Abstract
The use of mesenchymal stromal cells (MSCs) for treatment of bacterial infections, including systemic processes like sepsis, is an evolving field of investigation. The present study was designed to investigate the potential use of MSCs, harvested from compact bone, and their interactions with the innate immune system, during polymicrobial sepsis induced by cecal ligation and puncture (CLP). We also wanted to elucidate the role of endogenous HO-1 in MSCs during a systemic bacterial infection. MSCs harvested from the bones of heme oxygenase (HO)-1 deficient (−/−) and wild-type (+/+) mice improved the survival of HO-1−/− and HO-1+/+ recipient mice when administered after the onset of polymicrobial sepsis induced by cecal ligation and puncture (CLP), compared with the administration of fibroblast control cells. The MSCs, originating from compact bone in mice, enhanced the ability of neutrophils to phagocytize bacteria in vitro and in vivo, and to promote bacterial clearance in the peritoneum and blood after CLP. Moreover, after depleting neutrophils in recipient mice, the beneficial effects of MSCs were entirely lost, demonstrating the importance of neutrophils for this MSC response. MSCs also decreased multiple organ injury in susceptible HO-1−/− mice, when administered after the onset of sepsis. Taken together, these data demonstrate that the beneficial effects of treatment with MSCs after the onset of polymicrobial sepsis is not dependent on endogenous HO-1 expression, and that neutrophils are crucial for this therapeutic response.
Keywords: polymicrobial sepsis, mesenchymal stromal cells, compact bone, neutrophils, bacterial clearance, cytoprotective
Introduction
Mesenchymal stromal cells (MSCs) are a population of cells originally isolated from bone marrow [1], although MSCs have also been identified in other tissues and organs [2–5]. MSCs derived from the bone marrow contribute to the formation of hematopoietic stem cell (HSC) niches [6], and MSCs also exist in compact bone [7]. MSCs are considered to be a promising platform for cell-based therapy, and in preclinical animal models, MSCs have been shown to contribute to tissue repair despite limited levels of engraftment and transdifferentiation [8]. It appears that a critical property of MSCs, in limiting tissue injury, is modulation of the immune response. MSCs have the ability to regulate both the innate and the adaptive immune responses in a variety of disease models [6, 9, 10].
Investigators have recently begun to explore the potential of stem cell therapy in sepsis, a complex disease process due to a severe underlying infection leading to a systemic inflammatory response [11–14]. Even with standard therapeutic approaches for sepsis, morbidity and mortality rates remain high [15, 16]. Progression to severe sepsis, and the development of shock, often leads to multiple organ dysfunction/failure and death. Thus, new strategies are necessary to advance our understanding of the pathophysiology and treatment of sepsis. MSCs originating from either bone marrow [17, 18] or adipose tissue [19] have been shown to be beneficial in abdominal sepsis induced by cecal ligation and puncture (CLP) in mice. In addition, human bone marrow-derived MSCs have been studied in bacterial pneumonia models in mice [20]. Due to their immunomodulatory properties and their ability to protect organs from injury [9, 21], MSCs are being considered for therapy in sepsis.
A cytoprotective molecule that has also been shown to be advantageous during sepsis is heme oxygenase (HO)-1. HO-1 is highly inducible by inflammation, and important for heme degradation. The products of heme breakdown include carbon monoxide, biliverdin, and ferrous iron [22]. Expression of HO-1 is protective during CLP-induced sepsis in mice [23], and HO-1 and its products have been shown to have anti-inflammatory, anti-oxidant, and anti-apoptotic properties [24–28]. Since similar properties of MSCs are vital for tissue repair [8], we wanted to further explore the role of HO-1 in MSC function. HO-1 has been shown to be important for the immunosuppressive response of MSCs during heart allo-transplantation [29] and ischemic injury [30] in rats, and also in T-cell function [31]. However, the role of HO-1 in MSCs interacting with the innate immune response during sepsis is not known.
At the present time the predominance of MSC studies, including investigations in sepsis, endotoxemia, and lung injury, have focused on bone marrow-derived cells [17, 18, 20, 32–36]. The isolation of MSCs from bone marrow is more challenging in mice compared with humans or other animals, due to the lower frequency of MSC in bone marrow [37] and contamination of hematopoietic cells in culture. Recently, MSCs have been harvested in large numbers from compact bone of mice [7], and shown to have immunoregulatory properties in vivo [38–40]. However, the potential benefit of these MSCs in sepsis has not been investigated. The present study focused on MSCs from compact bone and their interactions with the innate immune response during CLP-induced sepsis, and elucidated the importance of endogenous HO-1 in these cytoprotective cells. To eliminate the effects of HO-1 expression in non-MSCs, our studies included the use of HO-1 null (−/−) mice [41], which are known to be very susceptible to tissue injury associated with sepsis in mice [23].
Materials and Methods
Reagents
Mouse (m) interleukin (IL)-1β, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and granulocyte-colony stimulating factor (G-CSF) were purchased from R&D Systems (Minneapolis, MN).
HO-1 deficient mice
HO-1−/− mice were generated as described [41], and bred back to a pure BALB/c genetic background. HO-1+/+ mice were littermate mice from heterozygous breeding.
Isolation and characterization of cells from compact bone
MSCs were harvested from mouse compact bone as described previously [7], with modifications. In brief, femurs and tibias of mice (either HO-1+/+ or HO-1−/− mice) were dissected and the marrow was flushed out. Cells from compact bone were isolated following mechanical digestion (crushing and enzymatic digestion with 0.1% collagenase I and 0.25% collagenase II with 2% FBS for 45 minutes). The isolated cells were filtered via a 70 μm cell strainer, and centrifuged at 300 g for 10 minutes at room temperature. The supernatant was removed, red blood cells lysed, and the cells were plated for expansion using MesenCult Proliferation Kit (StemCell Technologies, Vancouver, BC). Next, MSCs were isolated by negative selection using the EasySepR mouse mesenchymal stem/progenitor cell enrichment cocktail (StemCell Technologies). Lineage depleted cells were plated in full medium (StemCell Technologies) and expanded for two passages prior to immunophenotyping and use. For flow cytometric analyses, cells were analyzed using a BD FACS Canto II. At least 10,000 events were collected and data was analyzed using FlowJo software. Antibodies used for cytometric immunophenotyping of cells are listed in Supplemental Information, Table S1.
Isolation and characterization of lung fibroblasts
Lungs were harvested from wild-type BALB/c mice, and fibroblasts isolated (lineage negative/Sca1-depleted cells). The cells were plated in medium and expanded prior to immunophenotyping and use. See the Supplemental Information for further details.
Differentiation Assays
Multilineage differentiation capacity of MSCs harvested from compact bone of HO-1+/+ and HO-1−/− mice was performed as previously described [7]. The cells were differentiated into osteoblasts, adipocytes, and chondrocytes.
CLP model of polymicrobial sepsis
To induce polymicrobial sepsis, we utilized an established murine model of cecal ligation and puncture (CLP) as previously described [23, 42]. For experiments using HO-1−/− mice and mice after neutrophil depletion, two-thirds of the cecum was tied off and one 19-gauge hole was used for CLP. For experiments using wild-type mice, a more severe injury was performed, with two-thirds of the cecum tied off and two 21-gauge holes. Sham-operated mice underwent the same procedure, including opening of the peritoneum and exposing the bowel, but without ligation and needle perforation of the cecum. All mice undergoing CLP or sham procedures were male. Animal care and use for all experiments was approved by the Harvard Medical Area Standing Committee on Animals, Harvard Medical School.
Cell administration after CLP
To assess the efficacy of cellular treatment, mice received an intravenous (i.v.) injection via the tail vein of MSCs from HO-1+/+ or HO-1−/− mice, or lung-derived fibroblasts, at a dose of 5.0 × 105 cells in 200 μl of phosphate buffered saline (PBS) 2 hours post-CLP. Additional tail vein i.v. injections of 2.5 × 105 cells in 200 μl of PBS were given 24 and 48 hours post-CLP. As a non-cellular control group in survival experiments, PBS was administered in a volume of 200 μl, at the same time points. Since the half-life of MSCs administered i.v. is ≈24 hours [43], we gave a total dose of cells comparable to other studies [17, 44], but divided the cells into 3 separate doses to prolong the time of viable MSCs after the onset of sepsis.
Depletion of neutrophils
To deplete neutrophils, mice were injected intraperitoneally (i.p.) with Ly6G (clone 1A8) antibody at a dose of 10 mg/kg [45], 12 hours prior to the CLP surgery. In separate experiments, neutrophils were depleted as above, and CLP performed followed by i.v. administration of HO-1+/+ MSCs, fibroblasts, or PBS at 2, 24, and 48 hours after surgery. Rat IgG was used as a control antibody for the Ly6G antibody.
Bacterial phagocytosis and assessment of blood/peritoneal CFUs
In vitro phagocytosis
To isolate peritoneal neutrophils, HO-1−/− mice were given an i.p. injection of a 2% solution of Bio-Gel P100 polyacrylamide beads (Bio-Rad, Hercules, CA). After 24 hours, 10 ml of sterile PBS was used to lavage the peritoneal cavity, and cells were washed and labeled with Gr-1 microbeads (Miltenyi Biotec, Auburn, CA). Following magnetic selection, Gr-1 positive cells were placed in RPMI 1640 with 0.3% BSA and 10 mM HEPES. To activate the neutrophils, 10 ng/mL of m G-CSF was added to the medium, and incubated for an additional 2 hours. Following activation, MSCs from HO-1+/+ or HO-1−/− mice were added at a ratio of 1 MSC to 10 neutrophils. GFP labeled Escherichia (E.) coli (strain MMB1287) and Enterococcus (E.) faecalis (strain V583) were then added at 10 multiplicity of infection (MOI) per neutrophil. To examine the extent of phagocytosis [46], cells were harvested at 2 hours, washed, and incubated with Ly6G-APC conjugated antibody (BioLegend, San Diego, CA, clone 1A8). Following staining, cells were fixed (BD Cytofix/Cytoperm™, BD Biosciences) and flow cytometry was performed using a BD FACS Canto II. At least 10,000 events were collected and data was analyzed using FlowJo software and changes were documented as mean fluorescent intensity (MFI).
In vivo phagocytosis
To perform in vivo phagocytosis assays, HO-1−/− mice were injected i.p. with 2×107 live GFP-labeled E. coli (strain MMB1287). The mice were subsequently injected with either MSCs or fibroblasts, 1×106 cells per mouse. After one hour of incubation, the mice were anesthetized and a peritoneal lavage was performed. The cells from the lavage were treated with 0.2% of trypan blue for 1 minute to quench extracellular fluorescence, and then incubated with Ly6G-APC conjugated antibody (clone 1A8). Labeled cells were fixed, and flow cytometry was performed as described for the in vitro neutrophil phagocytosis assay.
Bacterial CFUs
Serial log10 dilutions of whole blood were performed, and aliquots were cultured on Luria-Bertani (LB) agar plates as described [23, 47]. CFUs were counted following overnight incubation at 37°C. In addition, serial log10 dilutions of peritoneal lavages were also performed, and CFUs counted.
Histology
Mice were sacrificed 24 hours following CLP and the spleens, livers, small bowels (ileum), and kidneys were harvested for histological evaluation. The tissues were fixed in 10% formalin, processed, embedded in paraffin, and sections (5 μm) were stained with hematoxylin-eosin (H&E) and periodic acid-Schiff staining (PAS) (Sigma-Aldrich, St. Louis, MO). Liver, kidney, small bowel, and spleen sections were stained with Gr-1 antibody (BD Biosciences). To assess injured/dying cells, formalin-fixed-paraffin sections were stained for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore, USA). Tissue sections stained with Gr-1 and TUNEL were scored by an investigator who was blinded to the treatment. The area of positively stained cells was calculated per 40X objective using FRIDA Software (FRamework for Image Dataset Analysis, [47]), and 10 fields were assessed per tissue section.
Tissue Injury
Tissue sections stained with H&E and PAS were evaluated by a pathologist who was blinded to the experimental procedures. An injury score was assigned to each tissue. For the ileum, scoring was performed for mucosal injury as recently described [47]. Mucosal scoring included grade 0, normal mucosal villi; grade 1, development of subepithelial space; grade 2, extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria; and grade 3, massive epithelial lifting down the sides of villi, possibly with a few denuded tips. Kidney injury was graded as previously described [48], 0 to 5 according to the percentage of tubules that displayed cell necrosis, loss of brush border, cast formation, and tubule dilation (0 = none, 1 = <10%, 2 = 11–25%, 3 = 26 to 45%, 4 = 46–75%, and 5 = ≥76%). Liver injury was scored according to the system developed by Sneed and colleagues [49]. Necrosis, hemorrhage, parenchymal inflammatory infiltrate, and sinusoidal inflammatory infiltrate were each individually scored from 0 (normal) to 4 (severe). The scores from each category were summed, providing a total hepatic injury score. Finally, for the spleen, injury was assessed according to changes in size and cellularity of the white pulp (periarteriolar sheaths, follicles, and marginal zones) and the red pulp as described [50]. Also, changes in germinal center development in lymphoid follicles were determined. From this assessment, injury was graded as 0 (normal), 1 (mild), 2 (moderate), and 3 (severe).
Statistical analysis
Data are expressed as mean ± SEM. Comparisons of mortality were made by analyzing Kaplan-Meier survival curves, and then log-rank test to assess for differences in survival. For comparisons between two groups, we used Student’s two-tailed unpaired t test, or Mann-Whitney U testing for non-parametric analyses. One-way analysis of variance followed by Newman-Keuls post test, or Kruskal-Wallis one-way analysis of variance followed by Dunn’s post test, was used for analysis of more than two groups. Nonparametric statistical analyses were performed in circumstances where the data were not normally distributed. The numbers of samples per group (n), or the numbers of experiments, are specified in the figure legends. Statistical significance is accepted at P<0.05.
Results
HO-1+/+ and HO-1−/− MSCs from Compact Bone are Phenotypically Similar
MSCs harvested from compact bone of HO-1+/+ and HO-1−/− mice were phenotyped by their ability to adhere to plastic and form CFU-Fs, to express specific cell surface markers, and to demonstrate multipotent differentiation potential (Supplemental Information, Figures S1 and S2). MSCs harvested from HO-1+/+ mice showed evidence for expression of mesenchymal markers including CD105, CD73, CD140b, CD44, CD29, and CD51 (Figure S1A). These cells also expressed Sca1, but not the receptor of stem cell factor, c-kit. There was no evidence for expression of hematopoietic lineage markers such as CD45 and CD34. The percentage of MSCs expressing MHC II was minimal. HO-1+/+ MSCs also expressed no CD133 and minimal Flk-1, the receptor for vascular endothelial growth factor. MSCs harvested from compact bone of HO-1−/− mice (Figure S1B) showed a very similar phenotype as wild-type cells, with the only clear difference being a higher percentage of HO-1−/− cells expressing Sca1. We also assessed the expression levels of mesenchymal markers in MSCs harvested from HO-1+/+ and HO-1−/− mice, using mean fluorescence intensity (Figure S2D). The expression levels of the majority of mesenchymal makers was not different between MSCs harvested from HO-1−/− and HO-1+/+ mice. Only expression of CD105 appeared to be higher in HO-1−/− cells compared with HO-1+/+ cells. In regard to differentiation potential, MSCs from both HO-1+/+ and HO-1−/− mice were able to differentiate in vitro into osteoblasts, adipocytes, and chondrocytes (Figure S1C). We quantitated the degree of osteoblast differentiation [51] in these bone-derived cells and found that HO-1+/+ and HO-1−/− MSCs differentiate to a similar degree (Figure S2E).
To further demonstrate the similarity of cell phenotypes, HO-1+/+ and HO-1−/− MSCs were exposed to pro-inflammatory mediators (IL-1β, TNF-α and IFN-γ) and assessed for the expression of CD54 and CD106 (Supplemental Information, Figure S3A and S3B). These molecules have been shown to be crucial for the immunoregulatory properties of MSCs [52]. The inflammatory stimuli resulted in an increased expression of CD54 and CD106, and the level of induction was not different in the presence or absence of HO-1 (Figure S3). These data suggest that MSCs harvested from compact bone of HO-1+/+ and HO-1−/− mice are similar phenotypically in vitro.
As a control cell for our studies, we used fibroblasts harvested from the lungs of wild-type mice. These cells expressed some mesenchymal markers, such as CD140b, CD105, CD44, and CD29 (Supplemental Information, Figure S4). However, they did not express CD73, and there was no evidence for expression of CD45, a marker of the leukocyte lineage of hematopoietic cells. Also, the cells harvested from the lungs were depleted for Sca1 to exclude contamination of fibroblasts with lung MSCs.
Exogenous Administration of HO-1+/+ and HO-1−/− MSCs Increases Survival of Mice After Onset of CLP-induced Sepsis
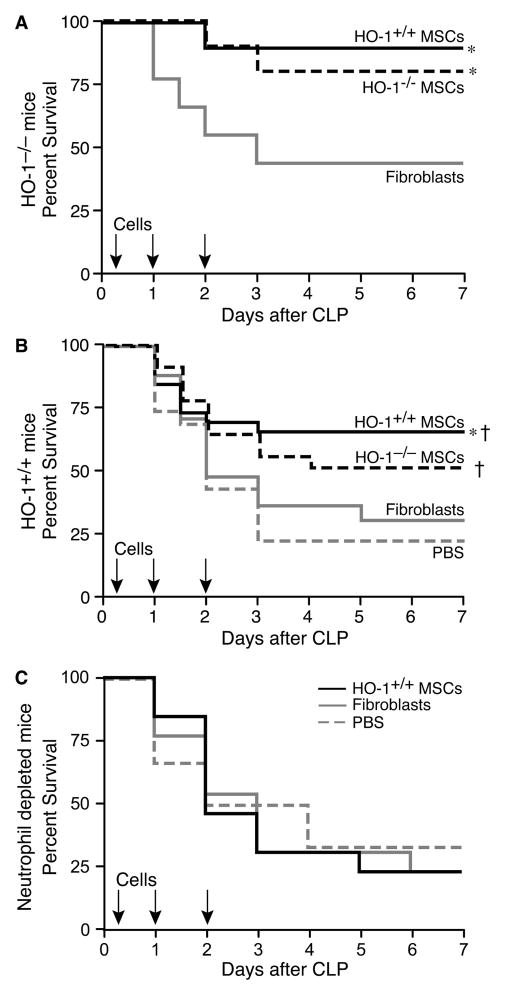
To understand the biological importance of HO-1 in MSCs in vivo, cells harvested from HO-1+/+ and HO-1−/− mice were administered to HO-1−/− mice after the onset of CLP-induced sepsis (two-thirds cecum ligated, one 19-gauge hole). HO-1−/− mice are very prone to organ injury and death in this clinically relevant model of sepsis [23]. When HO-1+/+ cells were administered (i.v.) 2 hours (5.0 × 105), 24 hours (2.5 × 105), and 48 hours (2.5 × 105) after the onset of polymicrobial sepsis, survival (90%) markedly increased compared with mice receiving fibroblast control cells (44%, Figure 1A). Treatment of HO-1−/− mice subjected to CLP-induced sepsis with HO-1−/− MSCs, administered at the same doses and time points, resulted in a comparable improvement in survival (80%) as mice treated with HO-1+/+ MSCs. These data revealed that expression of HO-1 in MSCs is not required for their beneficial effects during polymicrobial sepsis, even when mice deficient in HO-1 are the recipients of MSC treatment.
Figure 1.
MSCs improve survival following CLP-induced polymicrobial sepsis and neutrophils are critical for this response. A) HO-1−/− mice were treated with HO-1+/+ MSCs (solid black line, n=10), HO-1−/− MSCs (dashed black line, n=10) or fibroblasts (solid gray line, n=9) at 2, 24 and 48 hours post-CLP. Mice were monitored for survival over 7 days, and data are presented as a Kaplan-Meier survival curve. Survival for HO-1+/+ MSCs versus fibroblasts, * P=0.031; HO-1−/− MSCs versus fibroblasts, * P=0.045.
B) HO-1+/+ mice were treated with HO-1+/+ MSCs (solid black line, n=26), HO-1−/− MSCs (dashed black line, n=22), fibroblasts (solid gray line, n=17), or PBS (dashed gray line, n=18) at 2, 24 and 48 hours post-CLP. Mice were monitored for survival over 7 days, and data are presented as a Kaplan-Meier survival curve. Survival for HO-1+/+ versus fibroblasts (* P=0.049) and PBS († P=0.005); HO-1−/− MSCs versus PBS († P=0.032). C) HO-1+/+ mice receiving Ly6G antibody were treated with HO-1+/+ MSCs (solid black line, n=13), fibroblasts (solid gray line, n=13), or PBS (dashed gray line, n=6) administered 2, 24, and 48 hours post-CLP. Mice were monitored for survival over 7 days, and data are presented as a Kaplan-Meier survival curve.
We next administered MSCs to HO-1+/+ mice after the onset of CLP-induced sepsis (Figure 1B). A more severe model of CLP was induced in the cecum in wild-type mice (two-thirds cecum ligated, two 21-gauge hole), as they are not as susceptible to injury as HO-1−/− mice. The number of cells, volume of injections, and timing of cell administration was identical to the HO-1−/− recipients, and control groups included the administration of fibroblasts or vehicle (PBS). The lethality of mice receiving fibroblasts or PBS was not different, and the administration of HO-1+/+ MSCs increased survival by 36 to 49%, respectively (Figure 1B). Survival of mice receiving HO-1−/− MSCs was not significantly different from mice receiving HO-1+/+ MSCs, but was increased compared with mice receiving PBS.
To determine the importance of neutrophils—a critical cell involved in the immune response to bacterial infections—for the beneficial MSC response during sepsis, neutrophils were depleted in vivo by administering an antibody targeting Ly6G, as described previously [45]. The Ly6G antibody was given 12 hours prior to CLP (two-thirds cecum ligated, one 19-gauge hole), and circulating neutrophils (Supplemental Information, Figure S5A) were decreased 10-fold by the time of surgery, and remained depleted after 72 hours (Figure S5B). MSCs were not able to rescue mice from polymicrobial sepsis after depletion of neutrophils (Figure 1C), and their survival was comparable to mice receiving control fibroblasts or PBS. Moreover, when control rat IgG was administered instead of Ly6G antibody, there was no lethality in this less severe CLP model (data not shown). These data confirm the importance of neutrophils during the response to sepsis, and demonstrate that neutrophils are critical for the MSC-induced increase in survival.
MSCs Enhance Phagocytosis of Bacteria by Neutrophils, and Increase Bacterial Clearance During Polymicrobial Sepsis
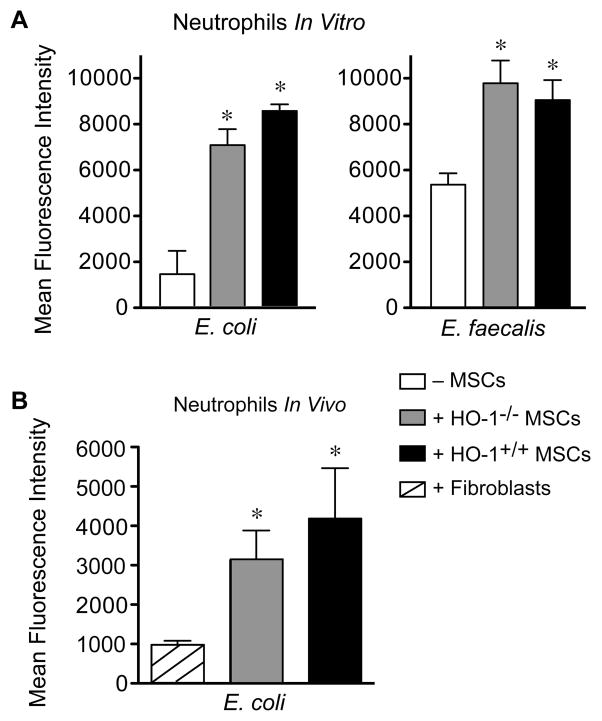
To further understand the beneficial effects of MSCs in CLP-induced sepsis, we performed bacterial phagocytosis assays, using neutrophils as important phagocytic cells involved in the response to infection. As shown in Figure 2A, neutrophil phagocytosis of E. coli was markedly increased in the presence of HO-1−/− (5-fold) or HO-1+/+ (6-fold) MSCs, as compared with neutrophils alone. Addition of fibroblasts did not alter neutrophil phagocytosis (data not shown). Phagocytosis of E. faecalis by neutrophils was also increased in the presence of HO-1−/− (1.8-fold) or HO-1+/+ (1.7-fold) MSCs. Representative images of flow cytometry pseudo-color density plots for neutrophil phagocytosis are shown in Supplemental Information, Figure S6A. MSCs alone did not phagocytize bacteria in the absence of neutrophils (Figure S6B). To translate this concept in vivo, E. coli were injected into the peritoneum of HO-1−/− mice, along with MSCs (either HO-1−/− or HO-1+/+) or fibroblasts. Neutrophil phagocytosis of E. coli was significantly increased in vivo in the presence of HO-1−/− (3.2-fold) or HO-1+/+ (4.4-fold) MSCs, as compared with neutrophils plus fibroblasts (Figure 2B). These data suggest that in an acute bacterial exposure, neutrophils play an important role in the ability of MSCs to increase phagocytosis, and this response is not dependent on expression of HO-1. Since HO-1+/+ and HO-1−/− MSCs were phenotypically and biologically similar in the setting of an exposure to bacteria, in vitro and in vivo, the remainder of studies were performed using HO-1+/+ MSCs.
Figure 2.
MSCs enhance the ability of peritoneal neutrophils to phagocytize bacteria. A) Activated peritoneal neutrophils were incubated with GFP-labeled E. coli or E. faecalis in the presence of MSCs (HO-1+/+, black bars or HO-1−/−, gray bars) or in the absence of MSCs (− MSCs, white bars) in vitro. Mean fluorescence intensity data are presented as mean±SEM from 3 independent experiments. *P<0.01 versus no MSCs, using Kruskal-Wallis one-way analysis of variance followed by the Dunn’s post test analysis. B) GFP-labeled E. coli were injected i.p. into HO-1−/− mice in the presence of MSCs (HO-1+/+, black bars or HO-1−/−, gray bars) or fibroblasts (striped bar) in vivo. Mean fluorescence intensity data are presented as mean±SEM of 3–4 mice per group. *P=0.043 versus fibroblasts, using Kruskal-Wallis one-way analysis of variance followed by the Dunn’s post test analysis.
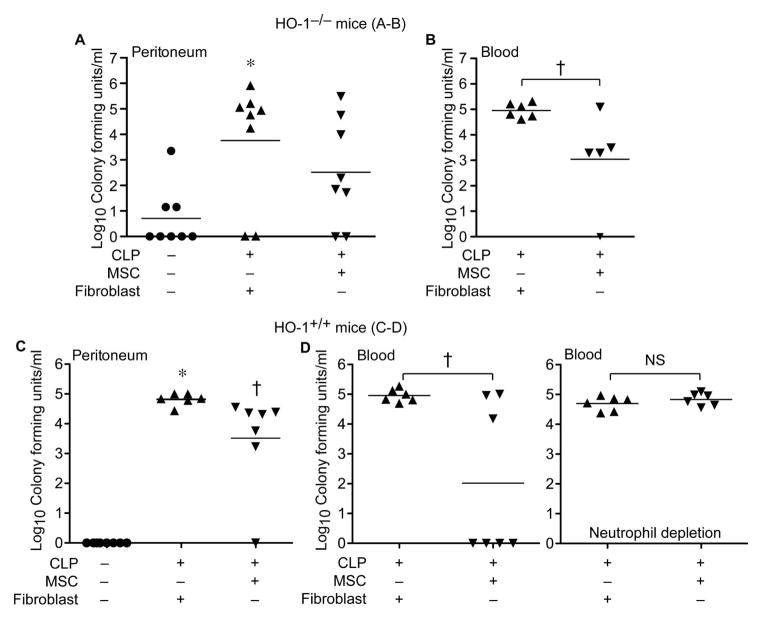
With evidence that enhanced bacterial phagocytosis by neutrophils contributes to the MSC response, peritoneal and blood bacterial counts were assessed in HO-1−/− mice undergoing sham or CLP surgery, in which mice received treatment with MSCs or fibroblast control cells. Cells were administered 2 hours (5.0 × 105 i.v.) after CLP, and bacterial CFUs from the peritoneum and blood were assessed at 24 hours. Compared with sham surgery, mice undergoing CLP that received fibroblasts had a significant increase in peritoneal bacteria (Figure 3A). Mice receiving MSCs after CLP did not have a significant difference in peritoneal bacteria compared with sham surgery. When bacterial CFUs from blood were subsequently assessed in HO-1−/− mice undergoing CLP, mice receiving MSCs had a significantly lower number of circulating bacteria compared with mice receiving fibroblasts (Figure 3B). These same studies were performed in HO-1+/+ mice, and administration of MSCs after CLP led to a reduction in bacterial CFUs from the peritoneum and blood compared with mice receiving fibroblasts (Figure 3C and 3D). Moreover, the ability of MSCs to promote bacterial clearance from the blood after CLP was lost in mice depleted of neutrophils (Figure 3D). Thus, MSCs increase neutrophil phagocytosis and improve bacterial clearance from the peritoneum and blood of HO-1−/− and HO-1+/+ mice, and neutrophils are critical for this clearance of bacteria by MSCs after CLP.
Figure 3.
MSCs promote bacterial clearance in mice after CLP, and this response is lost when neutrophils are depleted. HO-1−/− mice underwent sham (−) or CLP (+) surgery, and 2 hours after surgery were treated with MSCs or fibroblasts. At 24 hours after surgery, peritoneal fluid (A) and blood (B) were collected. CFUs were determined after incubating at 37°C overnight. Peritoneal fluid: sham, n=8; MSCs, n=8; fibroblasts, n=8. Blood: MSCs, n=6; fibroblasts, n=6. Horizontal bars represent mean values. Experiments were performed 2 independent times. *P<0.05 versus sham, using Kruskal-Wallis one-way analysis of variance followed by the Dunn’s post test analysis (A), and †P<0.05 MSC versus fibroblast, Mann-Whitney U testing (B). HO-1+/+ mice also underwent sham (−) or CLP (+) surgery, and 2 hours after surgery were treated with MSCs or fibroblasts. At 24 hours after surgery, peritoneal fluid (C) and blood (D) were collected. CFUs were determined after incubating at 37°C overnight. Peritoneal fluid: sham, n=8; MSCs, n=7; fibroblasts, n=6. Blood (no neutrophil depletion): MSCs, n=7; fibroblasts, n=6. Blood (neutrophil depletion): MSCs, n=6; fibroblasts, n=6. Horizontal bars represent mean values. Experiments were performed 2 independent times. *P<0.05 versus sham, and † P<0.05 versus fibroblasts, using Kruskal-Wallis one-way analysis of variance followed by the Dunn’s post test analysis (C), and †P<0.05 MSC versus fibroblast, Mann-Whitney U testing (D). NS, not significant.
MSCs Decrease Organ Injury During CLP-induced Sepsis
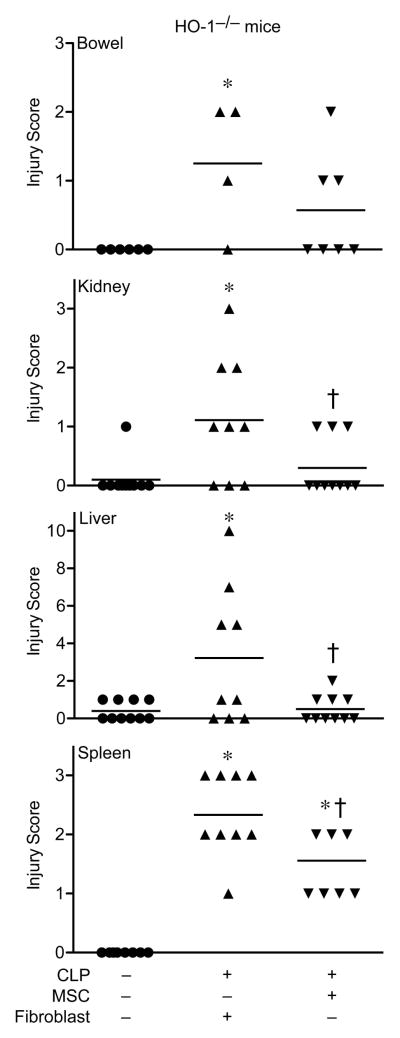
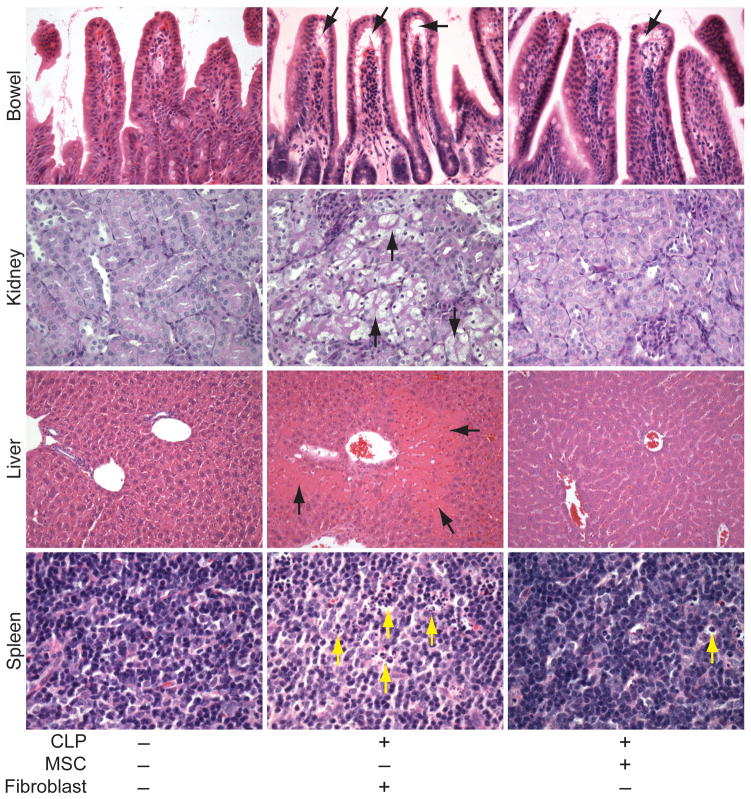
Histological analysis of organs 24 hours after CLP-induced sepsis in mice treated with fibroblast control cells demonstrated an increased injury score in small bowels (ileum), kidneys, livers, and spleens (Figure 4) compared with organs from sham operated mice. Bowel injury in mice receiving MSCs after CLP was not significantly different from sham mice, and in kidneys, livers, and spleens the administration of MSCs produced a significant decrease in injury scoring compared with fibroblast treated mice. Representative images form the injured organs showed evidence for subepithelial spaces in the villi of the small bowel, tubular epithelial cell swelling and degeneration in the kidney, liver necrosis, and numerous tingible bodies (macrophages engulfing apoptotic nuclei) and nuclear debris from dying cells in the white pulp of the spleen from mice receiving fibroblasts after CLP (Figure 5, arrows). In contrast, mice receiving MSCs demonstrated minimal subepithelial spaces, no evidence of tubular epithelial cell injury, no liver necrosis, and minimal evidence of tingible bodies. Circulating white blood cells (WBCs) were increased in HO-1−/− mice receiving fibroblasts after CLP, and treatment with MSCs produced a significant decrease in circulating WBCs compared with fibroblast treated mice (Supplemental Information, Figure S7A). Furthermore, we assessed Gr-1+ cells (predominantly neutrophils) in small bowels, kidneys, and livers of HO-1−/− mice after CLP. There was a significant decrease in Gr-1+ cells in small bowels of mice treated with MSCs compared with mice receiving fibroblasts after CLP (Figure S7B). In kidneys and livers, mice receiving MSCs had no difference in Gr-1+ infiltrating cells compared with sham operated mice (Figures S7C and S7D respectively). These data mirror the decrease in injury of organs from mice receiving MSCs after CLP.
Figure 4.
MSCs protect organs from injury after CLP-induced polymicrobial sepsis. HO-1−/− mice underwent sham (−) or CLP (+) surgery, and 2 hours after surgery were treated with MSCs or fibroblasts. At 24 hours after surgery, tissues were scored for injury (see Methods section). Horizontal bars represent mean values. Sham, n=8–10; MSCs, n=9–10; fibroblasts, n=9–10 for the kidney, liver and spleen. Sham, n=6; MSCs, n=7; fibroblasts, n=4 for the small bowel. *P<0.05 versus sham, †P<0.05 versus fibroblasts using Kruskal-Wallis one-way analysis of variance followed by the Dunn’s post test analysis.
Figure 5.
Representative images of organs from mice that received MSCs or fibroblasts after the onset of CLP-induced sepsis. H&E staining of sections from small bowel, liver, and spleen, and PAS staining of kidney, from mice 24 hours after sham (−) or CLP (+) surgery. In the CLP mice, 2 hours after the onset of sepsis, MSCs or fibroblasts were administered. The arrows point to subepithelial spaces in the villi (small bowel, black arrows), tubular epithelial cell swelling (kidney, black arrows), necrotic regions (liver, black arrows), and tingible body macrophages, predominantly in the white pulp, which have phagocytized apoptotic cells (spleen, yellow arrows). Light microscopy images, small bowel and kidney (20X objective), liver (10X objective), and spleen (40X objective).
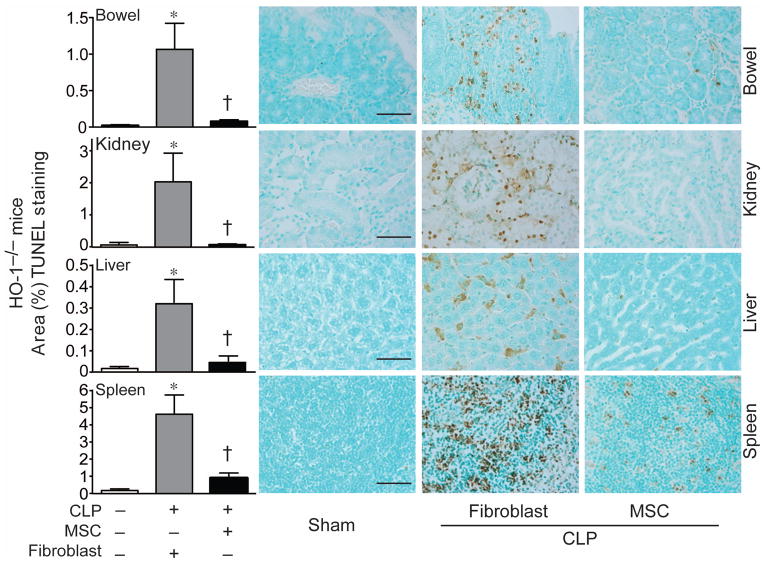
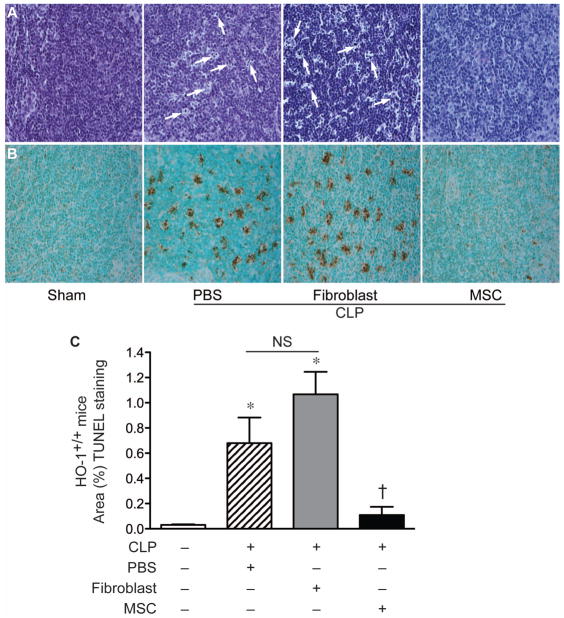
To use a second independent means of assessing tissue injury, TUNEL staining was performed in the organs of HO-1−/− mice undergoing sham or CLP surgery. As shown in Figure 6, evidence for TUNEL staining was dramatically increased in the organs (small bowels, kidneys, livers, and spleens) of HO-1−/− mice 24 hours after CLP when receiving fibroblasts. This staining was dramatically reduced in HO-1−/− mice receiving MSCs, demonstrating a significant reduction in injury. In fact, the level of TUNEL staining in septic mice that received MSCs was not significantly different from sham-operated mice. This decrease in organ injury produced by MSCs was also evident in tissue from HO-1+/+ mice after CLP. As seen in Figure 7, the increase in tingible bodies in splenic tissue of wild-type mice receiving PBS or fibroblasts after CLP was reduced in mice receiving MSCs. There was also a marked decrease in TUNEL staining in spleens of HO-1+/+ mice receiving MSCs. These data demonstrate that administration of MSCs after the onset of polymicrobial sepsis in mice leads to a decrease in end-organ injury.
Figure 6.
MSC treatment decreases tissue injury 24 hours after CLP-induced polymicrobial sepsis. HO-1−/− mice underwent sham (−) or CLP (+) surgery, and 2 hours after surgery were treated with MSCs or fibroblasts. At 24 hours after surgery, tissues were harvested and injury was evaluated by TUNEL staining (brown), and quantitated (left panels). Light microscopy images were taken with a 40X objective, and the bar in the sham panels represents a length of 50 μm. Data are represented as mean±SEM. Sham, n=6; MSCs, n=7–8; fibroblasts, n=6–8. *P<0.05 versus sham, †P<0.05 versus fibroblasts using Kruskal-Wallis one-way analysis of variance followed by the Dunn’s post test analysis. Representative small bowel, kidney, liver and spleen sections of HO-1−/− mice are to the right of the quantitative graphs.
Figure 7.
MSCs decrease splenic injury in wild-type mice after CLP-induced polymicrobial sepsis. A) PAS staining of spleen tissue from sham mice (− CLP) or mice 24 hours after CLP (+). In the CLP mice, 2 hours after the onset of sepsis, PBS, fibroblasts, or MSCs were administered. The white arrows point to representative tingible body macrophages, predominantly in the white pulp, which have phagocytized apoptotic cells. B) TUNEL staining was performed, in the same groups as described in (A), to identify apoptotic cells in splenic tissue after CLP. The brown staining identifies the apoptotic splenocytes. Light microscopy images, taken with a 20X objective. C) TUNEL staining was quantitated, and the data are represented as mean±SEM. For mice undergoing CLP, a minimum of 4–5 mice per group were analyzed. *P<0.05 versus sham, †P<0.05 versus fibroblasts and PBS using one-way analysis of variance followed by Newman-Keuls post test analysis. NS, not significant.
Discussion
MSCs have been harvested in large numbers from compact bone of mice [7, 38], and these cells are reported to support bone formation in vivo when cultured in basic fibroblast growth factor-conditioned medium [53]. Interestingly, when infused into mice, these MSCs also have immunoregulatory properties in vivo, prolonging skin grafts across major histocompatibility barriers [38] and inhibiting the development of acute graft-versus-host disease in mice [39, 40]. While the properties of these cells are suggested to be similar to their bone marrow counterparts in mice and humans [7, 38], to our knowledge MSCs harvested from compact bone have not been investigated for their functional importance in polymicrobial sepsis.
HO-1 is known to be a critical mediator in the response to sepsis, as shown previously by the enhanced lethality of HO-1−/− mice undergoing CLP [23] and exaggerated tissue injury. In the present study, we wanted to investigate the role of HO-1 specifically in MSCs, by administering either HO-1+/+ or HO-1−/− cells to mice deficient in HO-1 after the onset of polymicrobial sepsis. We utilized HO-1 deficient mice as recipients of MSCs because upregulation of HO-1 in endogenous cells during sepsis would increase production of CO, and then have an effect on the exogenously administered MSCs. The ability of CO to diffuse from one cell type to another, and produce a biologic response, has been demonstrated previously utilizing targeted cell expression of HO-1 [23, 54].
Prior studies have shown that overexpression of HO-1 can improve bone marrow-derived MSC function in disease models such as hypoxia-induced pulmonary hypertension [36] and myocardial ischemia [30]. However, the effect of genetic deficiency or inhibition of endogenous HO-1 in MSCs is less clear. In cisplatin-induced injury, HO-1−/− MSCs were not protective to the kidney [55] compared with wild-type MSCs. Also, in heart allograft studies, the protective effects of MSCs were lost after HO-1 inhibition [29]. However, during hypoxia-induced pulmonary hypertension, the therapeutic effects of bone marrow-derived MSCs harvested from HO-1 deficient mice were not dramatically different from wild-type MSCs, and both populations of cells were able to confer partial protection from the hypoxia [36]. This suggests that the importance of endogenous HO-1 in MSCs may depend on the model of disease. In the present study, we show that exogenous administration of either HO-1+/+ or HO-1−/− MSCs were capable of significantly improving survival after the development of CLP-induced sepsis in HO-1−/− mice (Figure 1A). This is a striking response, as HO-1−/− mice are very susceptible to sepsis-induced injury, resulting in multiple organ injury (Figures 4, 5, and 6). HO-1+/+ and HO-1−/− MSCs were also able to improve survival of wild-type mice after the onset of CLP-induced sepsis (Figure 1B). In addition, HO-1−/− MSCs have a similar response as HO-1+/+ MSCs to improve the phagocytosis of neutrophils, both in vitro and in vivo (Figure 2). Taken together, it does not appear endogenous HO-1 is required for MSC to promote their favorable effects during polymicrobial sepsis. However, this certainly does not exclude the possibility that genetic upregulation of HO-1 may be more beneficial for MSCs during sepsis, as has been shown when using bone marrow-derived cells in other disease models [30, 36].
Sepsis is a disease process that involves an exaggerated immune response, and MSCs are known to have important immunoregulatory properties [6, 9, 10, 56]. During the hyperimmune phase of sepsis, MSCs may protect the host by dampening the inflammatory response. Nemeth and colleagues reported that bone marrow-derived MSCs were able to reprogram macrophages in a prostaglandin E2-dependent manner, causing macrophages to increase production of IL-10, an anti-inflammatory cytokine [17]. Moreover, MSCs were able to decrease serum levels of the pro-inflammatory cytokines TNF-α and IL-6. However, if MSCs only suppressed the immune response during sepsis, the eradication of infecting organisms responsible for sepsis pathobiology would be jeopardized. Beyond this anti-inflammatory response, human MSCs have been shown to express the antimicrobial peptide LL-37, contributing to the clearance of gram-negative bacteria (E. coli) in the lung [20]. It has also been shown that MSCs (harvested from mouse bone marrow or human adipose tissue) can increase bacterial clearance in mouse models of sepsis [18, 19], and that CD11b+ cells from the peritoneum or spleens of CLP injured mice receiving MSCs have improved phagocytic activity [18]. In addition, recently it was reported that human MSCs reduced bacteremia in a gram negative model of sepsis in mice, in part by improving the phagocytic activity of blood CD11bhiCD115+ mononuclear cells [44].
Prior investigations assessing cellular therapy in sepsis have focused on the effects of MSCs on macrophage and monocyte function, however our interest was in the interactions between MSCs and neutrophils, one of the earliest immune responders to a bacterial infection. Neutrophils are clearly an important cell during sepsis, as shown by increased lethality when neutrophils are depleted prior to CLP [57]. Other than the ability of MSCs to decrease neutrophil infiltration into organs during models of sepsis and endotoxemia (Supplemental Information, Figure S7 and reviewed in [56]), less is known about the ability of MSCs to alter neutrophil function in vivo, or the importance of neutrophils to the MSC response. We demonstrate that MSCs (HO-1+/+ or HO-1−/−) were able to enhance the phagocytic activity of neutrophils, both in vitro and in vivo (Figure 2). Evidence for enhanced phagocytic activity of neutrophils helped to explain the improved clearance of bacteria in the peritoneum and blood by MSCs (Figure 3). Moreover, when neutrophils were depleted prior to CLP, MSC were unable to increase survival during polymicrobial sepsis, compared with fibroblasts or PBS (Figure 1C), and they were unable to promote bacterial clearance from the blood (Figure 3D). These studies provide new insight regarding the critical interactions between MSCs and neutrophils during sepsis.
Conclusions & Summary
MSCs harvested from compact bone are capable of decreasing lethality associated with CLP-induced polymicrobial sepsis. This beneficial MSC response does not require endogenous HO-1; however, the presence of neutrophils is essential for MSCs to improve survival. Furthermore, MSCs are able to improve bacterial clearance from the peritoneum and blood, and decrease tissue injury, after the onset of polymicrobial sepsis. Thus, MSCs are beneficial during a systemic bacterial infection, and the response of neutrophils to exogenous MSCs is crucial for the improved outcome.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants RO1HL060788 and PO1HL108801-01 (Project 3), and LRRI-BWH Consortium grant to M.A.P. S.R.H. was supported in part by funds from the Lovelace Respiratory Research Institute.
We are grateful to Dr. Lynn Hancock and Dr. Melanie B. Berkmen for providing the GFP labeled E. faecalis and E. coli respectively.
Footnotes
The authors have no disclaimers to disclose.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author contribution summary: S.R.H.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; K.T.: conception and design, collection and/or assembly of data, data analysis and interpretation; B.I.: collection and/or assembly of data; R.F.P.: data analysis and interpretation; J.A.L.: conception and design, data analysis and interpretation; Z.W.: collection and/or assembly of data; X.L.: conception and design, collection and/or assembly of data, data analysis and interpretation; M.A.P.: conception and design, financial and administrative support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
References
- 1.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Dennis JE, Merriam A, Awadallah A, et al. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 6.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Guo ZK, Jiang XX, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 8.Phinney DG, Prockop DJ. Mesenchymal stem/multi-potent stromal cells (MSCs): the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 9.Prockop DJ, Kota DJ, Bazhanov N, et al. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolar J, Le Blanc K, Keating A, et al. Hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;35:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 12.Baron RM, Baron MJ, Perrella MA. Pathobiology of sepsis. Are we still asking the same questions? Am J Respir Cell Mol Biol. 2006;34:129–134. doi: 10.1165/rcmb.F308. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 14.Vincent J-L, Abraham E. The last 100 years of sepsis. Am J Respir Crit Care Med. 2006;173:256–263. doi: 10.1164/rccm.200510-1604OE. [DOI] [PubMed] [Google Scholar]
- 15.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Sandrock CE, Albertson TE. Controversies in the treatment of sepsis. Semin Respir Crit Care Med. 2010;31:66–78. doi: 10.1055/s-0029-1246290. [DOI] [PubMed] [Google Scholar]
- 17.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 20.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 22.Tenhunen R, Marver H, Schmid R. Microsomal heme oxygenase, characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 23.Chung SW, Liu X, Macias AA, et al. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118:239–247. doi: 10.1172/JCI32730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 25.Maines MD, Gibbs PEM. 30 years of heme oxygenase: From a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun. 2005;338:568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 26.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic implications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 27.Ryter SW, Choi AM. Therapeutic applications of carbon monoxide in lung disease. Curr Opin Pharmacol. 2006;6:257–262. doi: 10.1016/j.coph.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Ryter SW, Morse D, Choi AMK. Carbon monoxide and bilirubin: Potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol. 2007;36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 30.Tsubokawa T, KY, Nakanishi C, et al. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298:1320–1329. doi: 10.1152/ajpheart.01330.2008. [DOI] [PubMed] [Google Scholar]
- 31.Mougiakakos D, Jitschin R, Johansson CC, et al. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 32.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 33.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JW, Gupta N, Serikov V, et al. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 2009;9:1259–1270. doi: 10.1517/14712590903213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang O, Mitsialis SA, Chang MS, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phinney DG, Kopen G, Isaacson RL, et al. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: Variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 38.Guo Z, Li H, Li X, et al. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells. 2006;24:992–1000. doi: 10.1634/stemcells.2005-0224. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Guo Z, Jiang X, et al. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26:2531–2541. doi: 10.1634/stemcells.2008-0146. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Guo Z, Zhu H, et al. Transplanted mesenchymal stem cells can inhibit the three developmental stages of murine acute graft-versus-host disease. In Vivo. 2010;24:659–666. [PubMed] [Google Scholar]
- 41.Yet S-F, Perrella MA, Layne MD, et al. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rittirsch D, Huber-Laang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krasnodembskaya A, Samarani G, Song Y, et al. Human Mesenchymal Stem Cells Reduce Mortality and Bacteremia in Gram Negative Sepsis in Mice in Part by Enhancing the Phagocytic Activity of Blood Monocytes. Am J Physiol Lung Cell Mol Physiol. 2012 doi: 10.1152/ajplung.00180.2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daley JM, Thomay AA, Connolly MD, et al. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 46.Gille C, Spring B, Tewes L, et al. A new method to quantify phagocytosis and intracellular degradation using green fluorescent protein-labeled Escherichia coli: comparison of cord blood macrophages and peripheral blood macrophages of healthy adults. Cytometry A. 2006;69:152–154. doi: 10.1002/cyto.a.20222. [DOI] [PubMed] [Google Scholar]
- 47.Fredenburgh LE, Velandia MM, Ma J, et al. Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J Immunol. 2011;187:5255–5267. doi: 10.4049/jimmunol.1101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W, Faubel S, Ljubanovic D, et al. Endotoxemic acute renal failure is attenuated in caspase-1-deficient mice. Am J Physiol Renal Physiol. 2005;288:F997–F1004. doi: 10.1152/ajprenal.00130.2004. [DOI] [PubMed] [Google Scholar]
- 49.Sneed RA, Grimes SD, Schultze AE, et al. Bacterial endotoxin enhances the hepatotoxicity of allyl alcohol. Toxicol Appl Pharmacol. 1997;144:77–87. doi: 10.1006/taap.1997.8115. [DOI] [PubMed] [Google Scholar]
- 50.Elmore SA. Enhanced histopathology of the spleen. Toxicol Pathol. 2006;34:648–655. doi: 10.1080/01926230600865523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregory CA, Gunn WG, Peister A, et al. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Ren G, Zhao X, Zhang L, et al. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamachika E, Tsujigiwa H, Matsubara M, et al. Basic fibroblast growth factor supports expansion of mouse compact bone-derived mesenchymal stem cells (MSCs) and regeneration of bone from MSC in vivo. J Mol Histol. 2011 doi: 10.1007/s10735-011-9385-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Minamino T, Christou H, Hsieh C-M, et al. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarjou A, Kim J, Traylor AM, et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol. 2011;300:F254–F262. doi: 10.1152/ajprenal.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wannemuehler TJ, Manukyan MC, Brewster BD, et al. Advances in Mesenchymal Stem Cell Research in Sepsis. J Surg Res. 2011 doi: 10.1016/j.jss.2011.09.053. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Hoesel LM, Neff TA, Neff SB, et al. Harmful and protective role of neutrophils in sepsis. Shock. 2005;24:40–47. doi: 10.1097/01.shk.0000170353.80318.d5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.