer, covering the mucous gel, contributing to GI injury.3 Our lab has provided insight into the molecular basis about this NSAID-induced biophysical transformation for the past 25 years. We demonstrated that the nonwettable hydrophobic properties of the GI surface layer are associated with the synthesis and secretion of surfactant-like phospholipids, such as phosphatidylcholine (PC).11 We have presented evidence that NSAIDs chemically interact with and associate with PC, thereby disrupting the mucosa's surface barrier properties.10 In addition, PC can provide significant protection against NSAID-induced GI injury and bleeding in both rodent model systems3 and pilot clinical trials.12,13 In the review that follows, we briefly present this PC-related pathophysiologic mechanism of NSAID-induced GI injury and its preventive and therapeutic strategy, based upon our accumulated research findings.
PHOSPHATIDYLCHOLINE AND THE HYDROPHOBIC LAYER OF THE GI TRACT
GI mucosal protection against injurious chemicals in the gut lumen is, in part, conferred by a surface barrier that is caused by the presence of a firmly adherent mucous gel that forms an unstirred layer between the luminal contents and the epithelium.14-22 This mucus gel layer has a thickness that ranges from 50 to 500 µm and has a uniquely nonwettable or hydrophobic luminal surface property that can be measured by contact angle analysis.3,4 We have performed a series of studies that suggests that this biophysical property originates from an extracellular lining of surfactant-like phospholipids, possibly a monolayer coating the surface of the mucus gel layer.4,5,11 Furthermore these phospholipids may be synthesized stored in a unique organelle of surface mucous cells and released in response to "cytoprotective" prostaglandins.15-18 Our lab has obtained evidence that a number of damaging agents (e.g., NSAIDs, Helicobacter pylori, bile salts) rapidly attenuate the hydrophobic properties of the GI mucosa of humans and laboratory animals, whereas this nonwettable characteristic appears to be fortified by protective agents, such as prostaglandins.3,8,21-27
As an example, our previous studies have shown that the PC-based hydrophobic surface layer of the GI mucosa is compromised after lipopolysaccharide (LPS) treatment.28-31 This loss of barrier integrity can lead to back-diffusion of injurious luminal substances such as acid, enzymes, and bacteria and be an enhancing factor in LPS-induced systemic inflammation. Therefore, maintenance of the GI barrier is desirable and can be a focus for therapeutic agents. Our permeability studies showed that an increase in gastric and intestinal phospholipase A2 was damaging, by degrading PC.28,30 In contrast, exogenous PC derived from purified soy lecithin was quite effective at preventing this mode of LPS injury, likely by enhancing the mucosal barrier.29
BILIARY PHOSPHATIDYLCHOLINE
Bile salts are present in human bile at concentrations ranging from 10 to 40 mM. Above their critical micellar concentration (~2 to 5 mM), bile salts can form micelles and act as detergents that can dissolve membrane lipids. Even below their critical micellar concentration, bile salt monomers can partition into membranes and exert damage.7,32,33 However, native bile is not normally damaging to the mucosal lining of the GI tract, suggesting there are mechanisms to protect the biliary and GI tract from these detergent effects of bile salts. Earlier studies suggest that PC, the major phospholipid in bile, may prevent bile salt-induced injury by promoting formation of less toxic mixed micelles and/or by enhancing/restoring the mucosa's hydrophobic properties.5,7
Biliary PC plays a physiologically important role in reducing the cytotoxic activity of bile salts, most likely by forming mixed micelles. Human bile normally contains PC at substantial concentrations and this phospholipid represents as much as 40% of its organic constitutents.33 Biliary PC mainly consists of unsaturated palmitoyl-linoleoyl PC. Our laboratory has shown that the PC in bile is crucial for protection of GI mucosal tissue from the membrane-disruptive actions of bile salts.7 One of the means by which this PC protection is accomplished is proposed to be through promotion of mixed micelles of bile salts and PC which are much less toxic, compared to pure bile acid micelles or monomers. Our study suggests that both saturated and unsaturated PCs protect erythrocytes, intestinal cells, and liposomes against bile salt toxicity.32,34-36 We also determined that unsaturated PC is even more effective than saturated PC in protecting cells and membranes against attack by bile salt.32
NSAID INDUCED SMALL INTESTINAL INJURIES AND BILE
The injurious effect of NSAIDs in the small intestine was not appreciated until the widespread use of capsule endoscopy. It is estimated that 50% to 80% of regular NSAID users develop small intestinal injury which is more common than NSAID-induced injury in the gastroduodenal mucosa.37,38 Recently, both prospective wireless capsule endoscopic studies and retrospective/epidemiological analyses showed that chronic aspirin consumption, much of which is enteric-coated aspirin, may also be associated with injury to the lower gut and be one of the main contributors to small bowel ulceration, hemorrhage, and strictures.39,40 The ability of aspirin and NSAIDs to inhibit activities of COX contributes to the drugs' cytotoxicity in the GI tract. However, many studies found that COX-independent mechanisms mainly contribute to NSAID cytotoxicity in the GI tract, especially small intestine.41-43
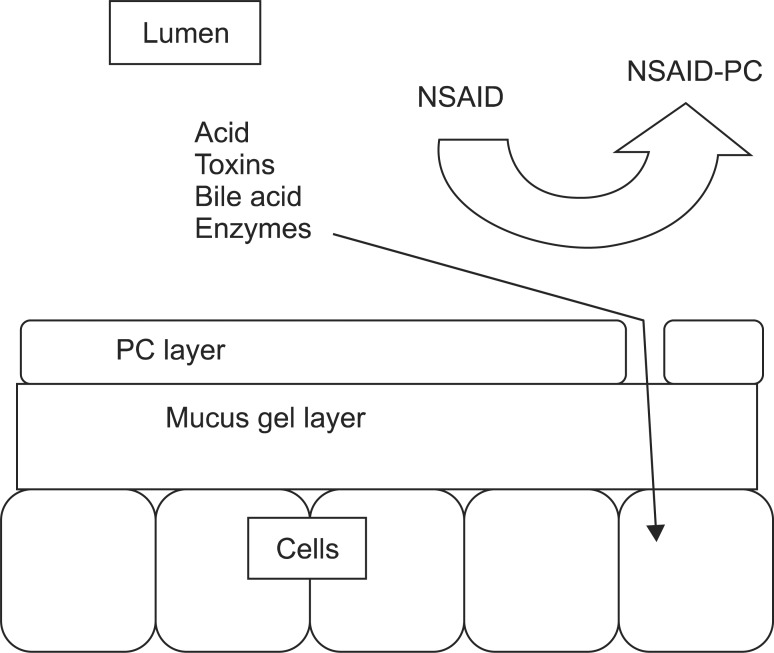
NSAIDs induce injury to the GI tract by a multifactorial process involving inhibition of both COX-1 and COX-2, topical injury to the mucosa, inhibition of local blood flow, leukocyte activation/adhesion to the endothelium and the induction of the apoptotic pathway of epithelial cells.1 NSAIDs may induce barrier disruption, in part, by chemically associating with and destabilizing PC from the surface of the mucous gel layer, thereby reducing the tissues' protective hydrophobic lining and making the mucosa more susceptible to injury. A schematic model of how topically applied NSAIDs can interact with and destabilize an extracellular phospholipid lining is depicted in Fig. 1.
Fig. 1.
Schematic model depicting the role of the extracellular lining of the phosphatidylcholine (PC) in generating a hydrophobic barrier in the gastrointestinal tract against aggressive luminal agents, as well as the mechanism by which nonsteroidal anti-inflammatory drugs (NSAIDs) may compromise the surface barrier by disrupting the PC layer allowing luminal agents access to the epithelium.
NSAIDs that are mainly amphiphiles can affect the stability of the GI mucosa by partitioning into both the extracellular and membrane phospholipid layers and inducing an alteration in their physical properties, which may ultimately lead to the formation of membrane pores in the mucosa to act as low resistance pathways for the back-diffusion of luminal acid.44-46 Amphiphilic NSAIDs have been shown to induce remarkable changes in the properties of phospholipid membranes with regards to changes in hydrophobicity, fluidity, thickness, bending stiffness, permeability, and pore formation. This concept may not only provide insight as to how NSAIDs may topically injure the GI mucosa by a mechanism independent from COX inhibition, but also may provide a new direction toward designing a novel class of NSAIDs that will have fewer side effects while retaining their therapeutic activity.47
Animal studies demonstrated that bile salts, which enter the small bowel as a component of the enterohepatic circulation, play an important role in the ability of NSAIDs to induce small intestinal injury, regardless of route of administration. The contribution of bile to the pathogenic process was highlighted by the finding that the ability of indomethacin (Indo) to induce severe injury and perforation to the distal small bowel of rats was prevented by bile duct ligation, which halted secretion of bile acids into the small intestine.48 A striking correlation between the ability of an NSAID to be secreted into the bile and its efficacy to induce small intestinal injury in rodent model systems has been found.49
The ability of combinations of bile salts and NSAIDs to induce injury has also been demonstrated in cell cultures derived from GI epithelial tissue. Because the individual toxicity of amphiphilic bile salts and NSAIDs directly correlates with their interactions with phospholipid membranes, the presence of both NSAIDs and bile salts alters their individual physicochemical properties and enhances the disruptive effect on cell membranes and overall cytotoxicity. Utilizing in vitro gastric AGS and intestinal IEC-6 cells, our lab found that combinations of bile acid, deoxycholic acid, taurodeoxycholic acid, glycodeoxycholic acid, and the NSAID Indo significantly increased cell plasma membrane permeability and became more cytotoxic than these agents alone.36 Indo associates with both bile salts and PC and promotes the potential formation of bile salt/NSAID complexes within either biomembranes or bile salt-lipid mixed micelles in the GI lumen, which leads to membrane disruption. We demonstrated increased cytotoxicity of combinations of bile salt and NSAID and provided a molecular mechanism for the observed toxicity. This mechanism potentially contributes to the NSAID-induced injury in the small bowel.
In summary, we have confirmed that NSAID-induced lower gut injury is dependent on the secretion of specific NSAIDs into the bile thereby altering the balance between the concentrations of damaging bile salts and protective PC/lecithin in bile.
With this information in hand, we reasoned that this ability of aspirin and other NSAIDs to topically injure the mucosa may be prevented if the drug was chemically pre-associated with synthetic or organic (soybean) PC. Accordingly, preclinical studies on experimental animals50-55 and clinical studies11,12 have demonstrated a remarkable reduction in the ability of a number of NSAIDs to induce GI ulceration and bleeding when the NSAIDs were chemically associated with PC before intragastric administration.
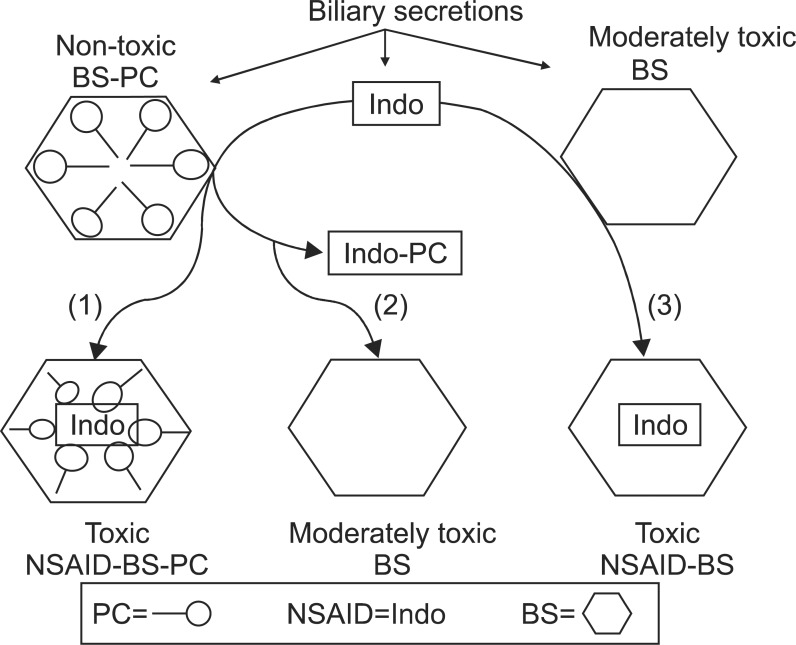
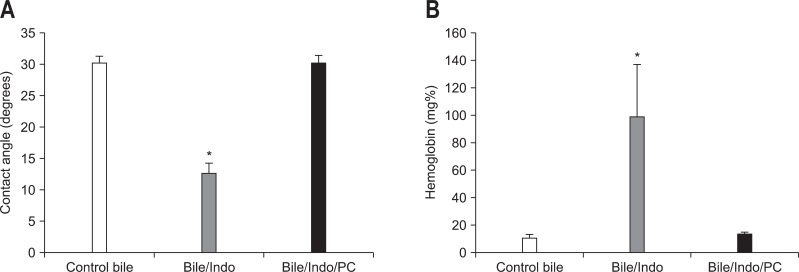
As schematically depicted in Fig. 2, secretion of NSAIDs into the bile abrogates the protective property of PC, possibly due to affinity of NSAIDs to chemically interact with PC. This interaction could either transform mixed micelles back into cytotoxic pure bile salt micelles (path 2) or form a more complex bile salt/NSAID-PC mixed micelle with cytotoxic properties (path 1). Still another possibility is that the NSAID can interact with pure bile salt micelles (path 3) to form toxic NSAID/bile salt micelles. In one of our earlier studies,7 bile collected from control rats had a negligible effect on the surface hydrophobicity of the ileal mucosa (Fig. 3). On the other hand, when bile from animals that had received one dose of Indo was used, the contact angle of the ileal mucosa decreased significantly over the 30-minute exposure period. The hydrophobicity of the mucosa was restored to control levels when the pretreated bile was sonicated with PC. The hemoglobin (Hb) concentration present in the ileal loop fluid collected from rats in the above groups is also shown in Fig. 3. There was a significant increase in the amount of Hb in the ileal loop when the tissue was exposed to bile from an Indo-pretreated rat. Again, this damage was completely reversed by the addition of PC to the sample. Similar to our observations with ileal surface hydrophobicity, the presence of Hb in the terminal ileal loop fluid, as an index of intestinal bleeding, was increased by the addition of sodium deoxycholate to the instillate; this damaging action was individually blocked by PC.7 Furthermore, the protective effect of PC against bile salts-induced ileal injury was partially reversed if the NSAID was added to the mixture.
Fig. 2.
Nonsteroidal anti-inflammatory drugs (NSAIDs) interact directly with the bile salts (BS) and phosphatidylcholine (PC) of pure and mixed micelles, converting the macromolecules into more toxic micelles. It is hypothesized that biliary secretions include nontoxic BS/PC micelles that can interact with secreted indomethacin (Indo) to yield either of the following: 1) toxic mixed micelles or 2) moderately toxic pure bile salt micelles where the PC has been removed to interact with Indo. Furthermore, an additional still another possibility is for that secreted pure bile salt micelles interact with Indo to form, 3) toxic Indo/bile salt micelles devoid of PC.
Fig. 3.
Protective effect of phosphatidylcholine (PC) against small intestinal injury induced by bile and indomethacin (Indo). The contact angle (A) of the ileal mucosa as an index of surface injury and the presence of hemoglobin (B) in the terminal ileal loop fluid as an index of intestinal bleeding were decreased and increased, respectively, by the addition of rat bile from an Indo-treated animal. This damaging action was reversed by the addition of PC.
*p<0.05 vs control and bile/Indo/PC.
NSAIDs at therapeutic concentrations are not toxic to the intestinal mucosa, and only become so in the presence of bile salts. This concept is also supported by our in vitro experiments on erythrocytes, in which Indo by itself did not cause hemolysis.32 However, the NSAID did partially block the protective effect of PC against the hemolytic effect of sodium deoxycholate. Our findings that this block could be overcome if we doubled the concentration of the phospholipid in the in vitro system supports the concept that the bile salts and NSAID compete for a limited number of biliary PC molecules.
PHOSPHATIDYLCHOLINE-NSAIDs
Over the past decade a number of pharmaceutical companies have developed and commercialized coxibs, as a safer alternative to conventional NSAIDs to treat pain and inflammation. These drugs have been designed to selectively inhibit COX-2 which is induced at sites of inflammation, and spare the COX-1 isoform that is constituitively expressed in the mucosa of the GI tract and plays a rate limiting role in the biosynthesis of cytoprotective prostaglandins and susceptibility to injury. However, the chronic use of these drugs has been limited due to cardiovascular concerns.2 Other approaches to protection against GI side effects of NSAIDs have been to add acid-inhibitory pharmaceuticals, such as proton pumps inhibitors (i.e., omeprazole) or H2 receptor antagonists (i.e., ranitidine, famotidine).56
Our approach of maintaining the surface barrier of the GI tract is quite different from the conventional approaches taken by the pharmaceutical industry. We have been developing a family of PC-associated NSAIDs, which appear to have improved GI safety with equivalent or better therapeutic efficacy.12,49-51
It is well established that certain NSAIDs such as Indo are readily excreted into the bile and enter the enterohepatic circulation, resulting in high concentrations of NSAID in the liver and bile. We showed that NSAIDs chemically associate with PC and this process can occur in the GI tract where the NSAID induced loss of surface-protective PC results in injury to the mucosa. We proposed that biliary PC normally protects hepatic and intestinal epithelia from bile salt induced injury, but in the presence of NSAIDs excreted into the bile, the defensive actions of PC are compromised. We showed that a submicellar concentration of the bile salt was toxic to hepatocytes and that this toxicity was overcome by mixing the bile salts with PC before exposure of the cells. In the presence of Indo, which is avidly taken up by the liver and excreted into bile, there is a tendency for the NSAID to associate with PC and bile salts in the mixed micelles, which may result in the formation of more toxic micelles. These NSAID-induced macromolecular changes will result in bile becoming more toxic to cells and membranes. This scheme thus supports the concept that one way to decrease the toxicity of NSAIDs is by preassociating the NSAID with PC. The presence of PC in bile converts simple bile salt micelles and/or monomers into mixed micelles, suppressing the bile salts from exerting their membrane disruptive actions while concentrated in the hepatic ducts and gall bladder. In addition to PC protection of epithelia from bile salts, PC also has protective effects against the topical actions of NSAIDs that are seen at the systems level. In the whole animal, the NSAID, Indo, produces GI bleeding, ulcerations and intestinal adhesions, depending on dosage. These injuries can be prevented by preassociation of Indo with PC. Thus, the PC-NSAIDs were developed as drugs that would be safer for the upper and lower GI tract.
ASPIRIN-PHOSPHATIDYLCHOLINE
Aspirin is effective not only in inhibiting fever, pain, and inflammation, but also in reducing the risk of developing heart disease, stroke, thrombosis, colon cancer, and Alzheimer's disease.57 Aspirin is a member of the NSAID family and blocks the formation of prostaglandins by inhibiting the rate-limiting enzyme, COX.
We have shown that aspirin can induce gastric injury in transgenic mice deficient in either COX-1 or COX-2, suggesting that a simple inhibition of COX-1 activity cannot provide the only explanation for NSAID-induced gastric pathogenesis.58 It is interesting to note that challenge with aspirin/HCl resulted in a significant increase in COX-2 mRNA, protein, and PGE2 concentrations in COX-1-null mice.58 This finding provides evidence for a role of the COX-2 isoform in the maintenance of mucosal prostanoid levels and repair, even in the presence of a COX-1-selective NSAID, such as aspirin. We also presented evidence that the linkage between gastric ulceration induced by aspirin/HCl and gastric prostaglandin concentration was more consistent with the possibility that COX plays a role in the mechanism of recovery from NSAID injury, rather than in its pathogenesis. In contrast, the inverse association between NSAID-induced gastric injury and mucosal surface hydrophobicity is consistent with the possibility that NSAIDs topically injure the mucosa by increasing the tissue's wettability to luminal acid. The role of surface phospholipids in the mechanism by which aspirin disrupts the gastric mucosal barrier was further supported by evidence that aspirin-induced gastric injury in COX-1 knockout mice could be prevented if the animals were administered aspirin-PC.58
Aspirin-induced injury to the gastric mucosa is markedly reduced or completely abolished if aspirin is chemically associated with the phospholipid, PC.43 The mechanism of mucosal protection provided by this compound is not related to any alteration in the ability of aspirin to inhibit mucosal COX activity. We believe this protection is attributable to the maintenance of the defensive hydrophobic barrier of the gastric mucosa. We also observed that the ability of daily aspirin administration to retard the healing of experimentally induced gastric ulcers could be overcome if rats were treated with PC-associated aspirin.52 Related studies using animal models of fever and inflammation indicate that PC-associated NSAIDs had equal or enhanced therapeutic activity, compared with the use of NSAID alone.3,55
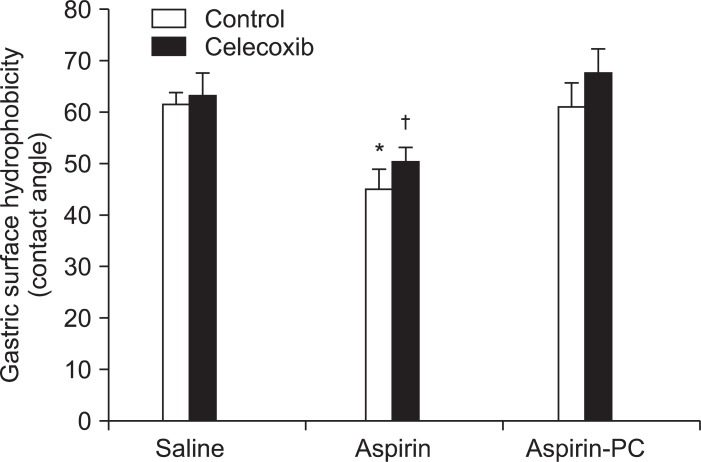
Clinical studies demonstrated that aspirin consumption reverses the GI benefits of coxibs, by an undefined mechanism.59 Rodent models were employed to investigate the effects of combinations of celecoxib and aspirin on gastric ulcerogenesis, bleeding, surface hydrophobicity (by contact angle analysis), and ulcer healing.60 We also evaluated the effects of PC-associated aspirin in these rodent models and confirmed its COX-inhibitory activity by measuring mucosal prostaglandin E2 (PGE2) concentration. Aspirin's ability to induce gastric injury and bleeding in rats, was exacerbated in the presence of a coxib and was dependent on its ability to reduce gastric surface hydrophobicity. In contrast, co-administration of PC-associated aspirin and celecoxib induced little or no gastric injury/bleeding and maintained the stomach's hydrophobic properties (Fig. 4). Interestingly, aspirin and aspirin-PC equally inhibited gastric mucosal PGE2 concentration. Aspirin in combination with a coxib retarded the healing of experimentally induced gastric ulcers, whereas healing rates of rats treated with celecoxib in combination with aspirin-PC were comparable to controls. If aspirin's ability to attenuate the surface barrier is an important component in the pathogenic mechanism, then how do coxibs further exacerbate this process? There is evidence from a number of laboratories that COX-2 is induced at sites of mucosal injury and that coxibs delay ulcer healing.61,62 These findings, which were further corroborated by our study, suggest that the ability of coxibs to disrupt the mucosal repair process is an important element in the pathogenic mechanism when these two classes of NSAIDs are administered in combination. Interestingly, when the surface barrier is maintained by the administration of PC-associated aspirin, the delay in ulcer healing with coxibs is no longer observed. These findings thereby suggest a potential therapeutic role for PC-associated aspirin and perhaps other PC-NSAIDs in both the prevention of mucosal injury and treatment of preexistent ulcers, when patients are placed on regimens that require the concomitant use of both a coxib and a conventional NSAID. This postulated mechanism, which would be independent of COX inhibition, is based upon the ability of aspirin (and other conventional NSAIDs) to attenuate the hydrophobic surface barrier of the GI tract.
Fig. 4.
Acute effects of orally administered celecoxib, aspirin, and aspirin-phosphatidylcholine (PC), either alone and or in combination on gastric mucosal surface hydrophobicity.
*p<0.01 vs saline, celecoxib, aspirin-PC, and celecoxib+aspirin-PC; †p<0.01 vs celecoxib+aspirin-PC.
Upper GI tract injury was prospectively evaluated in healthy human subjects 50 to 75 years of age at risk for developing aspirin-induced GI damage using a noncovalent complex of aspirin-PC (PL2200; PLx Pharma Inc., Houston, TX, USA).49 This agent and other PC-NSAIDs are under development by PLx Pharma Inc. PL2200 has equivalent antiplatelet efficacy to aspirin while reducing gastroduodenal ulcers by 70% when compared with aspirin. We demonstrated that local mechanisms of GI protection are important as aspirin's preassociation with surface-active phospholipids significantly reduced mucosal damage. PL2200 may be an attractive strategy in at-risk patients to reduce aspirin-induced GI injury.12
In recent studies, we have demonstrated in a rodent model system that intraduodenally administered aspirin induces mucosal injury, an increase in vascular permeability and intraluminal bleeding of the proximal small bowel that appears to be dependent on the presence of luminal bile salts.43 This aspirin-induced intestinal injury/bleeding can be abrogated by the intraduodenal administration of aspirin-PC (PL2200). The findings support the case that the presence of aspirin in the intestinal lumen as likely occurs with enteric coated aspirin does induce mucosal ulceration/bleeding of the lower gut, most likely due to a surface injurious action that is aggravated by bile acids and reversed by PC.43,49
IBUPROFEN-PHOSPHATIDYLCHOLINE
Ibuprofen was selected as a representative NSAID to associate with PC to examine its GI safety in a longer (6 weeks) study, as it is commonly used by osteoarthritis patients and is known to induce reproducible therapeutic efficacy and gastroduodenal injury when administered at an antiarthritic dose of 2,400 mg/day.13,55,63
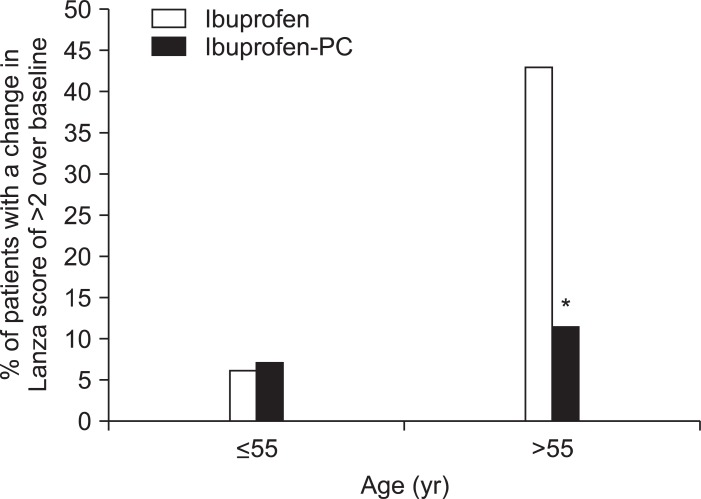
This Phase II trial was designed to evaluate the therapeutic efficacy and GI safety of ibuprofen-PC (PL1100; PLx Pharma Inc.) versus ibuprofen at therapeutic doses to treat osteoarthritis. Ibuprofen-PC was similar to ibuprofen with regard to both bioavailability and efficacy to treat arthritis symptoms, while reducing the NSAID's ability to induce gastroduodenal erosions and ulcers. However, in this total evaluable population, the GI protective effect did not reach statistical significance. Yet, ibuprofen-PC was safer in patients aged >55 years (with a mean age of 64 years) who are most at risk of developing NSAID-induced gastroduodenal injury. In this clinically relevant subgroup, ibuprofen treated patients had a significantly greater absolute change in Lanza scores and were 3.7 times more likely (or a 270% increased risk) to develop multiple gastroduodenal erosions (Lanza score >2) than the ibuprofen-PC-treated patients (Fig. 5). Ibuprofen-PC was well tolerated with no marked changes in the incidence of adverse events, compared with ibuprofen.
Fig. 5.
Comparison of patients less than or greater than 55 years of age in terms of their responses to ibuprofen or ibuprofen-phosphatidylcholine (PC). Younger subjects had fewer ibuprofen-associated gastroduodenal lesions than those >55 years. Those over 55 years taking ibuprofen were 3.7 times more likely to develop multiple gastroduodenal erosions than those taking an equivalent nonsteroidal anti-inflammatory drug (NSAID) dose of ibuprofen-PC. In brief, Lanza scores are broken down into the following values: 0, normal mucosa; 1, mucosal hemorrhages only; 2, one or two erosions; 3, three to ten erosions; 4, greater than 10 erosions or an ulcer (defined as a mucosal break with a diameter of 3 mm or more, with depth).
*p<0.02 vs ibuprofen.
The mechanism of age-related NSAID-induced GI toxicity remains unknown. However, age associated decreases in surface hydrophobicity, prostaglandin levels and impaired healing may contribute to the deterioration of the barrier property of the mucosa. Interestingly, Hackelsberger et al.64 observed an age related decrease in surface hydrophobicity in the antrum of the stomach.
INDO-PHOSPHATIDYLCHOLINE
Indo is possibly the most potent and GI-injurious of the NSAIDs, so that its use is limited to short-term circumstances. Yet because of its potency, it would be more useful clinically if the GI side effects could be reduced. We have shown that an Indo-PC formulation can be used parenterally and effectively to inhibit inflammation and pain, with significantly less GI bleeding and injury.50
CONCLUSIONS
NSAIDs are widely used for their anti-inflammatory, analgesic, and antipyretic effects. The limitation of NSAIDs usage is related to the GI side effect. Although GI side effects of NSAIDs are in part attributable to their property of COX-1 inhibition, we have devoted considerable effort to presenting evidence that NSAIDs induce surface damage to the mucosa and chemically interact with extracellular or membrane PC, thereby disrupting the mucosal barrier properties, which would be independent of COX inhibition.
PC-association provides significant protection against NSAID-induced GI injury in both rodent model systems and pilot clinical trials. We are developing a family of PC-associated NSAIDs, which appear to have improved GI safety and equivalent or better therapeutic efficacy.
Footnotes
Dr. Lichtenberger is a consultant and stockholder with PLx Pharma Inc. of Houston, Texas, a company that is developing the PC-NSAID class of drugs. Dr. Dial holds stock options at PLx Pharma. Dr. Lim has no conflicts to disclose.
References
- 1.Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39:433–464. doi: 10.1016/j.gtc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Watson DJ, Harper SE, Zhao PL, Quan H, Bolognese JA, Simon TJ. Gastrointestinal tolerability of the selective cyclooxygenase-2 (COX-2) inhibitor rofecoxib compared with nonselective COX-1 and COX-2 inhibitors in osteoarthritis. Arch Intern Med. 2000;160:2998–3003. doi: 10.1001/archinte.160.19.2998. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenberger LM, Wang ZM, Romero JJ, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenberger LM. Where is the evidence that cyclooxygenase inhibition is the primary cause of nonsteroidal anti-inflammatory drug (NSAID)-induced gastrointestinal injury? Topical injury revisited. Biochem Pharmacol. 2001;61:631–637. doi: 10.1016/s0006-2952(00)00576-1. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenberger LM, Romero JJ, Gibson GW, Blank MA. Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Dig Dis Sci. 2000;45:1792–1801. doi: 10.1023/a:1005574009856. [DOI] [PubMed] [Google Scholar]
- 7.Barrios JM, Lichtenberger LM. Role of biliary phosphatidylcholine in bile acid protection and NSAID injury of the ileal mucosa in rats. Gastroenterology. 2000;118:1179–1186. doi: 10.1016/s0016-5085(00)70371-4. [DOI] [PubMed] [Google Scholar]
- 8.Giraud MN, Motta C, Romero JJ, Bommelaer G, Lichtenberger LM. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: a possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs) Biochem Pharmacol. 1999;57:247–254. doi: 10.1016/s0006-2952(98)00303-7. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenberger LM, Ulloa C, Romero JJ, Vanous AL, Illich PA, Dial EJ. Nonsteroidal anti-inflammatory drug and phospholipid prodrugs: combination therapy with antisecretory agents in rats. Gastroenterology. 1996;111:990–995. doi: 10.1016/s0016-5085(96)70066-5. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenberger LM, Zhou Y, Jayaraman V, et al. Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: characterization of interaction of NSAIDs with phosphatidylcholine. Biochim Biophys Acta. 2012;1821:994–1002. doi: 10.1016/j.bbalip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kao YC, Lichtenberger LM. Localization of phospholipid-rich zones in rat gastric mucosa: possible origin of a protective hydrophobic luminal lining. J Histochem Cytochem. 1987;35:1285–1298. doi: 10.1177/35.11.2443559. [DOI] [PubMed] [Google Scholar]
- 12.Anand BS, Romero JJ, Sanduja SK, Lichtenberger LM. Phospholipid association reduces the gastric mucosal toxicity of aspirin in human subjects. Am J Gastroenterol. 1999;94:1818–1822. doi: 10.1111/j.1572-0241.1999.01211.x. [DOI] [PubMed] [Google Scholar]
- 13.Lanza FL, Marathi UK, Anand BS, Lichtenberger LM. Clinical trial: comparison of ibuprofen-phosphatidylcholine and ibuprofen on the gastrointestinal safety and analgesic efficacy in osteoarthritic patients. Aliment Pharmacol Ther. 2008;28:431–442. doi: 10.1111/j.1365-2036.2008.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenberger LM. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 1999;15:463–472. doi: 10.1097/00001574-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kao YC, Goddard PJ, Lichtenberger LM. Morphological effects of aspirin and prostaglandin on the canine gastric mucosal surface. Analysis with a phospholipid-selective cytochemical stain. Gastroenterology. 1990;98:592–606. doi: 10.1016/0016-5085(90)90278-9. [DOI] [PubMed] [Google Scholar]
- 16.Goddard PJ, Kao YC, Lichtenberger LM. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990;98:361–370. doi: 10.1016/0016-5085(90)90826-m. [DOI] [PubMed] [Google Scholar]
- 17.Dial EJ, Lichtenberger LM. Surface hydrophobicity of the gastric mucosa in the developing rat. Effects of corticosteroids, thyroxine, and prostaglandin E2. Gastroenterology. 1988;94:57–61. doi: 10.1016/0016-5085(88)90609-9. [DOI] [PubMed] [Google Scholar]
- 18.Kao YC, Lichtenberger LM. Phospholipid- and neutral lipid-containing organelles of rat gastroduodenal mucous cells. Possible origin of the hydrophobic mucosal lining. Gastroenterology. 1991;101:7–21. doi: 10.1016/0016-5085(91)90454-s. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenberger LM, Romero JJ, Kao YC, Dial EJ. Gastric protective activity of mixtures of saturated polar and neutral lipids in rats. Gastroenterology. 1990;99:311–326. doi: 10.1016/0016-5085(90)91011-t. [DOI] [PubMed] [Google Scholar]
- 20.Kao YC, Lichtenberger LM. A method to preserve extracellular surfactant-like phospholipids on the luminal surface of rodent gastric mucosa. J Histochem Cytochem. 1990;38:427–431. doi: 10.1177/38.3.1689341. [DOI] [PubMed] [Google Scholar]
- 21.Ottlecz A, Romero JJ, Lichtenberger LM. Helicobacter infection and phospholipase A2 enzymes: effect of Helicobacter felis-infection on the expression and activity of sPLA2 enzymes in mouse stomach. Mol Cell Biochem. 2001;221:71–77. doi: 10.1023/a:1010971910866. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenberger LM, Dial EJ, Ottlecz A, Romero JJ, Lechago J, Fox JG. Attenuation of hydrophobic phospholipid barrier is an early event in Helicobacter felis-induced gastritis in mice. Dig Dis Sci. 1999;44:108–115. doi: 10.1023/a:1026610418663. [DOI] [PubMed] [Google Scholar]
- 23.Giraud MN, Sanduja SK, Felder TB, Illich PA, Dial EJ, Lichtenberger LM. Effect of omeprazole on the bioavailability of unmodified and phospholipid-complexed aspirin in rats. Aliment Pharmacol Ther. 1997;11:899–906. doi: 10.1046/j.1365-2036.1997.00216.x. [DOI] [PubMed] [Google Scholar]
- 24.Serna JH, Genta RM, Lichtenberger LM, Graham DY, el-Zaatari FA. Invasive Helicobacter-like organisms in feline gastric mucosa. Helicobacter. 1997;2:40–43. doi: 10.1111/j.1523-5378.1997.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenberger LM, Romero JJ. Effect of ammonium ion on the hydrophobic and barrier properties of the gastric mucus gel layer: implications on the role of ammonium in H. pylori-induced gastritis. J Gastroenterol Hepatol. 1994;9(Suppl 1):S13–S19. doi: 10.1111/j.1440-1746.1994.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 26.Ottlecz A, Romero JJ, Hazell SL, Graham DY, Lichtenberger LM. Phospholipase activity of Helicobacter pylori and its inhibition by bismuth salts. Biochemical and biophysical studies. Dig Dis Sci. 1993;38:2071–2080. doi: 10.1007/BF01297087. [DOI] [PubMed] [Google Scholar]
- 27.Go MF, Lew GM, Lichtenberger LM, Genta RM, Graham DY. Gastric mucosal hydrophobicity and Helicobacter pylori: response to antimicrobial therapy. Am J Gastroenterol. 1993;88:1362–1365. [PubMed] [Google Scholar]
- 28.Dial EJ, Tran DM, Romero JJ, Zayat M, Lichtenberger LM. A direct role for secretory phospholipase A2 and lysophosphatidylcholine in the mediation of LPS-induced gastric injury. Shock. 2010;33:634–638. doi: 10.1097/SHK.0b013e3181cb9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dial EJ, Zayat M, Lopez-Storey M, Tran D, Lichtenberger L. Oral phosphatidylcholine preserves the gastrointestinal mucosal barrier during LPS-induced inflammation. Shock. 2008;30:729–733. doi: 10.1097/SHK.0b013e318173e8d4. [DOI] [PubMed] [Google Scholar]
- 30.Zayat M, Lichtenberger LM, Dial EJ. Pathophysiology of LPS-induced gastrointestinal injury in the rat: role of secretory phospholipase A2. Shock. 2008;30:206–211. doi: 10.1097/shk.0b013e318160f47f. [DOI] [PubMed] [Google Scholar]
- 31.Dial EJ, Romero JJ, Villa X, Mercer DW, Lichtenberger LM. Lipopolysaccharide-induced gastrointestinal injury in rats: role of surface hydrophobicity and bile salts. Shock. 2002;17:77–80. doi: 10.1097/00024382-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Dial EJ, Rooijakkers SH, Darling RL, Romero JJ, Lichtenberger LM. Role of phosphatidylcholine saturation in preventing bile salt toxicity to gastrointestinal epithelia and membranes. J Gastroenterol Hepatol. 2008;23:430–436. doi: 10.1111/j.1440-1746.2007.05153.x. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz MG, Renooij W. Phospholipids from rat, human, and canine gastric mucosa. Composition and metabolism of molecular classes of phosphatidylcholine. Gastroenterology. 1990;99:1292–1296. doi: 10.1016/0016-5085(90)91152-v. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Doyen R, Lichtenberger LM. The role of membrane cholesterol in determining bile acid cytotoxicity and cytoprotection of ursodeoxycholic acid. Biochim Biophys Acta. 2009;1788:507–513. doi: 10.1016/j.bbamem.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dial EJ, Darling RL, Lichtenberger LM. Importance of biliary excretion of indomethacin in gastrointestinal and hepatic injury. J Gastroenterol Hepatol. 2008;23(8 Pt 2):e384–e389. doi: 10.1111/j.1440-1746.2007.05266.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Dial EJ, Doyen R, Lichtenberger LM. Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G722–G731. doi: 10.1152/ajpgi.00387.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 38.Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178. doi: 10.1053/j.gastro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Lanas A, Sopeña F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am. 2009;38:333–352. doi: 10.1016/j.gtc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- 41.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Higuchi K, Kobata A, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–187. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenberger LM, Phan T, Okabe S. Aspirin's ability to induce intestinal injury in rats is dependent on bile and can be reversed if pre-associated with phosphatidylcholine. J Physiol Pharmacol. 2011;62:491–496. [PubMed] [Google Scholar]
- 44.Lichtenberger LM, Zhou Y, Dial EJ, Raphael RM. NSAID injury to the gastrointestinal tract: evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. J Pharm Pharmacol. 2006;58:1421–1428. doi: 10.1211/jpp.58.10.0001. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Plowman SJ, Lichtenberger LM, Hancock JF. The anti-inflammatory drug indomethacin alters nanoclustering in synthetic and cell plasma membranes. J Biol Chem. 2010;285:35188–35195. doi: 10.1074/jbc.M110.141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Hancock JF, Lichtenberger LM. The nonsteroidal anti-inflammatory drug indomethacin induces heterogeneity in lipid membranes: potential implication for its diverse biological action. PLoS One. 2010;5:e8811. doi: 10.1371/journal.pone.0008811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lichtenberger LM, Barron M, Marathi U. Association of phosphatidylcholine and NSAIDs as a novel strategy to reduce gastrointestinal toxicity. Drugs Today (Barc) 2009;45:877–890. doi: 10.1358/dot.2009.45.12.1441075. [DOI] [PubMed] [Google Scholar]
- 48.Yamada T, Hoshino M, Hayakawa T, et al. Bile secretion in rats with indomethacin-induced intestinal inflammation. Am J Physiol. 1996;270(5 Pt 1):G804–G812. doi: 10.1152/ajpgi.1996.270.5.G804. [DOI] [PubMed] [Google Scholar]
- 49.Cryer B, Bhatt DL, Lanza FL, Dong JF, Lichtenberger LM, Marathi UK. Low-dose aspirin-induced ulceration is attenuated by aspirin-phosphatidylcholine: a randomized clinical trial. Am J Gastroenterol. 2011;106:272–277. doi: 10.1038/ajg.2010.436. [DOI] [PubMed] [Google Scholar]
- 50.Lichtenberger LM, Romero JJ, Dial EJ. Gastrointestinal safety and therapeutic efficacy of parenterally administered phosphatidylcholine-associated indomethacin in rodent model systems. Br J Pharmacol. 2009;157:252–257. doi: 10.1111/j.1476-5381.2009.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichtenberger LM, Ulloa C, Vanous AL, et al. Zwitterionic phospholipids enhance aspirin's therapeutic activity, as demonstrated in rodent model systems. J Pharmacol Exp Ther. 1996;277:1221–1227. [PubMed] [Google Scholar]
- 52.Kurinets A, Lichtenberger LM. Phosphatidylcholine-associated aspirin accelerates healing of gastric ulcers in rats. Dig Dis Sci. 1998;43:786–790. doi: 10.1023/a:1018870131886. [DOI] [PubMed] [Google Scholar]
- 53.Lichtenberger LM, Romero JJ, Dial EJ, Moore JE. Naproxen-PC: a GI safe and highly effective anti-inflammatory. Inflammopharmacology. 2009;17:1–5. doi: 10.1007/s10787-008-8047-2. [DOI] [PubMed] [Google Scholar]
- 54.Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–860. [PubMed] [Google Scholar]
- 55.Lichtenberger LM, Romero JJ, de Ruijter WM, et al. Phosphatidylcholine association increases the anti-inflammatory and analgesic activity of ibuprofen in acute and chronic rodent models of joint inflammation: relationship to alterations in bioavailability and cyclooxygenase-inhibitory potency. J Pharmacol Exp Ther. 2001;298:279–287. [PubMed] [Google Scholar]
- 56.Wallace JL, Ferraz JG. New pharmacologic therapies in gastrointestinal disease. Gastroenterol Clin North Am. 2010;39:709–720. doi: 10.1016/j.gtc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 57.Scheiman JM. Prevention of damage induced by aspirin in the GI tract. Best Pract Res Clin Gastroenterol. 2012;26:153–162. doi: 10.1016/j.bpg.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Darling RL, Romero JJ, Dial EJ, Akunda JK, Langenbach R, Lichtenberger LM. The effects of aspirin on gastric mucosal integrity, surface hydrophobicity, and prostaglandin metabolism in cyclooxygenase knockout mice. Gastroenterology. 2004;127:94–104. doi: 10.1053/j.gastro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Laine L, Maller ES, Yu C, Quan H, Simon T. Ulcer formation with low-dose enteric-coated aspirin and the effect of COX-2 selective inhibition: a double-blind trial. Gastroenterology. 2004;127:395–402. doi: 10.1053/j.gastro.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Lichtenberger LM, Romero JJ, Dial EJ. Surface phospholipids in gastric injury and protection when a selective cyclooxygenase-2 inhibitor (Coxib) is used in combination with aspirin. Br J Pharmacol. 2007;150:913–919. doi: 10.1038/sj.bjp.0707176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuno H, Sakamoto C, Matsuda K, et al. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- 62.Schmassmann A, Zoidl G, Peskar BM, et al. Role of the different isoforms of cyclooxygenase and nitric oxide synthase during gastric ulcer healing in cyclooxygenase-1 and -2 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G747–G756. doi: 10.1152/ajpgi.00416.2005. [DOI] [PubMed] [Google Scholar]
- 63.Rahme E, Bernatsky S. NSAIDs and risk of lower gastrointestinal bleeding. Lancet. 2010;376:146–148. doi: 10.1016/S0140-6736(10)60839-2. [DOI] [PubMed] [Google Scholar]
- 64.Hackelsberger A, Platzer U, Nilius M, et al. Age and Helicobacter pylori decrease gastric mucosal surface hydrophobicity independently. Gut. 1998;43:465–469. doi: 10.1136/gut.43.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]