Abstract
Limitations with standard intradermal injections have created a clinical need for an alternative, low-cost injection device. In this study, we designed a hollow metal microneedle for reliable intradermal injection and developed a high-throughput micromolding process to produce metal microneedles with complex geometries. To fabricate the microneedles, we laser-ablated a 70 μm × 70 μm square cavity near the tip of poly(lactic acid-co-glyoclic acid) (PLGA) microneedles. The master structure was a template for multiple micromolded PLGA replicas. Each replica was sputtered with a gold seed layer with minimal gold deposited in the cavity due to masking effects. In this way, nickel was electrodeposited selectively outside of the cavity, after which the polymer replica was dissolved to produce a hollow metal microneedle. Force-displacement tests showed the microneedles, with 12 μm thick electrodeposition, could penetrate skin with an insertion force 9 times less than their axial failure force. We injected fluid with the microneedles into pig skin in vitro and hairless guinea pig skin in vivo. The injections targeted 90% of the material within the skin with minimal leakage onto the skin surface. We conclude that hollow microneedles made by this simple microfabrication method can achieve targeted intradermal injection.
Keywords: drug delivery, hollow microneedle, intradermal injection, micromolding, electrodeposition, skin
1 Introduction
Intradermal injection has pharmacological benefits over alternative injection methods for various different drug delivery scenarios. For vaccines, injection into the skin enables dose sparing and increased immunogenicity (Hickling et al. 2011; Kim and Prausnitz 2011a). For dermatological medicines, intradermal delivery targets drugs to the site of action in the skin with higher bioavailability than topical application (You et al. 2010). Newer applications of intradermal injection include improved lymphatic uptake of etanercept to treat autoimmune diseases like rheumatoid arthritis (Harvey et al. 2011) and expedited pharmacokinetics of insulin delivery to improve treatment of diabetes mellitus (Pettis et al. 2011; Gupta et al. 2011a).
Despite the pharmacological advantages, intradermal injections are not widely used. The standard Mantoux technique requires a specially trained healthcare provider to insert a hypodermic needle at a grazing angle above the skin surface into the skin, which is approximately 1 – 2 mm thick (Walker et al. 1990). Even when performed by trained professionals, intradermal localization is unreliable (Tarnow and King 2004).
To simplify intradermal delivery, we and others have fabricated hollow microneedles that target drug delivery into the skin with needles that are shorter than the thickness of the skin (Kim et al. 2004; Davis et al. 2005; Moon and Lee 2005; Stoeber and Liepmann 2005; Pérennès et al. 2006; Alarcon et al. 2007; Lippmann et al. 2007; Ovsianikov et al. 2007; Roxhed et al. 2007; Burton et al. 2011; Li et al. 2012). These devices are expected to reliably target injections into the skin, even by healthcare professionals without special training on their use (Laurent et al. 2007). Additionally, microneedles may improve patient acceptability compared to intradermal injections due to their small size and reduced pain (Gill et al. 2008; Birchall et al. 2011; Gupta et al. 2011b).
Previous research provides four guidelines to optimize microneedle design. First, microneedles should have a sharp tip to reduce the force required for insertion into the skin (Davis et al. 2004; Roxhed et al. 2007). Second, microneedles with side-opened lumens, off-center from the tip, may reduce injection pressure by initiating flow away from where the skin is most compressed (Griss and Stemme 2003; Martanto et al. 2006; Roxhed et al. 2007). Third, microneedles need an appropriate combination of material strength and geometry to survive insertion into skin without mechanical failure (Davis et al. 2004; Roxhed et al. 2007; Lee et al. 2008; Li et al. 2012). Fourth and finally, the fabrication process should rely only on inexpensive methods such as micromolding, ideally rejecting complicated two-piece molds or fragile demoldable cores. (Davis et al. 2005; Kim and Colton 2005; Xu et al. 2005; Lippmann et al. 2007; Matteucci et al. 2009; Burton et al. 2011). A microneedle meeting these criteria could easily insert into skin, inject at a moderate pressure to reduce patient pain, and compete on costs with needles and syringes.
In this study, we present a fabrication process based on sacrificial micromolding and selective electrodeposition meeting the design criteria listed above. In addition, we characterize the device’s insertion into skin and report in vitro and in vivo delivery studies with pig and hairless guinea pig skin.
2 Device Fabrication
We fabricated microneedle devices using a seven-step process: (i) fabrication of the master structure with a laser-ablated cavity, (ii) creation of a micromold based on the master structure (iii) creation of replicas of the master structure using the micromold, (iv) sputtering a gold seed layer onto the replicas, (v) selective electrodeposition to form the hollow microneedle structure, followed by (vi) dissolving of the sacrificial replicate structure to release the hollow metal microneedle and (vii) integration of the microneedle with a syringe-based pressure source for fluid delivery. Fig. 1 shows a schematic of the fabrication process, described in detail below.
Fig. 1.

Fabrication sequence. (A) Fabrication of the master structure with a laser ablated cavity. (B) Creation of a micromold based on the master structure featuring a protruding pillar that will become the lumen exit hole in the final device. (C) Creation of a replica using the micromold. (D) Sputtering a gold seed layer onto the replica. (E) Electrodeposition of metal everywhere except the cavity, followed by dissolving of the sacrificial base material to release the metal microneedle.
2.1 Master Structure
We lathed a brass rod (VS Lathe, Harrison, West Yorkshire, UK) to produce a disk measuring 7 mm in diameter and 1 mm in thickness with a tapered cylinder (i.e., solid microneedle) measuring 1.1 mm tall, 225 μm in radius at the base, and 20 μm in radius at the tip, which was centered on top of the disk. A poly(lactic acid) (PLA) replica of the lathed structure was made using PDMS molding and melt casting (Park et al. 2005). To accomplish this, the lathed structure was attached to an aluminum dish (5 cm diameter, 1.5 cm deep) using double-stick tape, and covered with 15 g of poly(dimethylsiloxane) (PDMS) (Sylgard 184, Dow Corning, Midland, MI). The PDMS was cured at 37°C for 24 h, and the PDMS mold was separated from the lathed structure by hand. PLA pellets (3051D, NatureWorks, Minnetonka, MN) were placed onto the PDMS mold, which was then placed in a 195°C vacuum oven. Vacuum was applied after the PLA had melted to degas the polymer and force it into the mold. After stopping the vacuum and cooling the samples, the PLA was separated from the mold by hand.
After molding, we laser-ablated a cavity in the PLA replica to create the feature that forms the lumen exit hole on hollow microneedles. The 70 μm × 70 μm cavity was ablated using a 248-nm excimer laser (Resonetics, Nashua, NH). The cavity was centered ~135 μm below the tip at a 45° angle relative to the base and was ~100 μm deep. The ablated microneedle served as the master structure and template for micromolding replicas.
2.2 Replicating the Master Structure
The PDMS molding and melt-casting procedures described above were used to create poly(lactic acid-co-glycolic acid) (PLGA) replicas of the master structure. The PLGA-filled mold was placed in a −20°C freezer for 5 min, taking advantage of differences in thermal expansion coefficients of PLGA and PDMS to facilitate demolding. Low molecular weight PLGA (7525 DLG 7A, Surmodics, Birmingham, AL) was used because it dissolves rapidly for sacrificial micromolding. Although the molds have a small, non-vertical core (Fig. 1) that could complicate demolding, we did not experience problems because the PDMS molds were sufficiently flexible.
2.3 Seed Layer and Electrodeposition
We sputtered a gold seed layer onto each PLGA replica using an EMS 500 sputter coater (Electron Microscopy Sciences, Hatfield, PA). To monitor the variance in seed layer thickness, we measured the resistance across the diameter of each sample (edge-to-edge resistance) using a standard Ohmmeter. Thicker seed layers would have reduced edge-to-edge resistance.
We expect, because of masking effects, much less gold was deposited in the cavity compared to the rest of the solid microneedle structure. Therefore, the resistivity inside the cavity would be higher compared to the rest of the sample, enabling selective electrodeposition outside the cavity. As described previously (Zhang et al. 2008), for a sample with heterogeneous resistivity, the reduction of metal from the electroplating bath is more difficult on the area of high resistivity compared to the area of low resistivity.
We electrodeposited nickel onto individual solid microneedle devices using a Watts nickel bath (Watts Semi-Bright RTU, Technic Inc., Cranston, RI) and a constant-current power supply (E3611A, Hewlett Packard, Palo Alto, CA) with a range of 0–850 mA. The bath solution was stirred at 700 rpm, and the solution and anode were replaced after 36 h of electrodeposition.
To optimize operating conditions for deposition of metal onto the solid microneedle structure, without depositing over the cavity, we varied the seed layer thickness (as determined by edge-to-edge resistance) and the starting current of electrodeposition (Table 1). We tested seed layers with edge-to-edge resistances between ~10 Ω (thick seed layer) to ~44 Ω (thin seed layer). We varied the starting current from 0–9 mA.
Table 1.
Effects of starting conditions on electrodeposition process yield
| Edge-to-Edge Resistance (Ω)1 | Starting Current (mA)2 | Sample Size | Yield3 | Mode of Electrodeposition Failure |
|---|---|---|---|---|
| Low 10 ± 2 |
Medium 1.65 ≤ I ≤ 3.0 |
3 | 0% | Immediate over-deposition4 |
| Medium 20 ± 2 |
Low 1.0 ≤ I ≤ 1.65 |
3 | 0% | No deposition |
| Medium 22 ± 6 |
Medium 1.65≤ < I ≤ 3.0 |
32 | 63% | Some over-deposition |
| Medium 18 ± 4 |
High 3.0 < I ≤ 9.0 |
5 | 20% | Over-deposition |
| High 44 ± 5 |
Medium 1.65 < I ≤ 3.0 |
9 | 11% | Under-deposition5 |
Edge-to-edge electrical resistance (mean ± standard deviation) was used as a proxy measure for seed layer thickness.
Electrodeposition was carried out at a starting current for 6 min at the beginning of the process.
Yield: percent of devices with complete metal deposition on the mold surface and a patent lumen exit hole.
Over-deposition: electrodeposition of metal covering the lumen exit hole.
Under-deposition: electrodeposition of some metal, insufficient to cover the microneedle surface.
Thick seed layers led to immediate over-deposition of metal, filling the cavity and preventing hollow microneedle formation. With thick seed layers, presumably even the cavity had sufficiently low resistivity for electrodeposition. Thin seed layers suffered the opposite problem: insufficient metal deposition to cover the microneedle surface.
For intermediate seed layer thicknesses (R ~ 20 Ω), the deposition pattern changed depending on the starting current. Low currents ( I < 1.65 mA) led to under-deposition, high currents ( I > 3.0 mA) led to over deposition. Optimal conditions were found at a starting current of 1.65 < I < 3.0 mA. The ability to switch between correct deposition and over-deposition suggests selective electrodeposition is driven by seed layer resistivity differences in the cavity.
After 6 min at the starting current, we expedited the electrodeposition process by ramping up the current by 0.125 mA/min to a peak current of 4.75 mA for 2 h. Although the current increased, we did not lose the selectivity of the electrodeposition. We believe this was because as metal was deposited outside the cavity, the resistivity of the microneedle surface decreased and thereby increased the selectivity of metal deposition outside the cavity.
2.4 Dissolving the Sacrificial Replicate Structure
After this electrodeposition process, samples were inspected using bright-field microscopy (SZX 16, Olympus, Center Valley, PA). By shining a light on the backside of the sample, we determined which samples had maintained a patent lumen exit hole by seeing if the light passed through (Fig. 2B). Samples passing inspection were suspended in an acetone bath stirred at 100 rpm for 1 h. The PLGA and the seed layer inside the cavity dissolved and disintegrated, releasing the metal hollow microneedle. We then electrodeposited a second time on these samples at 5.5 mA for 2 h to increase the metal thickness with no risk of over-deposition. The final metal thickness of five representative samples was 12.4 ± 1.4 μm, measured using brightfield microscopy.
Fig. 2.

Optical microscopy images of the microneedle device. (A) Hollow microneedle imaged with direct lighting. (B) Hollow microneedle imaged with light shining through the backside, which illuminates the lumen exit hole of the microneedle. (C) Cross-section of the PDMS micromold with arrow pointing to demoldable core that forms the lumen exit hole of the microneedle. (D) Device assembly used for injections with a hollow microneedle glued to a curved acrylic surface mounted on a luer lock.
2.5 Device Assembly
The fabricated microneedle was glued (Epoxy Plastic Bonder, Loctite, Rocky Hill, CT) to an acrylic device holder with a half-cylinder shape and aligned with a 3 mm diameter through-hole. The flat edge of the acrylic was glued using cyanoacrylate glue (Super Glue Gel, Loctite, Rocky Hill, CT) onto a nylon female luer lock cap (McMaster-Carr, Atlanta, GA) which also had an aligned 3 mm diameter through-hole. The finished assembly was mounted onto a syringe.
2.6 Final Device Imaging
Images of fabricated hollow microneedles are shown in Fig. 2. Fig. 2A shows a hollow microneedle after the fabrication process. Fig. 2B shows light passing through the lumen exit hole. Metal can also be seen inside the needle in Fig. 2B. This short, tubular vestige from the cavity reveals the boundary of selective electrodeposition. Fig. 2C shows a PDMS mold used for the micromolding-based fabrication. Fig. 2D is an image of a microneedle assembled onto a luer hub for connection to a syringe.
3 Force Displacement Measurement
We followed a method described previously (Park et al. 2005) to characterize the axial failure force of the microneedles. Briefly, the needles were pressed against a steel plate at a constant speed until a discontinuity was observed in the force versus displacement graph. The force at the onset of the discontinuity was the measured failure force.
3.1 Experimental Results
The axial failure force for the hollow microneedles was 0.74 ± 0.11 N (average ± standard deviation, n =5 replicate measurements). A representative force-displacement curve is shown in Fig. 3. Visual observation showedthe mode of failure was telescopic collapse of the needles similar to seen previously with hollow metal microneedle (Davis et al. 2004). The predicted force required to insert a needle with this tip geometry into human skin is 0.086 N (Davis et al. 2004). The measured failure force is close to an order of magnitude greater than the predicted insertion force, which provides a large margin of safety.
Fig. 3.

Representative force-displacement curve for a microneedle pressed against a rigid surface. A discontinuity representing microneedle failure is seen at 0.025 mm displacement with a peak force of 0.072 N.
4 Insertion and Injection into Pig Skin In Vitro
4.1 Insertion into Pig Skin
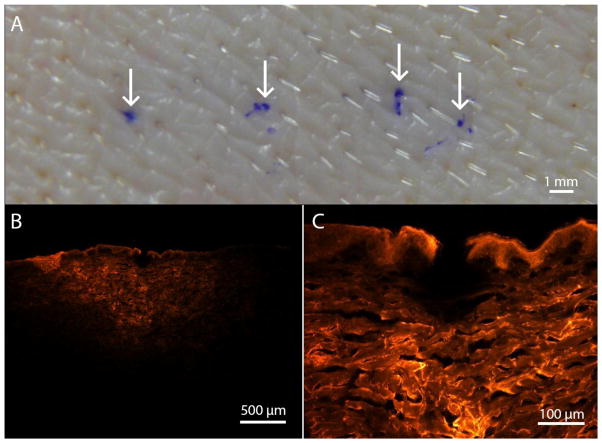
To validate the prediction that our microneedles should be able to be inserted into skin without mechanical failure, we pressed microneedles into pig skin in vitro, and then stained the insertion sites with gentian violet (Humco, Texarkana, TX), which selectively stains sites of microneedle penetration and not intact skin (Gill et al. 2008). The microneedles were mounted onto a 3 ml syringe and applied to the skin by hand. As shown in Fig. 4A, gentian violet staining confirmed the hollow microneedles were able to penetrate skin. We made four microneedle insertion attempts in this way each using a different microneedle, and all of them provided effective skin penetration. Additional successful microneedle insertions accompanied by fluid injection are described below.
Fig. 4.
Microneedle injection into pig skin in vitro. (A) Photograph of skin surface after insertion of four microneedles followed by gentian violet staining (arrows indicate insertion sites). The stain demonstrates penetration of the microneedles into skin. (B) Fluorescence microscopy image of a skin cross-section after injection of red-fluorescent sulforhodamine B using a microneedle. (C) Magnified view showing the site of needle insertion into skin.
4.2 Injection Into Pig Skin
We also verified the microneedles could inject fluid into skin. Using microneedles mounted onto 3 ml syringes, we applied thumb pressure for 20 s to inject 0.1 ml of a 1 mM solution of a fluorescent dye, sulforhodamine B (Sigma-Aldrich, St. Louis, MO), in phosphate-buffered saline (PBS) (Sigma-Aldrich). After injection, 1 cm x 1 cm pieces of skin were cut out, sectioned, embedded in Optimal Cutting Temperature compound (Sakura Finetek, Torrance, CA), and frozen on dry ice. The skin was cut into 10 μm sections using a Microm cryostat (Thermo Fisher, Walldorf, Germany). Skin sections, excited by a BH2-RFL-T3 light source (Olympus, Center Valley, PA), were photographed using a SZX16 microscope and DP71 camera (Olympus) to examine the distribution of sulforhodamine B in the skin.
Fig. 4B shows a representative histological cross-section of the sulfrohodamine B delivery confirming these microneedles can deliver fluid into skin. The needle insertion site is shown at greater magnification in Fig. 4C. We examined three skin sites after injection in this way, each using a different microneedle, which resulted in intradermal injection.
5 Insertion and Injection Into Hairless Guinea Pig Skin In Vivo
We tested insertion and injection using microneedles in vivo on a hairless guinea pig (Charles River Laboratory, Wilmington, MA) with approval from the Georgia Tech Institutional Animal Care and Use Committee (IACUC). The guinea pig was anesthetized using isofluorane gas in oxygen and sustained in anesthesia using a nose cone. The microneedle was manually inserted into the skin on the back of the guinea pig, and pressure was applied to the syringe plunger by hand. We delivered approximately 50 – 75 μL of a 1 mM solution of sulforhodamine B in PBS. We measured the peak pressure using a digital manometer (Model 220-95, Netech, Farmingdale, NY). After delivery, we applied gauze to the delivery site for 10 s and then placed the gauze in 4 mL of PBS for 30 min. The PBS solution was analyzed using a fluorometer (Spectramax Gemini XS, Molecular Devices, Sunnyvale, CA) to determine the amount of dye leaked onto the skin surface. At the end of the experiment, the guinea pig was euthanized using carbon dioxide and 1 cm × 1 cm pieces of skin and subcutaneous tissue were cut into 14 μm-thick sections as frozen sections and imaged as described above. Images were split into red, green, and blue channels using Image J (Rasband 1997–2009). The fraction of dye delivered to the skin was calculated as the red intensity in the epidermis and dermis divided by the red intensity of the whole tissue section.
5.1 Experimental Results
Fig. 5 shows a representative photographic image and histological cross-section of an injection site after microneedle injection into a guinea pig. The peak pressure for intradermal injection with microneedles was 16.4 ± 2.6 psig (average ± standard deviation, n = 3). Using the image analysis technique described above, the mean fraction of dye delivered to the skin is 90 ± 7%(n = 4; two from guinea pig, two from pig in vivo – data not shown).
Fig. 5.

Microneedle injection into hairless guinea pig skin in vivo. (A) Photograph of the skin surface on the back of the animal showing injection of red-colored sulforhodamine B into the skin. (B) Fluorescence microscopy image of a skin cross-section after injection of red-fluorescent sulforhodamine B using a microneedle. On average, 90% of the dye was localized in the skin, which demonstrates effective targeting to the skin.
6 Discussion
We fabricated and tested hollow microneedles with the following advantageous features: (i) sharp tips, (ii) lumen exit holes located on the side of needles just below the tips, (iii) metal as the material of construction, and (iv) a micromolding-based fabrication process.
Human skin thickness varies across the body, but is generally 1 – 2 mm thick (Ploin et al. 2011). Here, we used microneedles measuring 1.1 mm in length to target injection into the skin by pressing the needle perpendicularly against the skin surface. This method should improve upon the conventional Mantoux technique that is unreliable even for trained experts.
The choice of fabricating a single, conical microneedle with a 20–25° taper was based on the observation that similarly shaped glass microneedles fabricated by pulled-glass micropipette techniques could insert into skin and deliver up to 1 mL of fluid in human subjects (Gupta et al. 2011b). The needles we fabricated were similarly able to insert into skin and inject fluid without leaks. The ratio of microneedle failure force to predicted skin insertion force of approximately 9 provides a large safety margin, allowing reliable insertion without fracture. This large safety margin was enabled by reducing insertion force by having a sharp needle tip and increasing needle strength by using metal as the material of construction.
Micromolding of hollow microneedles is challenging. For this reason, most previous studies have used direct fabrication methods: high precision etching, grinding, or laser ablation in the production stage. (Moon and Lee 2005; Stoeber and Liepmann 2005; Roxhed et al. 2007; van Damme et al. 2009; Li et al. 2012). These methods are less attractive for inexpensive mass production because they require the use of sophisticated fabrication equipment often in a high-cost cleanroom environment to make each device. Direct fabrication of master structures followed by indirect molding-based replication should be much less expensive and enable the large-scale mass production needed for disposable needles. Among molding methods in the published literature, most have lumen exits at the microneedle tip which reduces sharpness and may impede fluid flow into tissue (Kim et al. 2004; Davis et al. 2005; Lippmann et al. 2007; Lee et al. 2010). Others have made molded hollow microneedles with side-opening lumen exit holes, but the molds were complex: either two-piece, aligned molds (Xu et al. 2005) or molds with seemingly fragile demoldable cores (Pérennès et al. 2006).
We introduced a fabrication method that lends itself to simpler mass production and produces microneedles with a sharp tip and side-opening lumen exit hole. After laser fabrication of a master structure, the master structure was repeatedly used to produce female micromolds. These micromolds were in turn used to make replicate structures employed as male micromolds for electrodeposition to produce metal microneedles. While this approach involves an additional step to make the male micromolds compared to prior methods, it is an improvement because (i) it uses high-throughput molding methods to produce the microneedle devices and employs the lower-throughput laser etching process only to make the re-usable master structures and (ii) is able to mold metal microneedles with improved geometry that enable insertion and injection into skin.
The lumen exit hole was created by using a laser-generated divot in the master structure, which appeared to inhibit seed layer deposition and later electrodepositon. The divot in the master structure became a protruding core in the intermediate replicate mold, which might be expected to introduce complications in molding, especially during demolding. However, PDMS as the mold material provided sufficient flexibility and allowed reliable demolding with damaging the mold or the demolded structure.
We needed a balance to deposit metal onto the surface of the male mold, but not deposit metal within the cavity forming the lumen exit hole. A thin seed layer or a high starting current both led to over-deposition that covered the cavity. A medium seed layer thickness and a moderate starting current minimized over-deposition and enabled fabrication of suitable structures with reasonable yields. Further optimization of the microneedle geometry and electrodeposition process could improve yield.
Overall, this fabrication process produces metal hollow microneedles with simple, one-piece molds. Using an excimer laser to define the master geometry allowed precise positioning of the microneedle hole, and the PDMS-based molds were reproducible and reusable even with the angled pillar. The selective electrodeposition process may have other uses in microfabrication. Potential uses include depositing metal up to a certain distance/elevation, avoiding deposition in high-aspect-ratio holes, or bonding two conducting materials with different resistivities as in (Zhang et al. 2008).
7 Conclusion
This study presented, for the first time, a fabrication process based on sacrificial micromolding and selective electrodeposition to produce side-opened, sharp-tipped, hollow, metal microneedles. The resulting microneedles were shown to be sufficiently strong to permit reliable insertion into skin without failure and to enable injection into skin in vitro and in vivo. The micromolding approach presented here is expected to enable low-cost, mass production of microneedles, which is an advance over prior methods that relied on direct fabrication of microneedles or more complicated molds. With additional research and development, this microneedle design could enable simple, reliable intradermal injections.
Acknowledgments
The authors would like to thank Donna Bondy for administrative assistance, Richard Shafer for assistance with lasers, the Georgia Tech Physiological Research Laboratory staff for assistance and advice with animal studies, the Georgia Tech Research Institute for machining services, and the Georgia Tech Electron Microscopy Center for sputtering services. This work was supported by the National Institutes of Health. Mark Prausnitz is an inventor of patents that have been licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products.
Footnotes
This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University.
References
- Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Clin Vaccine Immunol. 2007;14:4. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchall JC, Clemo R, Anstey A, John DN. Pharm Res. 2011;28:1. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- Burton SA, Ng CY, Simmers R, Moeckly C, Brandwein D, Gilbert T, Johnson N, Brown K, Alston T, Prochnow G, Siebenaler K, Hansen K. Pharm Res. 2011;28:1. doi: 10.1007/s11095-010-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. J Biomech. 2004;37:8. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Davis SP, Martanto W, Allen MG, Prausnitz MR. IEEE Trans Biomed Eng. 2005;52:5. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- Gill HS, Denson DD, Burris BA, Prausnitz MR. Clin J Pain. 2008;24:7. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griss P, Stemme G. J Microelectromech Syst. 2003;12:3. [Google Scholar]
- Gupta J, Felner EI, Prausnitz MR. Diabetes Technol Ther. 2011a;13:4. doi: 10.1089/dia.2010.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J, Park SS, Bondy B, Felner EI, Prausnitz MR. Biomaterials. 2011b;32:28. doi: 10.1016/j.biomaterials.2011.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Pharm Res. 2011;28:1. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- Hickling J, Jones K, Friede M, Zehrung D, Chen D, Kristensen D. Bull WHO. 2011;89:3. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Colton JS. J Med Eng Technol. 2005;29:4. doi: 10.1080/03091900412331289898. [DOI] [PubMed] [Google Scholar]
- Kim K, Park DS, Lu HM, Che W, Kim K, Lee JB, Ahn CH. J Micromech Microeng. 2004;14:4. [Google Scholar]
- Kim YC, Prausnitz MR. Drug Delivery Transl Res. 2011a;1:1. doi: 10.1007/s13346-010-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Vaccine. 2007;25:52. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park JH, Prausnitz MR. Biomaterials. 2008;29:13. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Lee HC, Lee DS, Jung H. Adv Mater. 2010;22:4. doi: 10.1002/adma.200902418. [DOI] [PubMed] [Google Scholar]
- Li CG, Lee CY, Lee K, Jung H. Biomed Microdevices. 2012 doi: 10.1007/s10544-012-9683-2. [DOI] [PubMed] [Google Scholar]
- Lippmann JM, Geiger EJ, Pisano AP. Sens Actuators, A. 2007;134:1. [Google Scholar]
- Martanto W, Moore JS, Couse T, Prausnitz MR. J Controlled Release. 2006;112:3. doi: 10.1016/j.jconrel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Matteucci M, Fanetti M, Casella M, Gramatica F, Gavioli L, Tormen M, Grenci G, De Angelis F, Di Fabrizio E. Microelectron Eng. 2009;86:4–6. [Google Scholar]
- Moon SJ, Lee SS. J Micromech Microeng. 2005;2005:5. [Google Scholar]
- Ovsianikov A, Chichkov B, Mente P, Monteiro-Riviere NA, Doraiswamy A, Narayan RJ. Int J Appl Ceram Technol. 2007;4:1. [Google Scholar]
- Park JH, Allen MG, Prausnitz MR. J Controlled Release. 2005;104:1. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Pérennès F, Marmiroli B, Matteucci M, Tormen M, Vaccari L, Di Fabrizio E. J Micromech Microeng. 2006;16:3. [Google Scholar]
- Pettis RJ, Ginsberg B, Hirsch L, Sutter D, Keith S, McVey E, Harvey NG, Hompesch M, Nosek L, Kapitza C, Heinemann L. Diabetes Technol Ther. 2011;13:4. doi: 10.1089/dia.2010.0184. [DOI] [PubMed] [Google Scholar]
- Ploin D, Schwarzenbach F, Dubray C, Nicolas JF, Goujon C, Trong MD, Laurent PE. Vaccine. 2011;29:46. doi: 10.1016/j.vaccine.2011.07.111. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, MD: 1997–2009. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Roxhed N, Gasser TC, Griss P, Holzapfel GA, Stemme G. J Microelectromech Syst. 2007;16:6. [Google Scholar]
- Stoeber B, Liepmann D. J Microelectromech Syst. 2005;14:3. [Google Scholar]
- Tarnow K, King N. Appl Nurs Res. 2004;17:4. [PubMed] [Google Scholar]
- van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Vaccine. 2009;27:3. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Walker HK, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. Butterworths; Boston: 1990. [PubMed] [Google Scholar]
- Xu Y, Chen MM, Li Z, Lim CY, Tan PYJ. 2005/087305. WO. :A1.
- You SK, Noh YW, Park HH, Han M, Lee SS, Shin SC, Cho CW. J Drug Targeting. 2010;18:1. doi: 10.3109/10611860903115274. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhou Z, Yang X, Ye X, Wang ZL. Electrochem Solid-State Lett. 2008;11:9. [Google Scholar]