Abstract
The anticholinesterase (antiChE) organophosphorus (OP) and methylcarbamate (MC) insecticides have been used very effectively as contact and systemic plant protectants for seven decades. About 90 of these compounds are still in use – the largest number for any insecticide chemotype or mode of action. In both insects and mammals, AChE inhibition and acetylcholine accumulation leads to excitation and death. The cholinergic system of insects is located centrally (where it is protected from ionized OPs and MCs) but not at the neuromuscular junction. Structural differences between insect and mammalian AChE are also evident in their genomics, amino acid sequences and active site conformations. Species selectivity is determined in part by inhibitor and target site specificity. Pest population selection with OPs and MCs has resulted in a multitude of modified AChEs of altered inhibitor specificity some conferring insecticide resistance and others enhancing sensitivity. Much of the success of antiChE insecticides results from a suitable balance of bioactivation and detoxification by families of CYP450 oxidases, hydrolases, glutathione S-transferases and others. Known inhibitors for these enzymes block detoxification and enhance potency which is particularly important in resistant strains. The current market for OPs and MCs of 19% of worldwide insecticide sales is only half of that of 10 years ago for several reasons: there have been no major new compounds for 30 years; resistance has eroded their effectiveness; human toxicity problems are still encountered; the patents have expired reducing the incentive to update registration packages; alternative chemotypes or control methods have been developed. Despite this decline, they still play a major role in pest control and the increasing knowledge on their target sites and metabolism may make it possible to redesign the inhibitors for insensitive AChEs and to target new sites in the cholinergic system. The OPs and MCs are down but not out.
Keywords: Acetylcholinesterase, Anticholinesterase, Cytochrome, P450 Insecticide, Resistance, Selective toxicity
1. Introduction
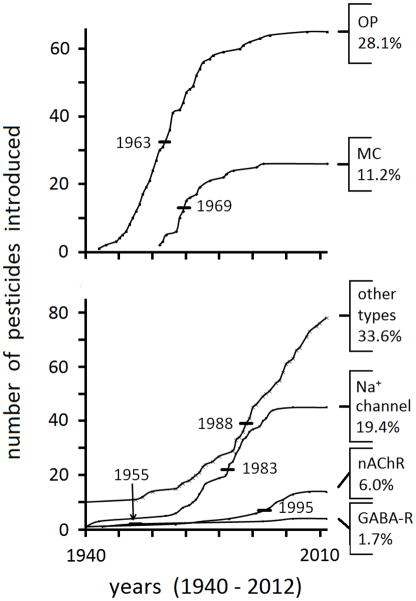
Insecticides in 1940 consisted of inorganics and botanicals (including pyrethrum, rotenone and nicotine) applied to crops at high levels to achieve moderate pest control. The chlorinated hydrocarbons including DDT, lindane, cyclodienes and toxaphene were introduced in the 1940s and 1950s to control chewing pests on crops and insect vectors of human disease. Organophosphorus compounds (OPs) were also introduced in the 1940s and 1950s and methylcarbamates (MCs) in the 1960s to control sucking insects on crops and fill other gaps in pest management programs [1]. Almost all pests could finally be controlled, in large part because of highly effective OPs and MCs selected from the thousands of active compounds for use as contact and systemic insecticides (see Supplemental Fig. 1 for six important examples). The OPs and MCs quickly became the major compounds by number, a position they retain even today with 232 insecticides in current use including 91 OPs and MCs, i.e. 40% of the total [2] (Fig. 1). The OPs and MCs act as acetylcholinesterase (AChE) inhibitors or anticholinesterases (antiChEs). Other principal insecticide targets are the nicotinic acetylcholine (ACh) receptor (nAChR) for the neonicotinoids (e.g. imidacloprid and thiamethoxam), the γ-aminobutyric acid (GABA) receptor (GABA-R) for the cyclodienes, lindane, toxaphene and fiproles (example fipronil), and the voltage-dependant Na+ channel for DDT and the pyrethroids (e.g. permethrin and deltamethrin) [1–3]. The number of compounds acting at other targets has increased in the last three decades to reach 34% of the total (Fig. 1). Despite all these changes the OPs and MCs, and their AChE target, remain at the top in number among the commercial insecticides.
Fig. 1.
Chronology for introduction of the current insecticides. The year of introduction is from The Pesticide Manual [3] if reported there or otherwise from multiple sources. Compounds introduced before 1940 are shown as 1940.
2. Insect cholinergic pharmacology
The insect and mammalian nervous systems are very similar in biochemical components but differences in localization of function allow selective toxicant action. The insect central nervous system (CNS) is cholinergic (ACh, AChE, and nAChR) and shielded in most insects by an ion barrier whereas the insect neuromuscular junction lacks this protection and has glutamate as the activator and GABA as the inhibitory neurotransmitter [2,4,5]. Thus OPs and MCs act in the insect’s CNS but not at the neuromuscular junction. The insect cholinergic system also contains the targets for nAChR agonists (nicotine, neonicotinoids), channel blockers (nereistoxin analogs) or allosteric activators (spinosad) which are important and selective insecticides and for muscarinic channel agonists, antagonists and blockers which, thus far, are not adequately effective or acceptably selective as insecticides (Supplemental Fig. 2) [4].
3. Acetylcholinesterase
3.1 Insect versus mammal
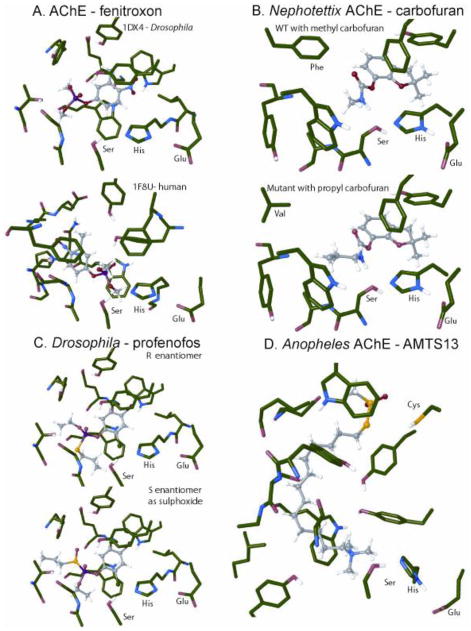
The phylogenetic relationships of invertebrate and vertebrate AChEs suggest that major diversifications occurred early during evolution of this enzyme [6,7]. AChE sequences are known for more than 600 arthropods including many insects of agricultural and medical importance. Drosophila and vertebrate AChEs are structurally-defined at high resolution (Fig. 2A) [8,9] allowing confident deductions on how structural changes influence OP and MC action. OPs and MCs vary considerably in insect specificity and selectivity between insects and mammals [10,11] due in part to species differences in target site structure. The His-Ser-Glu catalytic triad is always the same but the active site varies in the acyl gorge and other pockets. A single OP substituent change can confer selectivity, e.g. a 3-chloro or 3-methyl substituent on O,O-dimethyl O-(4-nitrophenyl) phosphate greatly reduces the potency on mammalian but not insect AChE [12]. The 3-methyl compound (fenitroxon) docks better in Drosophila than human AChE by more that 1 kcal/mol attributable to clean aromatic stacking in Drosophila not possible in the much more crowded human AChE (Fig. 2A).
Fig. 2.
Models for binding site interactions of OPs and MCs at the AChE active site. A. Fenitroxon with Drosophila and human AChE. B. Carbofuran with green rice leafhopper (Nephotettix cincticeps) AChE of susceptible strain and propyl analog of carbofuran with AChE of resistant strain. C. R-Profenofos and S-profenofos sulfoxide with Drosophila AChE. D. Cysteine-targeting S-sulfonate AMTS-13 with African malaria mosquito Anopheles gambiae AChE. (See Supplementary Information on binding site interactions.)
3.2 Target site resistance
A major type of antiChE insecticide resistance is selection for mutations conferring reduced OP and/or MC sensitivity, first noted in spider mites [13] with well over 20 examples in insects involving at least 14 specific identified mutations [7,14–16]. In Musca domestica, the modified amino acids conferring resistance are larger than the corresponding wild type (WT) residues close to the catalytic triad [17]. Each mutation that reduces sensitivity alters the OP and MC binding site and may therefore change the antiChE inhibitor specificity [7,17]. Cross-resistance results when the modified AChE is less sensitive than the WT to not only the selecting compound but also to other OPs and MCs. The green rice leafhopper is an interesting example where N-methylcarbamate (MC) insecticide resistance was negatively correlated with increased N-propylcarbamate analog (PC) sensitivity both in lethal dose and in vitro enzyme inhibition [16,18,19] assigned to a F290V mutation [20]. In binding site models carbofuran shows favorable Phe WT hydrophobic interactions with both MC and PC whereas the Val mutant leaves too much space in that region for the MC to effectively bind (Fig. 2B). N-Propyl carbofuran could be used to control N-methyl carbofuran resistant strains and vice versa. This MC/PC relationship has also been noted in Colorado potato beetles and fruitflies, thus providing an expanded model for negatively-correlated cross resistance [14,21]. Another example of high specificity is the S431F mutation in several species of aphids that confers resistance to the dimethylcarbamate insecticide pirimicarb but not to the MCs [22]. Profenofos resistance develops slowly possibly because the R-enantiomer acts directly and the S-enantiomer after sulfoxidation with a reversal of AChE inhibitor stereospecificity on S-oxide formation, i.e. two toxicants are involved [23] (Fig. 2C). More generally target site resistance may in theory develop more slowly for a racemic OP with both enantiomers contributing to the toxicity.
The catalytic serine residue is not the only site for derivatizing AChE leading to insect mortality. In mosquitoes, aphids and 14 other insects there is also an insect-specific cysteine residue (absent in mammals) located at the opening of the AChE active site as an alternative target site for inhibition that can be reversibly blocked with thiol reagents such as AMTS13 in Anopheles [24] (Fig. 2D). Although no adequately potent inhibitors have been reported, cysteine-targeting antiChE insecticides could potentially provide selective toxicity and avoid current cross-resistance patterns. [24]. Word Count: 465
4. Insecticide metabolism
4.1 Proinsecticides for stability and selective toxicity
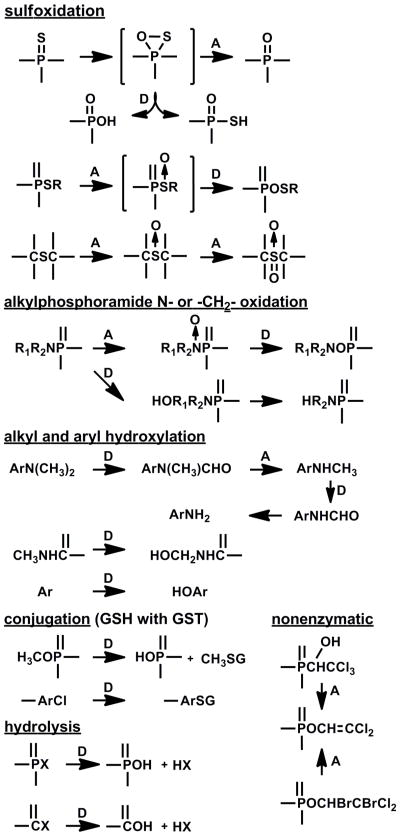
The OPs were the first readily-biodegradable synthetic organic insecticides. They must be persistent to achieve prolonged control yet reactive as AChE inhibitors, an apparent anomaly solved by using proinsecticides undergoing bioactivation reactions, a relationship illustrated for mammals (Supplemental Fig. 3) but also applicable to insects. Substituent contributions to some of the bioactivation and detoxification reactions (Fig. 3) become more interesting when multiple biodegradable sites appear in the same molecule, particularly when there is facile bioactivation in insects and detoxification in mammals, the latter illustrated by malathion and acephate (Supplemental Fig. 4), or the bioactivation reaction forms an inhibitor for the detoxification, resulting in major selectivity for acute toxicity but much less in chronic toxicity where the detoxification phase is turned off.
Fig. 3.
Substituents of OP and MC insecticides showing some sites of reaction leading to activation (A) or detoxification (D) as AChE inhibitors. Specific insecticides for each type of reaction are given in relevant reviews [3, 4, 10, 11, 26, 27].
4.2 Metabolic resistance
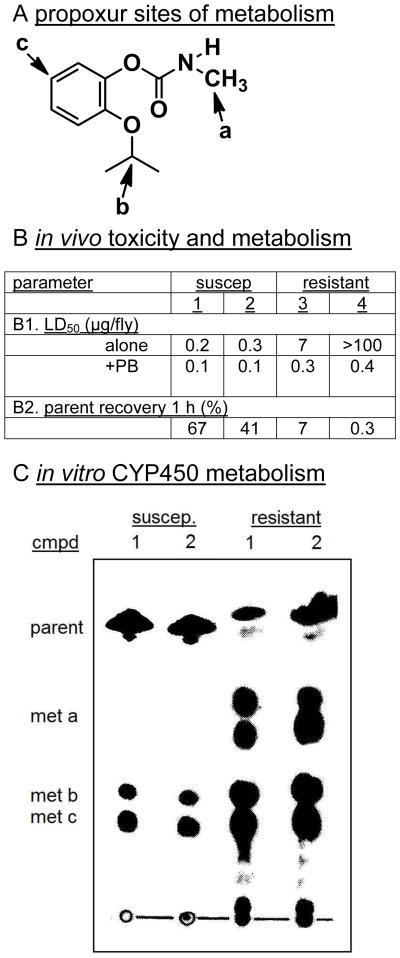
The relationship of CYP450 metabolism to resistance and synergist action was first shown in a single experiment with Musca adults and the MC propoxur by comparing two susceptible strains with two strains resistant to propoxur by 23 to >500-fold [25]. The resistance factor was reduced to 3- to 4-fold by piperonyl butoxide (PB) synergist which is diagnostic for CYP450 involvement. The rank order for the strains in resistance to propoxur was the same as that for metabolism of the parent compound in vivo and in vitro with microsome-NADPH incubations (CYP450 assay conditions) establishing that PB-sensitive microsomal oxidases were the limiting factor in determining susceptibility and resistance. This type of experiment has been repeated with several OPs and MCs using WT and resistant strains from natural selection or with specific expressed mutants. The genomic era has made it possible to define the CYP450 isoforms involved (e.g. CYP6A1 and CYP6A2 for Musca and Drosophila) [26,27] and interface the results with cross resistance and the development of pest management systems. Insect aliesterases also play a role in resistance with mutations conferring enhanced activity in OP hydrolytic detoxification established with toxicological and genomic approaches [26,27].
5. Summary
There has been a marked decline but not a demise in OP and MC (antiChE) insecticide use relative to the neonicotinoids, pyrethroids, fiproles, and non-neuroactive (other) chemotypes (Fig. 5). Four features keep the OPs and MCs in major use for pest control and integrated pest management programs: decades of experience in their use; highly cost effective on many pests; diverse physicochemical properties not duplicated in any other chemotype; and old compounds going off patent become generic and less expensive. However, safety is of continuing concern since many of the most toxic compounds to mammals are widely used, e.g. carbofuran and aldicarb. Compounds causing major toxicological problems from secondary targets (including neuropathy target esterase) were dropped in development or withdrawn from the market as more thorough testing protocols were developed and enforced [4]. Exposure monitoring and the effectiveness of antidotes have been improved. The common mode of action of OPs and MCs has led in some cases to considering a summation of residues and acute and chronic toxicity risks. Resistance is a critical and apparently intractable problem for major economic pest populations. This is a major discouraging factor for development of additional antiChE insecticides unless they are highly effective, safe, and of a new chemotype. Avoiding or circumventing resistance can only be achieved by introducing compounds with new targets and novel modes of action [2] (“other” in Fig. 5) or genetically-modified crops expressing Bacillus thuringiensis endotoxin. Although the antiChE insecticides are being replaced by what are now better and more effective chemotypes, they played the major role for decades in providing food, fiber and health protection for an ever-expanding human population. This review is a retrospective not a post mortem for antiChE insecticides.
Fig. 5.
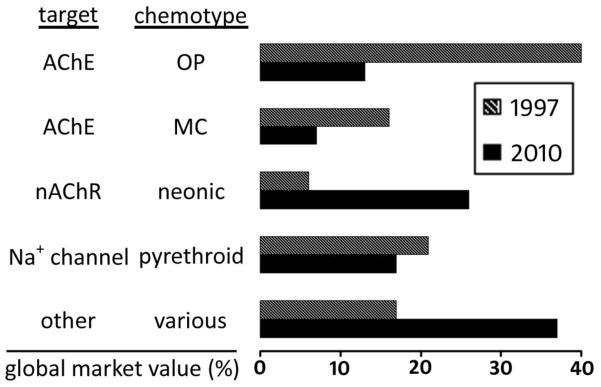
Declining use of antiChE insecticides between 1997 and 2010 [4].
Supplementary Material
Two of the early important OPs and MCs (parathion and carbaryl) and the two current leaders of each chemotype in global market sales, each 120 to 400 million dollars annually according to the Agranova 2010 database (provided by Thomas C. Sparks of Dow AgroScience). Chlorpyrifos is also known as chlorpyrifos-ethyl.
Insecticide target sites in the AChE and nicotinic receptor/cation channel excitatory synapse (Casida and Durkin 2013).
Relation for OPs between in vitro antiChE activity (IC50) and toxicity to mice and rats (LD50) (Casida 1956). The direct-acting OPs are alkyl phosphates and phosphonates and the bioactivated compounds are phosphoramidates and phosphorothionates. The bioactivation reactions include some of those shown in Fig. 3.
Selective toxicity of malathion and acephate from preferential bioactivation in insects and detoxification in mammals. The detoxifying malathion carboxyesterase is inhibited by malaoxon (Eto, 1974; Main and Dauterman, 1963). The activating acephate amidase is inhibited by the product methamidophos (Mahajna et al, 1997).
Fig. 4.
Propoxur (A) resistance in housefly adults is dependant on the strain (susceptible 1 and 2 versus resistant 3 and 4) and reversed by PB (B1) and related to parent metabolism in vivo (B2) and CYP450 metabolism in vitro (C). (TLC radioautogram for [14C]carbonyl(*) label with metabolites involving three sites of oxidation). [27]
Highlights.
For seven decades most insecticides by number were cholinesterase inhibitors
Current market of 19% global insecticide sales is only half of 10 years ago
Target site resistant pests have altered inhibitor specificity
Critical balance of bioactivation and detoxification required
Expanded knowledge on target sites and metabolism may allow redesign of inhibitors
Acknowledgments
This review is based on a lecture presented at the 11th International Meeting on Cholinesterases held June 2012 in Kazan, Russia. The Berkeley research was supported by the University of California, the National Institutes of Health, and the Environmental Protection Agency. We are indebted to our laboratory colleagues Sean Kodani, Elodie Tong-Lin, Alex Laihsu, and Ellen Key for valuable suggestions and assistance.
Abbreviations
- ACh
acetylcholine
- AChE
acetylcholinesterase
- antiChE
anticholinesterase
- CYP450
cytochrome P450
- GABA
γ-aminobutyric acid
- MC
methylcarbamate
- nAChR
nicotinic acetylcholine receptor
- OP
organophosphorus
- PC
propylcarbamate
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John E. Casida, Environmental Chemistry and Toxicology Laboratory Department of Environmental Science, Policy and Management, University of California, Berkeley, California, 94720-3112 USA
Kathleen A. Durkin, Molecular Graphics and Computational Facility, College of Chemistry, University of California, Berkeley, 94720-1460
References
- 1.Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu Rev Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Nauen R, Elbert A, Mccaffery A, Slater R, Sparks TC. IRAC: insecticide resistance, and mode of action classification of insecticides. In: Krämer W, Schirmer U, Jeschke P, Witschel M, editors. Modern Crop Protection Compounds. 2. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2012. pp. 935–955. [Google Scholar]
- 3.Tomlin CDS, editor. The Pesticide Manual: a World Compendium. 15. Alton, Hampshire: British Crop Protection Council; 2009. pp. 1–1457. [Google Scholar]
- 4.Casida JE, Durkin KA. Annu Rev Entomol. 2013. Neuroactive insecticides: targets, selectivity, resistance and secondary effects. pending publication. [DOI] [PubMed] [Google Scholar]
- 5.Sattelle DB, Breer H. Cholinergic nerve terminals in the central nervous system of insects. J Neuroendocrinol. 1990;2:241–256. doi: 10.1111/j.1365-2826.1990.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang Z, Chen J, Chen Y, Jiang S. Molecular cloning and characterization of an acetylcholinesterase cDNA in the brown planthopper, Nilaparvata lugens. J Insect Sci. 2008;10:102. doi: 10.1673/031.010.10201. available online: insectscience.org/10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oakeshott JG, Claudianos C, Campbell PM, Newcomb RD, Russell RJ. Biochemical genetics and genomics of insect esterases. In: Gilbert LI, Gill SS, editors. Insect Pharmacology: Channels, Receptors, Toxins and Enzymes. Academic Press; London, UK: 2010. pp. 229–301. [Google Scholar]
- 8.Harel M, Kryger G, Rosenberry TL, Mallender WD, Lewis T, Fletcher RJ, Guss JM, Silman I, Sussman JL. Three-dimensional structures of Drosophila melanogaster acetylcholinesterase and of its complexes with two potent inhibitors. Protein Sci. 2000;9:1063–1072. doi: 10.1110/ps.9.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvir H, Silman I, Harel M, Rosenberry TL, Sussman JL. Acetylcholinesterase: from 3D structure to function. Chem-Biol Interact. 2010;187:10–22. doi: 10.1016/j.cbi.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eto M. Organophosphorus Pesticides: Organic and Biological Chemistry. CRC Press; Cleveland, Ohio: 1974. pp. 1–387. [Google Scholar]
- 11.Kuhr RJ, Dorough HW. Carbamate Insecticides: Chemistry, Biochemistry and Toxicology. CRC Press; Cleveland, Ohio: 1976. pp. 1–301. [Google Scholar]
- 12.Metcalf RA, Metcalf RL. Selective toxicity of analogs of methyl parathion. Pestic Biochem Physiol. 1973;3:149–159. [Google Scholar]
- 13.Smissaert HR. Cholinesterase inhibition in spider mites susceptible and resistant to organophosphate. Science. 1964;143:129–131. doi: 10.1126/science.143.3602.129. [DOI] [PubMed] [Google Scholar]
- 14.Villatte F, Ziliani P, Estrada-Mondaca S, Menozzi P, Fournier D. Is acetyl/butyrylcholine specificity a marker for insecticide-resistance mutations in insect acetylcholinesterase? Pest Manag Sci. 2000;56:1023–1028. [Google Scholar]
- 15.Fournier D, Mutero A, Pralavorio M, Bride JM. Drosophila acetylcholinesterase: mechanisms of resistance to organophosphates. Chem-Biol Interact. 1993;87:233–238. doi: 10.1016/0009-2797(93)90047-3. [DOI] [PubMed] [Google Scholar]
- 16.Fournier D. Mutations of acetylcholinesterase which confer insecticide resistance in insect populations. Chem-Biol Interact. 2005;157–158:257–261. doi: 10.1016/j.cbi.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Walsh SB, Dolden TA, Moores G, Kristensen M, Lewis T, Devonshire AL, Williamson M. Identification and characterization of mutations in housefly (Musca domestica) acetylcholinesterase involved in insecticide resistance. Biochem J. 2001;359:175–181. doi: 10.1042/0264-6021:3590175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto I, Kyomura N, Takahashi Y. Negatively correlated cross resistance: combinations of N-methylcarbamate with N-propylcarbamate or oxadiazolone for green rice leafhopper. Arch Insect Biochem Physiol. 1993;22:277–288. [Google Scholar]
- 19.Yoo JK, Lee SW, Ahn YJ, Nagata T, Shono T. Altered acetylcholinesterase as a resistance mechanism in the brown planthopper (Homoptera: Delphacidae), Nilaparvata lugens Stål. Appl Entomol Zool. 2002;37:37–41. [Google Scholar]
- 20.Ott S, Kozaki T, Tomita T, Kono Y. Biochemical properties of recombinant acetylcholinesterases with amino acid substitutions in the active site. Appl Entomol Zool. 2007;42:367–373. [Google Scholar]
- 21.Kim HJ, Yoon KS, Clark JM. Functional analysis of mutations in expressed acetylcholinesterase that result in azinphosmethyl and carbofuran resistance in Colorado potato beetle. Pestic Biochem Physiol. 2007;88:181–190. [Google Scholar]
- 22.Andrews MC, Callaghan A, Field LM, Williamson MS, Moores GD. Identification of mutations conferring insecticide-insensitive AChE in the cotton-melon aphid, Aphis gossypii Glover. Insect Mol Biol. 2004;13:555–561. doi: 10.1111/j.0962-1075.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 23.Wing KD, Glickman AH, Casida JE. Oxidative bioactivation of S-alkyl phosphorothiolate pesticides: stereospecificity of profenofos insecticide activation. Science. 1983;219:63–65. doi: 10.1126/science.6849116. [DOI] [PubMed] [Google Scholar]
- 24.Pang YP, Brimijoin S, Ragsdale DW, Zhu KY, Suranyi R. Novel and viable acetylcholinesterase target site for developing effective and environmentally safe insecticides. Curr Drug Targets. 2012;13:471–482. doi: 10.2174/138945012799499703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrivastava SP, Tsukamoto M, Casida JE. Oxidative metabolism of C14-labeled Baygon by living house flies and house fly enzyme preparations. J Econ Entomol. 1969;62:483–498. [Google Scholar]
- 26.Bergé JB, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Phil Trans R Soc Lond B. 1998;353:1701–1705. doi: 10.1098/rstb.1998.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott JG, Wen Z. Cytochromes P450 of insects: the tip of the iceberg. Pest Manag Sci. 2001;57:958–967. doi: 10.1002/ps.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two of the early important OPs and MCs (parathion and carbaryl) and the two current leaders of each chemotype in global market sales, each 120 to 400 million dollars annually according to the Agranova 2010 database (provided by Thomas C. Sparks of Dow AgroScience). Chlorpyrifos is also known as chlorpyrifos-ethyl.
Insecticide target sites in the AChE and nicotinic receptor/cation channel excitatory synapse (Casida and Durkin 2013).
Relation for OPs between in vitro antiChE activity (IC50) and toxicity to mice and rats (LD50) (Casida 1956). The direct-acting OPs are alkyl phosphates and phosphonates and the bioactivated compounds are phosphoramidates and phosphorothionates. The bioactivation reactions include some of those shown in Fig. 3.
Selective toxicity of malathion and acephate from preferential bioactivation in insects and detoxification in mammals. The detoxifying malathion carboxyesterase is inhibited by malaoxon (Eto, 1974; Main and Dauterman, 1963). The activating acephate amidase is inhibited by the product methamidophos (Mahajna et al, 1997).