Abstract
Objective
The renin-angiotensin-aldosterone system (RAAS), vitamin D, and parathyroid hormone have all been implicated as regulators of adipocytokines and inflammation. We evaluated human interventional study protocols to investigate whether controlled modulations of these calcium- and sodium-regulatory hormones could influence adipocytokines and inflammation in obesity and diabetes.
Methods
Post-hoc analyses of two separate human protocols (Protocol 1, n=14; Protocol 2, n=24) conducted in a clinical research setting after rigorous control of diet, posture, medications, and diurnal rhythm, were performed. Protocol 1 evaluated obese hypertensives with vitamin D deficiency who received an infusion of angiotensin II (AngII) before and after 1 month of vitamin D3 therapy. Protocol 2 evaluated obese subjects with type 2 diabetes who also received AngII. Adipocytokines and inflammatory markers were measured before and after vitamin D3 therapy, and also before and after infusions of AngII.
Results
Vitamin D3 therapy significantly raised 25(OH)D and 1,25(OH)2D concentrations, and lowered parathyroid hormone, but had no effect on concentrations of adiponectin, resistin, leptin, IL-6, PAI-1, urinary TGFβ1, or HOMA-IR. AngII infusions, despite significant elevations in blood pressure and serum aldosterone, did not influence adipocytokine concentrations in either protocol.
Conclusion
In contrast to prior studies conducted in healthy populations, or those that could not control major regulators of the RAAS or adipocytokines, we observed that robust modulations in calcium- and sodium-regulatory hormones did not influence adipocytokines or inflammation in obesity or diabetes. Adipose-tissue physiology in these conditions may alter the hormonal regulation of inflammatory parameters.
Keywords: Adiponectin, Resistin, Obesity, Renin-angiotensin-aldosterone system, vitamin D
INTRODUCTION
Adipose-tissue is an endocrine organ that is dysfunctional in obesity[1, 2]. Adipose-tissue derived proteins (adipocytokines) have been associated with important roles in metabolism, vascular function, and inflammation[2–4]. The dysregulation of adipocytokine physiology in obesity (such as adiponectin deficiency) has been associated with an inflammatory state and obesity-related morbidities such as hypertension and diabetes[2, 5].
The increased renin-angiotensin-aldosterone system (RAAS) milieu that occurs with increasing adiposity may contribute to adipocytokine and inflammatory dysfunction[6–9]. Higher RAAS activity is associated with cardiovascular disease and inflammation[10–12], and has been implicated as a negative regulator of adiponectin and positive regulator of resistin[13–18]. Animal studies have linked local adipose-tissue RAAS activity with reductions in adiponectin and an increase in resistin[13, 15]. In turn, human studies have shown that an exogenous infusion of angiotensin II (AngII) in healthy lean men acutely lowers adiponectin, whereas RAAS-antagonist therapy increases it[16]. In contrast, higher RAAS activity, particularly higher aldosterone levels, are associated with higher resistin levels[14, 18]. To evaluate whether these relationships persist in human obesity and diabetes, where RAAS activity and adipocytokines are most abnormal [7–9, 19], interventional studies are needed.
Calcium-regulatory hormones represent another potential regulator of inflammatory status in obesity, and also have an intertwined relationship with the RAAS[20]. Vitamin D therapy has been implicated as an anti-inflammatory agent in vitro[21–23] and in vivo[24–26], that also negatively regulates RAAS activity[27–30]; this may be particularly relevant in obese and type 2 diabetes populations where vitamin D insufficiency is common. Prior studies have associated higher vitamin D levels with higher levels of adiponectin[17, 31–33] and resistin[18], higher and lower levels of leptin[33–35], and improved glycemic parameters[33].
Dedicated interventional studies in human obesity and type 2 diabetes are needed to better characterize potential relationships between the RAAS and calcium-homeostatic hormones with adipocytokines and inflammatory markers. In particular, physiology studies conducted under well-controlled settings are needed since many environmental factors may significantly influence measurements and interpretations of the RAAS and adipocytokines, including: medications (particularly RAAS antagonists), dietary sodium and electrolyte content, changes in body weight, renal function and other co-morbid medical conditions[32, 36]. We performed post-hoc analyses on two previously completed interventional study protocols that were conducted under strict conditions of a Clinical Research Center to evaluate whether controlled modulations in calcium- and sodium-regulatory hormones influenced adipocytokine and inflammatory marker concentrations in individuals with obesity and diabetes[19, 27].
MATERIALS AND METHODS
Study Populations
We evaluated study populations from two separate interventional protocols that included aggressive vitamin D supplementation to modulate calcium-regulating hormones and exogenous infusions of AngII to modulate the RAAS. Protocol 1 was comprised of 14 subjects with obesity and stage I hypertension who received infusions of AngII before and after high-dose vitamin D3 therapy, as previously described[27]. Protocol 2 was comprised of 24 subjects with type 2 diabetes and obesity who were studied within the HyperPATH cohort, and previously described[19], who also received infusions of AngII to modulate the RAAS. Adipocytokine and inflammatory marker measurements from the stored blood of these previously conducted study protocols were used to evaluate the effects of vitamin D3 and RAAS interventions, and the interaction between these interventions.
As previously described, subjects were recruited into Protocol 1 if they were between the ages of 18–65 years, obese (BMI ≥ 30 kg/m2), had stage I hypertension, and 25-hydroxyvitamin D (25[OH]D) levels < 25 ng/mL[27]. Subjects were recruited into Protocol 2 if they had a known history of type 2 diabetes that was documented by a clinician, or met any of the American Diabetes Association diagnostic criteria (fasting blood glucose ≥ 126 mg/dL or random blood glucose ≥ 200 mg/dL or hemoglobin A1c ≥ 6.5% or 2-hour glucose following a 75 gram glucose load of ≥ 200 mg/dl)[19]. The 24 subjects from Protocol 2 with available frozen blood for adipocytokine and inflammatory maker analyses were included in the current study. Subjects were excluded from either protocol if they had an estimated glomerular filtration rate of < 60 mL/min, a serum creatinine of > 1.5 mg/dL, a history of coronary heart disease, stroke, liver disease, secondary cause of hypertension, retinopathy, neuropathy, or active tobacco use. Subjects with proteinuria were excluded from Protocol 2. Although characteristics of these two study protocols and populations have been previously described[19, 27], the analyses and results reported in the current study are unique and have not been previously reported. All subjects were studied after informed consent was obtained at the Brigham and Women’s Hospital (Boston, USA) using study protocols that were approved by the Institutional Review Board.
Protocol 1 Study Design
The Protocol 1 study design has been previously described[27]. Subjects who used angiotensin converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid antagonists, beta-blockers, or diuretics were withdrawn of these medications for up to 3 months to ensure that measurements of the RAAS reflected the native underlying hormonal equilibrium. Daily home blood pressure monitoring was used with established parameters for safety[27]. Subjects were then provided a study diet for 1 week that included > 200 mmol of sodium, 100 mmol of potassium, and 20 mmol of calcium to minimize diet-induced variability in RAAS components, adipocytokines, and glycemic parameters[17, 18, 37]. After completing this diet for 1 week, subjects were admitted to the inpatient clinical research center for their first study visit where they remained in supine position overnight to minimize posture-induced variability in RAAS activity. In the morning while fasting, they received an infusion of AngII (1 ng/kg/min) for 90 minutes. Blood measurements, including measurements of RAAS components and adipocytokines were taken before and after AngII infusion. Subjects were then discharged home with instructions to take 15,000 IU/daily of vitamin D3 for 4 weeks. During this time, they continued to be maintained off all interfering medications. They returned for their second study visit after completing another 1 week of the study diet, and repeated the exact same aforementioned AngII protocol in the clinical research center to evaluate baseline and AngII-induced changes in adipocytokines after vitamin D3 therapy. After completing study visit 2, subjects resumed medications they took prior to the initiation of the study, and were returned to the care of their routine medical care providers.
Protocol 2 Study Design
Protocol 2 followed a very similar study design that has also been previously described[19]. All subjects were withdrawn of interfering medications as described above. Subjects who were being treated with sulfonylureas, insulin, biguanides, or HMG-CoA reductase inhibitors remained on these medications for the duration of the study. Subjects reported their home blood glucose measurements to study staff which were used to titrate oral hypoglycemic agents and insulin doses to maintain a fasting blood glucose value of ≤ 160 mg/dL; all participants achieved this prior to their inpatient study visit. Subjects were prescribed the same study diet described above 1 week prior to their admission to the clinical research center, where they were maintained supine overnight. In the morning while fasting, they received an infusion of AngII (3 ng/kg/min) for 60 minutes. Blood measurements, including measurements of RAAS components and adipocytokines, were taken before and after the infusion.
Laboratory Measurements
Blood samples in each study protocol were collected while fasting in the morning of all study visits, after overnight supine rest while on the study diet during the inpatient clinical research center admission, and in the case of adipocytokines, also immediately following each AngII infusion. These blood samples were used to measure: parathyroid hormone (PTH) (Beckman Coulter, Fullerton, CA; intra-assay variation [IAV]=1.6–2.6%, inter-assay variation [IEV]=2.8–5.8%), 25-hydroxyvitamin D (25[OH]D) (Diasorin, Inc., Stillwater, MN; IAV=4.4–8.3%, IEV=6.2–12.5%), 1,25-dihydroxyvitamin D (1,25[OH]2D) (Diasorin, Inc., Stillwater, MN), serum aldosterone (Siemens, Los Angeles, Ca; IAV=2.5–5.4%, IEV=3.8–15.7%), plasma glucose and insulin. The homeostasis model assessment index (HOMA-IR) was calculated from plasma glucose and insulin values ([glucose] × [insulin] / 405), as a general representation of insulin resistance. For the current study, collected samples from Protocols 1 and 2 that were frozen were used to measure: plasma total adiponectin (ALPCO Diagnostics Inc., Salem, NH; IAV=5.0–5.4%, IEV=6.0%), plasma resistin (R&D Systems, Minneapolis, MN; IAV=1.8–7.7%, IEV=3.4–9.3%), serum leptin (LINCO Research, St. Charles, MS; IAV=5.2–7.5%, IEV=3.2–8.9%), serum IL-6 (Beckman Coulter, Fullerton, CA; IAV=1.7–4.6%, IEV=3.1–12.0%), PAI-1 (R&D Systems, Minneapolis, MN; IAV=5.8–7.4%, IEV=5.6–7.2%), hemoglobin A1c (Roche Diagnostics Corporation).
Urinary TGFβ1 was measured by ELISA (R&D Systems, Minneapolis, MN) from previously collected and frozen urine samples from Protocol 1. Urine samples were concentrated 2-, 10- and 50-fold by centrifugation using Ultracel YM-10 filters (Milipore, Billerica, MA) for measurement. Only experimental values that fell within the linear range of the TGFβ1 standard curve were used to calculate urinary TGFβ1 levels. To account for variability in urine collection volumes, TGFβ1 values were normalized to urine creatinine (mg/dL). Samples were run in duplicate (2-fold and 10-fold concentrated) or singleton (50-fold concentrated).
Statistical Methods
The primary objective of the current study was to evaluate whether controlled modulations in calcium- and sodium-regulatory hormones (performed in previously reported interventional protocols) influenced adipocytokine and inflammatory marker concentrations in obesity and type 2 diabetes.
Study population characteristics are presented as means ± standard error of means. Blood measurements of adipocytokines and inflammatory markers before and after interventions with vitamin D3 or AngII infusion were compared using paired t-tests. Correlations between calcium-regulatory hormones and adipocytokines are represented by Pearson correlation coefficients. The level for significance for all tests conducted was set at α=0.05 and reported P-values are two-tailed. Data analyses were performed using SAS v9.2 (Cary, NC) statistical software.
Power
The sample sizes in Protocol 1 and 2 were adequately powered to see potential changes in outcome measures with RAAS and vitamin D3 modulations. Prior studies with similar sample sizes, but performed in populations not enriched for obesity or diabetes, have shown dramatic reductions in adiponectin with acute AngII infusions (~50% decrease)[16] and elevations in resistin with higher RAAS activity[14]. Furthermore, raising 25(OH)D concentrations from approximately 20 ng/mL to 40 ng/mL has been associated with 50–70% higher concentrations of adiponectin[17, 26, 32] and resistin[18].
RESULTS
Modulation of Calcium-Regulatory Hormones via Vitamin D3 Intervention in Protocol 1
Descriptive data of obese hypertensives with low 25(OH)D concentrations from Protocol 1 are shown in Table 1, and have been previously described[27]. Vitamin D3 intervention significantly raised 25(OH)D (18.2 ± 1.8 to 51.9 ± 3.1 ng/mL; P<0.0001), 1,25(OH)2D (38.1 ± 3.3 to 57.7 ± 4.5 pg/mL; P<0.0001), calcium (8.9 ± 0.1 to 9.1 ± 0.8 mg/dL; P<0.01), and lowered PTH (46.4 ± 4.3 to 37.1 ± 3.1 pg/mL; P<0.01). In the current study, we evaluated the correlation between baseline calcium-regulatory hormones and inflammatory metabolic markers before and after vitamin D3 therapy (Table 2). Notable cross-sectional trends included an inverse association between 1,25(OH)2D with fasting blood glucose and adiponectin, and a positive correlation between PTH with PAI-1 and urinary TGFβ1. In contrast to these cross-sectional associations, after substantial modulation of calcium-regulatory hormones with high-dose vitamin D3 intervention, there was no change in glycemic parameters, adipocytokines, or other inflammatory markers (Table 3). These interventional findings are in sharp contrast to prior studies that did not employ similar dietary, environmental, and medication restrictions and reported changes in TGFβ1, glucose homeostasis, inflammatory markers, and adipocytokines with vitamin D interventions[24–26].
Table 1.
Population Demographics.
| Protocol 1 Study Population: | Protocol 2 Study Population: | |
|---|---|---|
| N | 14 | 24 |
| Age (years) | 50 ± 2.5 | 51.4 ± 9.2 |
| Gender (%female) | 64 | 46 |
| Race (%white/%black/%other) | 30/70/0 | 44/39/17 |
| BMI (kg/m2) | 36.3 ± 1.2 | 30.3 ± 5.4 |
| HbA1c (%) | 6.0 ± 0.08 | 7.0 ± 0.8 |
| MAP (mmHg) | 105.6 ± 1.9 | 109.9 ± 3.0 |
| 24 hour urine creatinine (mg) | 1599.5 ± 103.4 | 1523.8 ± 99.2 |
Table 2.
Correlations between calcium-regulatory hormones and adipocytokines and inflammatory markers in the Protocol 1 study population.
| Study Visit 1 (Pre-Vitamin D3 Intervention) | Study Visit 2 (Post-Vitamin D3 Intervention) | |||||
| 25(OH)D | 1,25(OH)2D | PTH | 25(OH)D | 1,25(OH)2D | PTH | |
| Fasting glucose (mg/dL) | −0.49† | −0.54* | 0.40 | 0.10 | −0.58* | 0.16 |
| Fasting insulin (μIU/mL) | −0.08 | 0.03 | −0.27 | 0.14 | −0.37 | −0.00 |
| HOMA-IR | −0.16 | −0.05 | −0.20 | 0.15 | −0.42 | 0.02 |
| IL-6 (pg/mL) | 0.33 | 0.37 | −0.48 | −0.04 | −0.45 | −0.29 |
| PAI-1 (ng/mL) | −0.31 | −0.33 | 0.66* | −0.29 | 0.38 | 0.59* |
| Urinary TGF-β1/urine Creatinine (μg/dL) | −0.09 | 0.42 | 0.62* | −0.39 | −0.30 | 0.31 |
| Adiponectin (μg/mL) | −0.31 | −0.53* | 0.34 | −0.05 | −0.68* | 0.15 |
| Resistin (μg/L) | 0.22 | 0.52† | 0.16 | 0.23 | 0.40 | 0.33 |
| Leptin (ng/mL) | 0.02 | −0.17 | 0.41 | 0.19 | −0.05 | 0.31 |
Table displays Pearson correlation coefficients (r) before and after vitamin D3 therapy (
P<0.10,
P<0.05).
25(OH)D=25-hydroxyvitamin D; 1,25(OH)2D = 1,25-dihydroxyvitamin D; PTH=parathyroid hormone; HOMA-IR=homeostatic model for insulin resistance; IL-6=interleukin 6; PAI-1=plasminogen activator inhibitor 1.
Table 3.
The influence of high-dose vitamin D3 therapy on adipocytokines and inflammatory markers in Protocol 1.
| Before Vitamin D3 Intervention | After Vitamin D3 Intervention | P-value | |
|---|---|---|---|
| Fasting glucose (mg/dL) | 93.4 ± 2.07 | 93.4 ± 2.03 | 0.99 |
| Fasting insulin (μIU/mL) | 7.63 ± 1.02 | 10.49 ± 1.85 | 0.11 |
| HOMA-IR | 1.77 ± 0.25 | 2.47 ± 0.46 | 0.14 |
| IL-6 (pg/mL) | 4.66 ± 1.18 | 4.08 ± 0.86 | 0.76 |
| PAI-1 (ng/mL) | 5.17 ± 0.71 | 5.81 ± 0.90 | 0.51 |
| Urinary TGFβ1/Urine Creatinine (μg/dL) | 94.2 ± 12.0 | 108.3 ± 18.4 | 0.48 |
| Adiponectin (μg/mL) | 3.87 ± 0.53 | 3.57 ± 0.40 | 0.28 |
| Resistin (μg/L) | 5.80 ± 0.35 | 5.98 ± 0.49 | 0.53 |
| Leptin (ng/mL) | 41.11 ± 6.52 | 42.71 ± 7.58 | 0.42 |
Table displays mean values (± standard error of means) before and after vitamin D3 intervention.
Modulation of the RAAS via AngII infusion in Protocol 1
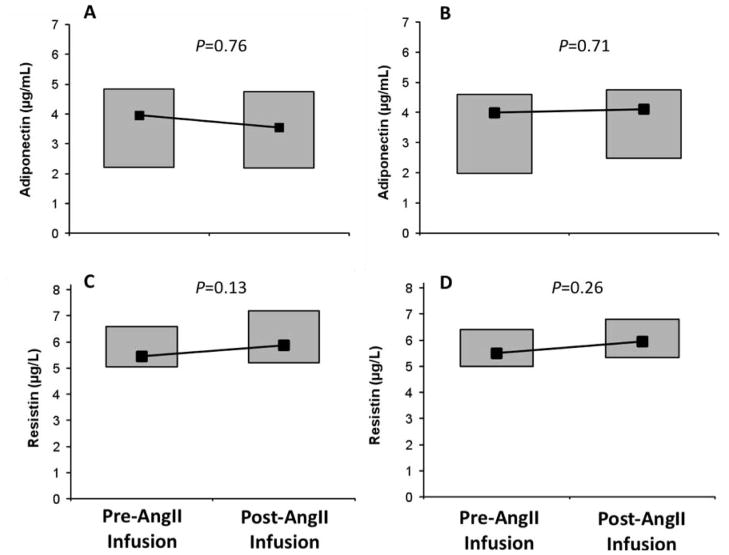
As previously reported, AngII infusion resulted in expected acute elevations in mean arterial pressure (MAP) and serum aldosterone before (MAP: 105.6 ± 7.2 to 115.9 ± 10.7 mmHg and aldosterone: 3.3 ± 0.2 to 8.2 ± 0.8 ng/dL; both P<0.0001) and after (MAP: 102.7 ± 8.1 to 115.9 ± 10.2 mmHg, aldosterone: 3.7 ± 0.4 to 9.8 ± 1.0; both P<0.0001), vitamin D3 intervention[27]. In contrast to prior studies in healthy lean men[16], AngII infusion did not acutely modulate adiponectin concentrations in this obese hypertensive population (Figures 1A and 1B). Similarly, resistin concentrations did not change in response to AngII infusion (Figures 1C and 1D). Although prior studies demonstrated a positive relationship between aldosterone concentrations and resistin in primary hyperaldosteronism[14], we observed no association between the AngII-induced change in serum aldosterone and the AngII-induced changes in adiponectin or resistin (r<0.10 and P=NS for both).
Figure 1.
Changes in adiponectin in response to low-dose AngII infusion before (A) and after (B) vitamin D3 therapy, and changes in resistin in response to low-dose AngII infusion before (C) and after (D) vitamin D3 therapy. Box plots depict the 25th–75th percentile (grey boxes) and the median (black square).
Modulation of the RAAS via AngII infusion in Protocol 2
The relationship between the RAAS and adipocytokines was examined in subjects with type 2 diabetes and obesity who underwent an AngII infusion in Protocol 2 (Table 1). These subjects received a more potent dosing of AngII when compared to subjects in Protocol 1 (3 ng/kg/min versus 1 ng/kg/min), and as a result had greater increases in MAP and serum aldosterone (MAP 95.0 ± 8.1 to 111.5 ± 11.1 mmHg; P<0.0001 and serum aldosterone 2.5 ± 2.5 to 11.5 ± 5.5 ng/dL; P<0.0001). Despite these anticipated AngII-induced changes, AngII infusion did not acutely influence either adiponectin or resistin levels (Figure 2). Similarly, no correlation between AngII-induced changes in aldosterone and AngII-induced changes in adiponectin or resistin were observed (r<0.10 and P=NS for both).
Figure 2.
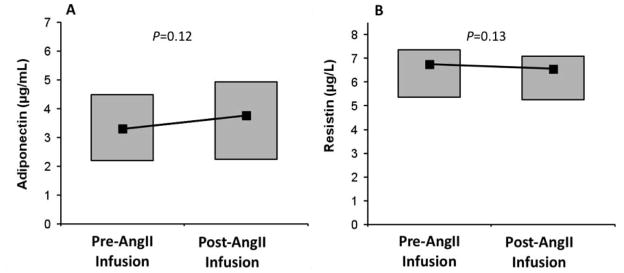
Changes in adiponectin in response to high-dose AngII infusion (A) and changes in resistin in response to high-dose AngII infusion (B). Box plots depict the 25th–75th percentile (grey boxes) and the median (black square).
DISCUSSION
Obesity is an inflammatory state characterized by adiponectin deficiency and increased resistin and leptin; these dysfunctional parameters have been implicated as factors contributing to obesity-related cardio-metabolic diseases such as diabetes[2, 3, 12, 38]. Prior studies have described intertwined relationships between adipose-derived peptides (adipocytokines), the RAAS, and calcium-regulatory hormones[16–18, 26, 31–33]. In the current study, we evaluated the effect of interventions that modulated calcium-regulatory hormones, using high-dose vitamin D3 therapy, and sodium-regulatory hormones, using exogenous AngII infusions, in a clinical research center. The primary objective was to evaluate whether modulating these hormonal systems influenced adipocytokines and inflammatory markers in the pathological conditions of human obesity and diabetes, as has been suggested before by interventions in healthy lean individuals [16] and non-interventional studies in similar disease states[14]. The reliability of our observations reside in the study protocols that were meticulously designed to account for dietary electrolytes, interfering medications, posture, diurnal variation, and other co-morbid medical conditions; all of these factors are known to alter the measurements or interpretations of the RAAS, adipocytokines, and calcium-regulatory hormones[17, 18, 36, 39, 40]. Although cross-sectional associations in the current, and previously reported, studies may suggest potential relationships between adipocytokines and sodium- and calcium-regulatory hormones[14, 17, 18, 32], robust interventions to modulate these hormonal systems using vitamin D3 therapy and exogenous AngII infusions did not influence adipocytokines or relevant inflammatory markers in either the obese hypertensive or obese type 2 diabetes populations.
The Influence of Calcium-Regulatory Hormone Interventions on Adipocytokines and Inflammation in Obese Hypertensives
Prior cross-sectional studies have suggested that 25(OH)D concentrations are positively associated with adiponectin and resistin[17, 18, 33], and possibly also with leptin[33–35]. Furthermore, interventional studies using vitamin D3 supplementation[26] or vitamin D receptor agonists[24, 25] have suggested that raising vitamin D levels (with concomitant decrements in PTH) may raise adiponectin and lower inflammatory markers such as IL-6, TGF-β, and C-reactive protein. A potential limitation of these prior studies may reside in either their observational nature or study design. One such study worthy of discussion is that conducted by Neyestani et al. which evaluated the effect of vitamin D on cardio-metabolic profiles in subjects with type 2 diabetes[26]. In this randomized and double-blinded trial, subjects were treated for 3 months with one of the following interventions (n=30 each): 1) a yogurt drink with 150 mg of calcium and no vitamin D3; 2) a yogurt drink with 150 mg of calcium and 500 IU of vitamin D3; or 3) a yogurt drink with 250 mg of calcium and 500 IU of vitamin D3. Although they observed an increase in adiponectin concentrations with vitamin D fortified yogurt drinks, it is worth noting that increments in adiponectin were seen even in the non-vitamin D-fortified group. Furthermore, the group randomized to a vitamin D-fortified yogurt drink with the highest calcium content (250 mg) had the greatest increment in adiponectin. In contrast, our current study argues that the effect of high-dose vitamin D3 on adiponectin levels, and other inflammatory markers, is null. Although the vitamin D3 intervention in our Protocol 1 was shorter than that conducted by Neyestani et al. (1 month versus 3 months), 25(OH)D concentrations in Protocol 1 were raised to a much higher degree, and in a population with a significantly larger BMI[26, 27]. In addition, Protocol 1 accounted for dietary sodium content and the use of RAAS and adipocytokine modulating medications (such as RAAS antagonists), which are known to skew the measurements and interpretations of these variables[17, 18].
It is well established that adipocytokines and inflammatory markers are influenced by a host of demographic, environmental, and hormonal variables in addition to renal function[16, 32]; interventional studies that evaluated populations using a heterogeneous combination of medications, variable co-morbid conditions, and ad-lib diets may have been susceptible to spurious or non-specific findings[24–26]. In contrast, the evaluation of adipocytokine and RAAS relationships in normal, healthy, lean individuals has established a physiologic baseline from which to compare the abnormalities in metabolic disease states such as obesity and type 2 diabetes[16]. However, since adipocytokine abnormalities are most apparent and most associated with obesity-related co-morbidities, observations in non-obese populations may not directly translate to the pathophysiology seen in obesity and type 2 diabetes. Protocol 1 was designed to modulate calcium-regulatory hormones using high-dose vitamin D3 therapy in a morbidly obese population, and we previously observed that these alterations reduced RAAS activity[27]. The fact that these potent changes in the current study did not translate to changes in adipocytokines or inflammatory markers may have several potential explanations: 1) prior observational studies in non-healthy populations reporting positive findings with vitamin D interventions may have been vulnerable to any number of confounders (such as total body adiposity); 2) favorable changes in adipocytokine and inflammatory profiles may not be readily induced by vitamin D interventions in the state of morbid obesity or diabetes for reasons that remain unclear (indications for the latter null effect have been suggested in prior reports evaluating obese populations[41]); 3) although modulating calcium-regulatory hormones did not appreciably influence circulating adipocytokines or inflammatory markers, whether the local adipose-tissue levels of these components were changed is not known.
The Influence of Sodium-Regulatory Hormone Interventions on Adipocytokines and Inflammation in Obesity with and without Diabetes
Observational studies have suggested that higher aldosterone concentrations may raise resistin[14], whereas physiology studies have suggested that acute alterations in the RAAS induced by modulations in dietary sodium intake could significantly influence adiponectin and resistin levels[16–18, 42]. Specifically, lowering RAAS activity using a liberal sodium diet raises adiponectin and lowers resistin, whereas raising RAAS activity with sodium restriction induces the opposite. Although these human observations supported elegant animal studies that reported similar observations when RAAS activity was abolished or heightened[13, 15], they were limited in concluding that RAAS-activity specifically (as opposed to the other direct effects of dietary sodium manipulation) was causal in influencing these adipocytokines. Lely et al. demonstrated that an infusion of exogenous AngII in healthy humans acutely lowered adiponectin in a dose-dependent manner; in contrast, treatment with enalapril raised adiponectin after 1 week[16]. The population evaluated in this interventional study was comprised of young, lean, healthy men who therefore had high adiponectin concentrations. Since adiponectin deficiency in obesity is implicated as a contributor to cardiovascular and metabolic diseases and is hypothesized to occur due to excess local-RAAS activity[15], we evaluated whether acute modulations in RAAS activity could similarly influence adipocytokines in our obese and “non-healthy” populations. No changes in adiponectin in response to low dose (1 ng/kg/min) or the higher dose (3ng/kg/min) of exogenous AngII infusion were observed. Furthermore, in contrast to prior studies positively associating aldosterone and resistin concentrations, we observed no changes in resistin levels despite substantial increases in aldosterone using AngII stimulation[14]. These observations may suggest that in contrast to healthy lean individuals, a milieu of excess adipose-tissue may result in sufficiently saturated endogenous adipose tissue-RAAS activity (AngII and/or aldosterone)[43, 44] to maximally lower adiponectin and raise resistin. Whether intervention with pharmacologic RAAS antagonists early in the progression to obesity could improve adipocytokine profiles or clinical outcomes is not known.
Strengths and Limitations
The strengths of our study include the evaluation of obese hypertensive and obese diabetic participants where adipocytokine dysfunction, inflammation, vitamin D insufficiency, and excess tissue-RAAS activity may be most prevalent and clinically relevant. We studied participants who were withdrawn of all relevant interfering medications, under controlled dietary conditions, and studied under controlled posture, after accounting for diurnal rhythm; these interventions increased the reliability of hormonal measurements and allowed greater confidence in paired comparisons. Although our study sample sizes were small, the study designs employed robust interventions to modulate calcium- and sodium-regulatory hormones in sample sizes that have previously been used to detect changes in healthy population interventions[16] and observational settings[14]. The evaluation of adipocytokine and inflammatory markers was not the primary objective of either Protocol 1 or 2; rather, it was the primary aim of the current post-hoc analysis, which should be considered as a limitation of the current findings and may be confirmed in future studies. The two study protocols described were not completely comparable in that they were comprised of different subjects, the RAAS modulations were slightly different (different AngII doses and duration of infusions), and only Protocol 1 included a calcium-regulatory hormone modulation. However, the fact that similar observations were made following AngII infusions in two separate populations may add confidence to the reliability of our findings. We studied a pre-determined obese and diabetic demographic, therefore the generalizability of our observations is limited to these populations. Our study was not designed to elucidate the biology for our negative findings, rather raises new hypotheses for future pursuits.
Conclusions
In summary, we observed that neither significant alterations in calcium-regulatory hormones (including large increases in vitamin D metabolites and concomitant decrements in PTH), or sodium-regulating hormones (including acute dose-dependent increases in AngII and aldosterone levels), influenced adipocytokines or inflammatory markers in obesity or diabetes. These findings are in sharp contrast to many prior observational and interventional studies that either evaluated healthy individuals, or may not have been optimally designed to minimize potential modulators of these hormonal systems and outcome variables. Our findings may suggest that the influence of calcium- and sodium-regulatory hormones on the modulation of adipocytokines and inflammation in states of severe adipose-tissue dysregulation are either marginalized or nonexistent. Evaluating the factors that contributed to these abnormal relationships warrants investigation.
Acknowledgments
FUNDING AND ACKNOWLEDGMENTS
We thank our funding sources: F32 HL104776-02, K23 HL111771-01, an American Medical Association Foundation Seed Grant, and a Brigham and Women’s Hospital Biomedical Research Institute Grant (to A.V.), K23 HL08236-05 (to J.S.W.), F31 NR011108 and F32 NR013318 (to PCU), K08 Dk084206 (to JPA), and R01 HL085224 (GHW). This project was supported by Clinical Translational Science Award U54LM008748 and grant M01-RR02635 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources, and the Specialized Center of Research in Molecular Genetics of Hypertension Grant P50HL055000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ABBREVIATIONS
- RAAS
renin-angiotensin-aldosterone system
- AngII
angiotensin II
- BMI
body-mass index
- 25(OH)D
25-hydroxyvitamin D
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- PTH
parathyroid hormone
- PAI-1
plasminogen activator inhibitor 1
- IL-6
interleukin 6
- HOMA-IR
homeostatic model assessment
- TGFβ1
transforming growth factor β1
- MAP
mean arterial pressure
- IAV
intra-assay variation
- IEV
inter-assay variation
Footnotes
DISCLOSURES: The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS: This study was designed and supervised by AV and JSW. Study methods, assays, and analyses were performed by AV, PCU, BS, and JPA. Data interpretation and manuscript writing received contributions from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. Journal of Clinical Endocrinology and Metabolism. 2004 Jun;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 2.Brochu-Gaudreau K, Rehfeldt C, Blouin R, et al. Adiponectin action from head to toe. Endocrine. 2010 Feb;37:11–32. doi: 10.1007/s12020-009-9278-8. [DOI] [PubMed] [Google Scholar]
- 3.Boydens C, Maenhaut N, Pauwels B, et al. Adipose Tissue as Regulator of Vascular Tone. Curr Hypertens Rep. 2012 Mar 14; doi: 10.1007/s11906-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 4.Choi HY, Kim S, Yang SJ, et al. Association of adiponectin, resistin, and vascular inflammation: analysis with 18F-fluorodeoxyglucose positron emission tomography. Arterioscler Thromb Vasc Biol. 2011 Apr;31:944–949. doi: 10.1161/ATVBAHA.110.220673. [DOI] [PubMed] [Google Scholar]
- 5.Siitonen N, Pulkkinen L, Lindstrom J, et al. Association of ADIPOQ gene variants with body weight, type 2 diabetes and serum adiponectin concentrations: the Finnish Diabetes Prevention Study. BMC Med Genet. 2011;12:5. doi: 10.1186/1471-2350-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Underwood PC, Chamarthi B, Williams JS, et al. Replication and meta-analysis of the gene-environment interaction between body mass index and the interleukin-6 promoter polymorphism with higher insulin resistance. Metabolism. 2011 Nov 8; doi: 10.1016/j.metabol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yasue S, Masuzaki H, Okada S, et al. Adipose Tissue Specific Regulation of Angiotensinogen in Obese Humans and Mice: Impact of Nutritional Status and Adipocyte Hypertrophy. American Journal of Hypertension. 2010;23:425–431. doi: 10.1038/ajh.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007 Nov;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaidya A, Forman JP, Williams JS. Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. J Hum Hypertens. 2010 Dec 2; doi: 10.1038/jhh.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamarthi B, Williams GH, Ricchiuti V, et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens. 2011 Oct;24:1143–1148. doi: 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000 Jan 20;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 12.Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2010 Oct 13; doi: 10.1038/ki.2010.391. [DOI] [PubMed] [Google Scholar]
- 13.Ran J, Hirano T, Fukui T, et al. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism. 2006 Apr;55:478–488. doi: 10.1016/j.metabol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Petramala L, Cotesta D, et al. Adipokines and cardiometabolic profile in primary hyperaldosteronism. J Clin Endocrinol Metab. 2010 May;95:2391–2398. doi: 10.1210/jc.2009-2204. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Soltani-Bejnood M, Quignard-Boulange A, et al. The Adipose Renin-Angiotensin System Modulates Systemic Markers of Insulin Sensitivity and Activates Intrarenal Renin-Angiotensin System. Journal of Biomedicine and Biotechnology. 2006;5 doi: 10.1155/JBB/2006/27012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lely AT, Krikken JA, Bakker SJ, et al. Low dietary sodium and exogenous angiotensin II infusion decrease plasma adiponectin concentrations in healthy men. J Clin Endocrinol Metab. 2007 May;92:1821–1826. doi: 10.1210/jc.2006-2092. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya A, Forman JP, Underwood PC, et al. The influence of body mass index and renin-angiotensin-aldosterone system activity on the relationship between 25-hydroxyvitamin D and adiponectin in Caucasian men. Eur J Endocrinol. 2011 Jun;164:995–1002. doi: 10.1530/EJE-11-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidya A, Pojoga L, Underwood PC, et al. The Association of Plasma Resistin with Dietary Sodium Manipulation, the Renin-Angiotensin-Aldosterone System, and 25-Hydroxyvitamin D(3) in Human Hypertension. Clin Endocrinol (Oxf) 2010 Nov 2; doi: 10.1111/j.1365-2265.2010.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Underwood PC, Chamarthi B, Williams JS, et al. Nonmodulation as the Mechanism for Salt Sensitivity of Blood Pressure in Individuals with Hypertension and Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2012 Aug 3; doi: 10.1210/jc.2012-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaidya A, Williams JS. The relationship between vitamin D and the renin-angiotensin system in the pathophysiology of hypertension, kidney disease, and diabetes. Metabolism. 2011 Nov 9; doi: 10.1016/j.metabol.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao D, Trayhurn P, Bing C. 1,25-Dihydroxyvitamin D(3) inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes (Lond) 2012 Apr 17; doi: 10.1038/ijo.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorente-Cebrian S, Eriksson A, Dunlop T, et al. Differential effects of 1alpha,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr. 2012 Apr;51:335–342. doi: 10.1007/s00394-011-0218-z. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Kong J, Sun T, et al. 1,25-Dihydroxyvitamin D(3) suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kappaB activation. Arch Biochem Biophys. 2011 Mar 15;507:241–247. doi: 10.1016/j.abb.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension. 2008 Aug;52:249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 25.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010 Nov 6;376:1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 26.Neyestani TR, Nikooyeh B, Alavi-Majd H, et al. Improvement of Vitamin D Status via Daily Intake of Fortified Yogurt Drink Either with or without Extra Calcium Ameliorates Systemic Inflammatory Biomarkers, including Adipokines, in the Subjects with Type 2 Diabetes. J Clin Endocrinol Metab. 2012 Mar 22; doi: 10.1210/jc.2011-3465. [DOI] [PubMed] [Google Scholar]
- 27.Vaidya A, Sun B, Larson C, et al. Vitamin D3 Therapy Corrects the Tissue Sensitivity to Angiotensin II Akin to the Action of a Converting Enzyme Inhibitor in Obese Hypertensives: An Interventional Study. J Clin Endocrinol Metab. 2012 Apr 26; doi: 10.1210/jc.2012-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaidya A, Forman JP. The Future of Vitamin D in Vascular Disease: Reviewing the Role of Vitamin D in Hypertension and Kidney Disease. Current Hypertension Reports. 2012 doi: 10.1007/s11906-012-0248-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaidya A, Forman JP, Seely EW, et al. 25-hydroxyvitamin D is Associated with Plasma Renin Activity and the Pressor Response to Dietary Sodium Intake in Caucasians. Journal of the Renin-Angiotensin-Aldosterone System. 2011 Feb 17; doi: 10.1177/1470320310391922. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomaschitz A, Pilz S, Ritz E, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Clin Chim Acta. 2010 May 29; doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, et al. The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine. 2009 Oct;36:205–210. doi: 10.1007/s12020-009-9216-9. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya A, Williams JS, Forman JP. The Independent Association Between 25-Hydroxyvitamin D and Adiponectin and Its Relation With BMI in Two Large Cohorts: The NHS and the HPFS. Obesity (Silver Spring) 2011 Jul 14; doi: 10.1038/oby.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikh S, Guo DH, Pollock NK, et al. Circulating 25-hydroxyvitamin d concentrations are correlated with cardiometabolic risk among american black and white adolescents living in a year-round sunny climate. Diabetes Care. 2012 May;35:1133–1138. doi: 10.2337/dc11-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009 Oct;94:4023–4030. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 35.Grethen E, Hill KM, Jones R, et al. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J Clin Endocrinol Metab. 2012 May;97:1655–1662. doi: 10.1210/jc.2011-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilarrasa N, Vendrell J, Maravall J, et al. Distribution and determinants of adiponectin, resistin and ghrelin in a randomly selected healthy population. Clin Endocrinol (Oxf) 2005 Sep;63:329–335. doi: 10.1111/j.1365-2265.2005.02346.x. [DOI] [PubMed] [Google Scholar]
- 37.Garg R, Williams GH, Hurwitz S, et al. Low-salt diet increases insulin resistance in healthy subjects. Metabolism. 2011 Jul;60:965–968. doi: 10.1016/j.metabol.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vendrell J, Broch M, Vilarrasa N, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004 Jun;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 39.Pilz S, Kienreich K, Drechsler C, et al. Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J Clin Endocrinol Metab. 2012 Jan;97:E75–79. doi: 10.1210/jc.2011-2183. [DOI] [PubMed] [Google Scholar]
- 40.Tomaschitz A, Ritz E, Pieske B, et al. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res. 2012 Apr 1;94:10–19. doi: 10.1093/cvr/cvs092. [DOI] [PubMed] [Google Scholar]
- 41.Vilarrasa N, Vendrell J, Maravall J, et al. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine. 2010 Oct;38:235–242. doi: 10.1007/s12020-010-9379-4. [DOI] [PubMed] [Google Scholar]
- 42.Krikken JA, Dallinga-Thie GM, Navis G, et al. Short term dietary sodium restriction decreases HDL cholesterol, apolipoprotein A-I and high molecular weight adiponectin in healthy young men: Relationships with renal hemodynamics and RAAS activation. Nutr Metab Cardiovasc Dis. 2010 Jul 31; doi: 10.1016/j.numecd.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000 Jun;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- 44.Briones AM, Nguyen Dinh Cat A, Callera GE, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012 May;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]