Abstract
Aims
The mechanisms mediating kidney injury and repair in humans with atherosclerotic renal artery stenosis (ARAS) remain poorly understood. We hypothesized that the stenotic kidney releases inflammatory mediators and recruits progenitor cells to promote regeneration.
Methods and results
Essential hypertensive (EH) and ARAS patients (n= 24 each) were studied during controlled sodium intake and antihypertensive treatment. Inferior vena cava (IVC) and renal vein (RV) levels of CD34+/KDR+ progenitor cells, cell adhesion molecules, inflammatory biomarkers, progenitor cell homing signals, and pro-angiogenic factors were measured in EH and ARAS, and their gradient and net release compared with systemic levels in matched normotensive controls (n= 24). Blood pressure in ARAS was similar to EH, but the glomerular filtration rate was lower. Renal vein levels of soluble E-Selectin, vascular cell adhesion molecule-1, and several inflammatory markers were higher in the stenotic kidney RV vs. normal and EH RV (P< 0.05), and their net release increased. Similarly, stem-cell homing factor levels increased in the stenotic kidney RV. Systemic CD34+/KDR+ progenitor cell levels were lower in both EH and ARAS and correlated with cytokine levels. Moreover, CD34+/KDR+ progenitor cells developed a negative gradient across the ARAS kidney, suggesting progenitor cell retention. The non-stenotic kidney also showed signs of inflammatory processes, which were more subtle than in the stenotic kidney.
Conclusion
Renal vein blood from post-stenotic human kidneys has multiple markers reflecting active inflammation that portends kidney injury and reduced function. CD34+/KDR+ progenitor cells sequestered within these kidneys may participate in reparative processes. These inflammation-related pathways and limited circulating progenitor cells may serve as novel therapeutic targets to repair the stenotic kidney.
Keywords: Renovascular hypertension, Progenitor cells, Inflammatory biomarkers, Renal artery stenosis, Cytokines
Introduction
Hypertension represents a major-public health problem that affects ∼1 billion individuals worldwide.1 Essential hypertension (EH) accounts for the majority of cases, while in ∼5% secondary causes of hypertension can be detected. Atherosclerotic renal artery stenosis (ARAS) remains an important entity that acclerates secondary hypertension and renal injury and can be identified in >6.8% of individuals older than 65.2 Renovascular hypertension in ARAS patients and decreased glomerular filtration rate (GFR) are associated with increased cardiovascular morbidity and mortality3 and target organ injury compared with EH.4
In patients with ARAS, a pressure gradient across the stenosis leads to activation of the renal-angiotensin–aldosterone system to increase systemic arterial pressure, which also increases oxidative stress and leads to lipid peroxidation and platelet aggregation, contributing to kidney and target organ injury.5 Experimental data suggest that angiotensin II elicits a chronic vascular inflammatory reaction characterized by Th-1 lymphocyte activation and macrophage infiltration, which is mediated by and contributes to release of pro-inflammatory cytokines.6,7 We have previously shown that the stenotic swine kidneys exhibit increased tissue levels of the pro-inflammatory chemokine monocyte chemoattractant protein (MCP-1), associated with endothelial dysfunction and microvascular loss.8 Similarly, an increased production of the inflammatory factors tumour necrosis factor (TNF)-α and interleukin (IL)-6 was observed in 2K1C rats 4 days after renal artery clipping.9 Furthermore, elevated systemic levels of these inflammatory markers are associated with an increased risk for developing chronic kidney disease (CKD) in humans, suggesting a role for inflammatory mechanisms in its aetiology.10
Tissue inflammation activates a mixture of adaptive responses and repair mechanisms in the stenotic kidney (STK). Mobilized endogenous circulating endothelial progenitor cells, recruited and directed by homing factors and cytokines released from damaged tissues, play a critical role in healing tissues by mediating neovascularization and attenuating tissue injury.11 We have demonstrated that the experimental ARAS kidney recruits endogenous circulating progenitor cells to stimulate its reparative processes.12 However, whether this repair mechanism is activated in the human kidney has not been determined. Thus, the present study was designed to test the hypothesis that in renovascular hypertensive patients the injured kidney releases inflammatory mediators and retains circulating progenitor cells. For this purpose, we measured bilateral renal vein (RV) and inferior vena cava (IVC) levels of inflammatory biomarkers and CD34+/KDR+ progenitor cells in essential and renovascular hypertensive patients.
Methods
Patient population
Patients identified with EH (n= 24) or unilateral ARAS (n= 24), participating in inpatient protocol studies,13 were prospectively enrolled from 1 August 2008 to 11 October 2010. Informed written consent was obtained after receiving approval from the Institutional Review Board of the Mayo Clinic. Atherosclerotic renal artery stenosis patients were included using entry criteria analogous to enrolment in Cardiovascular Outcomes for Renal Atherosclerotic Lesions (CORAL).14 Imaging criteria included renal artery Doppler ultrasound velocity acceleration (peak systolic velocity >200 cm/s), or MR/CT angiography with evident stenosis >60% and/or post-stenotic dilation.
Exclusion criteria included serum creatinine >1.7 mg/dL, uncontrolled hypertension [systolic blood pressure (SBP) >180 mmHg, despite antihypertensive therapy], diabetes requiring medications, recent cardiovascular event (myocardial infarction, stroke, congestive heart failure within 6 months), pregnancy, and kidney transplant.
Clinical data collection and laboratory measurements
Patients were admitted to the Clinical Research Unit for 3 days. Dietary intake of 150 mEq of sodium was maintained throughout the duration of the study. To standardize antihypertensive treatment in EH and ARAS subjects, in all patients blockade of the renin–angiotensin system with angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARBs) was continued or initiated at usual recommended daily dose (equivalent: 40 mg lisinopril). Diuretics were continued. One day before RV sampling, a single dose of furosemide was administered for other protocol studies.13 Normotensive control subjects (SBP <130 and diastolic blood pressure <80 mmHg) were prospectively recruited and serum samples collected through the Mayo Clinic Biobank, and matched to EH and ARAS according to age, gender, and body mass index.
Clinical data collected by physical examination or via the electronic medical records included age, sex, height, weight, body mass index, systolic, diastolic, and mean arterial pressure. Serum creatinine, estimated GFR (eGFR), proteinuria, plasma renin activity (PRA), low density lipoprotein, high density lipoprotein, total cholesterol, and triglyceride levels were determined by standard procedures. In addition, use of concomitant medication, comorbidities, and smoking status were recorded.
Renal vein sampling and renal haemodynamics measurement
Blood samples were obtained prior to the measurement of kidney blood flows by multi-detector computer tomography (MDCT). In brief, a catheter was placed via the femoral or internal jugular vein and 120 mL of blood obtained from the right and left RV and IVC. These samples were subsequently analysed for inflammatory cytokines, CD34+/KDR+ (kinase insert domain receptor) progenitor cell number and homing signals, adhesion molecules, and pro-angiogenic factors. The catheter was moved to the superior vena cava for contrast media injections during MDCT imaging. Stenotic-kidney and contralateral-kidney (CLK) cortical perfusion, medullary perfusion, and renal blood flow (RBF) were measured in ARAS patients using MDCT. In EH patients, individual kidney (right and left) cortical perfusion, medullary perfusion, and RBF were averaged. The estimated glomerular filtration rate was measured using the modification of diet in renal disease (MDRD) study equation. In healthy volunteers, only peripheral (antecubital) blood samples were collected.
Inflammatory biomarkers
Renal vein and IVC levels of soluble E-Selectin (sE-Selectin), vascular cell adhesion molecule (sVCAM-1), TNF-α and its receptor (sTNFR-1), IL-6, IL-10, MCP-1, granulocyte colony-stimulating factor (G-CSF), interferon (IF)-γ, tissue plasminogen activator inhibitor (PAI)-1, vascular endothelial growth factor (VEGF) and its receptors (VEGFR-1 and VEGFR-2), stromal-derived factor (SDF)-1, and stem cell factor (SCF) were measured by luminex (Millipore, Billerica, MA, USA). Based on the assumption that the difference between infra-renal IVC and RV levels reflects the net release of cytokines within the affected kidney,15 we estimated a renal cytokine gradient (RV-IVC) and the net release (gradient × RBF) for each measured product.
Characterization of CD34+/KDR+ progenitor cells
Peripheral (for controls), RV, and IVC blood mononuclear cells were isolated from fresh blood by the density-gradient method and characterized for antigen expression of the endothelial progenitor markers CD34 and KDR.16 Systemic and RV levels of CD34+/KDR+ progenitor cells were determined by fluorescence-activated cell sorting, as previously described.15,17,18
Statistical analysis
Statistical analysis was performed using JMP software package version 8.0 (SAS Institute, Inc., Cary, NC, USA). Results were expressed as mean ± SD for normally distributed data and as median (range) for data that did not show a Gaussian distribution. Comparisons within patients with severe (≥75%) and moderate (<75%) degree of stenosis were performed using paired Student's t-test or the Kruskal–Wallis test, when appropriate. All tests were two-tailed, and P-values ≤0.05 were considered statistically significant.
For detailed methods regarding blood sampling, single-kidney haemodynamics assessment, statistical analysis, and cytofluorimetric CD34+/KDR+ progenitor cell detection, please see the Supplementary material online.
Results
Table 1 shows the clinical, laboratory, and demographic characteristics of the patients included in the study. Systolic blood pressure was higher in EH and ARAS compared with normal (P= 0.001 and P= 0.019, respectively), with no significant differences in antihypertensive regimens between the groups. Serum creatinine levels were higher and eGFR lower in ARAS patients compared with normal and EH, but cholesterol fractions and medication intake were not different. The estimated glomerular filtration rate calculated by the Chronic Kidney Disease Epidemiology Collaboration equation19 yielded similar results (data not shown). Single-kidney cortical perfusion was decreased in the STK compared with the CLK and EH patients (P= 0.001 and P= 0.042, respectively), which were not different from each other. Single-kidney medullary perfusion levels were similar among the groups. Single-kidney RBF was reduced in the STK-ARAS compared with EH (Table 1, P= 0.009) and to the CLK (P< 0.0001). Systemic PRA levels were elevated in ARAS compared with normal and EH patients, and RV PRA levels were higher in the STK-ARAS compared with EH (P= 0.007) and the CLK (P= 0.02). Conversely, urinary protein excretion did not differ among the groups. Four ARAS patients presented modest lesions in the CLK, considered haemodynamically insignificant (<50%, peak systolic velocity <200 cm/s).
Table 1.
Clinical, laboratory, and demographic data of normal, essential hypertensive, and atherosclerotic renal artery stenosis patients
| Normal | EH | ARAS (STK) | ARAS (CLK) | |
|---|---|---|---|---|
| Demographics | ||||
| No. of patients | 24 | 24 | 24 | |
| Age (years) | 67 (52–79) | 68 (26–78) | 66.5 (51–79) | |
| Gender (male/female) | 12/12 | 12/12 | 12/12 | |
| Body mass index | 26.7 ± 4.1 | 27.1 ± 3.8 | 28.1 ± 4.5 | |
| Duration of the disease (years) | 9.8 ± 6.5 | 10.7 ± 10.3 | ||
| Smoking status | ||||
| Current smoker (n, %) | 2/8.3 | 3/12.5 | 3/12.5 | |
| Former smoker (n, %) | 5/20.8 | 7/29.2 | 10/41.7 | |
| Related laboratory measures | ||||
| Systolic blood pressure (mmHg) | 120.8 ± 11.2 | 139.7 ± 19.3* | 131.9 ± 18.5* | |
| Diastolic blood pressure (mmHg) | 71.5 ± 8.7 | 70.7 ± 13.3 | 68.9 ± 8.8 | |
| Mean blood pressure (mmHg) | 87.9 ± 8.0 | 92.9 ± 12.0 | 89.9 ± 9.8 | |
| Total cholesterol (mg/dL) | 170.9 ± 32.1 | 189.4 ± 31.3 | 171.7 ± 22.4 | |
| Concomitant medication (n, %) | ||||
| No. of antihypertensive drugs median (range) | 0 | 3 (1–5) | 3 (1–4) | |
| Diuretic | 0/0 | 16/66.7 | 15/62.5 | |
| Calcium-channel blocker (CCB) | 0/0 | 8/33.3 | 9/37.5 | |
| Beta-blocker (BB) | 0/0 | 11/45.8 | 12/50.0 | |
| Angiotensin-converting enzyme inhibitor (ACE-I) | 0/0 | 16/66.7 | 12/50.0 | |
| Angiotensin receptor blocker (ARB) | 0/0 | 10/41.7 | 13/54.2 | |
| Alpha-blocker (AB) | 0/0 | 2/8.3 | 0/0 | |
| Statins/lipid-lowering drugs | 0/0 | 11/45.8 | 16/66.7 | |
| Hormone replacement therapy/oestrogens | 1/4.2 | 2/8.3 | 1/4.2 | |
| Renal function | ||||
| Serum creatinine (mg/dL) | 0.95 (0.7–1.2) | 0.9 (0.5–1.5) | 1.0 (0.7–1.9)*,** | |
| eGFR-MDRD (mL/min/1.73/m2) | 80.4 ± 10.6 | 78.9 ± 22.5 | 64.5 ± 23.5*,** | |
| Cortical perfusion (mL/min/cc) | 3.3 ± 1.2 | 2.6 ± 1.0**,*** | 3.0 ± 0.9 | |
| Medullary perfusion (mL/min/cc) | 1.3 (0.21–3.11) | 1.1 (0.49–2.14) | 1.1 (0.55–1.7) | |
| Single-kidney RBF (mL/min) | 358.9 ± 146.0 | 241.1 ± 150.8**,*** | 431.1 ± 131.3 | |
| PRA systemic (ng/mL/h) | 0.5 (0.1–1.8) | 5.2 (0.1–25.2)* | 10.1 (0.3–43.3)*,** | |
| PRA RV (ng/mL/h) | 6.2 (0.1–30.8) | 17.8 (0.3–57.2)**,*** | 13.7 (0.2–46.3) | |
| Proteinuria (mg/24 h) | 90.2 ± 81.1 | 92.9 ± 83.8 | 80.0 ± 50.2 | |
EH, essential hypertensive patients; ARAS, atherosclerotic renal artery stenosis patients; PRA, plasma renin activity; STK, stenotic kidney; CLK, contralateral kidney; RV, renal vein; RBF, renal blood flow; eGFR-MDRD, estimated glomerular filtration rate-modification of diet in renal disease.
*P≤ 0.05 vs. normal.
**P≤0.05 vs. EH.
***P< 0.05 vs. ARAS (CLK).
Cell adhesion molecules
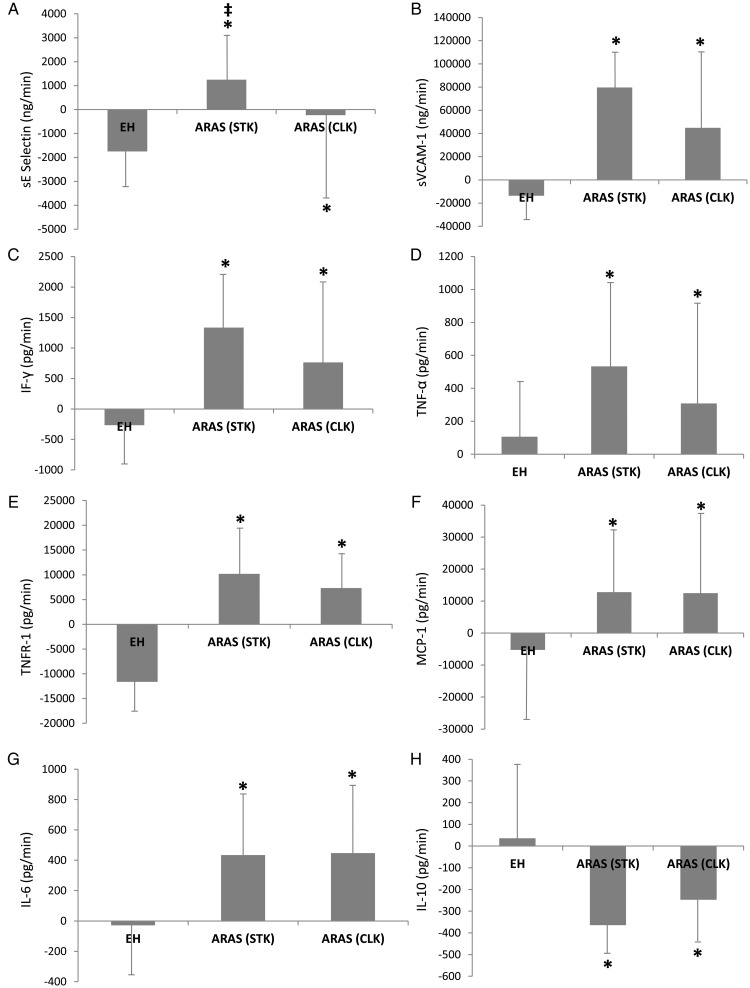
Renal vein levels of E-Selectin and sVCAM-1 were higher in the STK-RV compared with EH (Table 2, P= 0.005 and P= 0.034, respectively), and their renal net release was elevated (P< 0.0001 and P< 0.0001 vs. EH, Figure 1), whereas CLK-RV levels of cell adhesion molecules were not different than in EH (P= 0.060 and P= 0.356, respectively). The stenotic kidney-net release of sE-Selectin, but not sVCAM-1, was higher compared with the CLK (Figure 1A and B, P= 0.048 and P= 0.951 vs. CLK).
Table 2.
Systemic and renal vein cytokine levels in normal volunteers, essential hypertensive and atherosclerotic renal artery stenosis patients
| Normal | EH |
ARAS |
||||
|---|---|---|---|---|---|---|
| Systemic | Systemic | RV | Systemic | CLK RV | STK RV | |
| Cell adhesion molecules | ||||||
| sE-Selectin (ng/mL) | 15.0 (7.0–32.9) | 16.6 (3.9–43.9) | 24.3 (2.4–39.4) | 22.1 (6.2–63.9) | 12.3 (11.9–41.2) | 31.3 (9.3–67.6)*,** |
| sVCAM-1 (ng/mL) | 617.8 (319–2021) | 992.5 (622–1505) | 658.9 (599–21370) | 858.6 (526–1515) | 770.6 (526–1724) | 1055.1 (559–1847)*,**,*** |
| Inflammatory markers | ||||||
| MCP-1 (pg/mL) | 120.7 (88.7–191.2) | 140.7 (57–245) | 128.5 (49–193) | 122.4 (46–272) | 124.0 (45–452) | 169.0 (52–363)*,**,*** |
| IF-γ (pg/mL) | 4.3 (2.2–12.7) | 4.6 (3.6–17.2) | 3.9 (1.4–20.6) | 6.0 (2.2–16.0) | 6.1 (0.1–18.8) | 7.4 (6.1–22.4)*,**,***,**** |
| TNF-α (pg/mL) | 3.5 (1.5–7.7) | 3.3 (1.0–8.4) | 3.9 (2.0–9.9) | 5.3 (1.8–14.2) | 5.3 (3.0–11.9) | 6.4 (3.5–11.6)*,**,*** |
| sTNFR-1 (pg/mL) | 945.7 (554–1483) | 942.3 (482–2110) | 947.8 (383–1919) | 910.5 (737–2052) | 1235.8 (768–3487) | 1313.5 (722–3570)*,** |
| IL-6 (pg/mL) | 1.17 (1.17–9.91) | 1.7 (1.3–37.8) | 1.5 (1.5–37.8) | 2.3 (2.3–12.3) | 2.4 (0.4–16.0) | 4.4 (2.8–14.0)*,*** |
| Anti-inflammatory markers | ||||||
| IL-10 (pg/mL) | 2.83 (2.83–38.5) | 1.2 (0.5–17.3) | 1.8 (1.3–15.7) | 3.8 (0.4–12.6) | 1.6 (0.7–11.4) | 1.5 (0.5–9.7)*,*** |
| Homing factors | ||||||
| SDF-1 (pg/mL) | 1468.2 (892–6740) | 1487.1 (118–3876) | 1581.5 (149–3574) | 1460.6 (1093–7614) | 2305.1 (1262–5785) | 1868.6 (1278–6792)*,** |
| SCF (pg/mL) | 6.4 (1.3–37.0) | 2.0 (1.3–66.6) | 2.3 (2.1–40.4) | 11.5 (2.9–51.9) | 9.0 (2.8–50.1) | 11.8 (3.4–49.4)*,** |
CLK, contralateral kidney; STK, stenotic kidney.
*P ≤ 0.05 vs. normal.
**P ≤ 0.05 vs. EH (RV).
***P ≤ 0.05 vs. ARAS (systemic).
****P ≤ 0.05 vs. ARAS (CLK).
Figure 1.
The net release of cytokines in essential hypertension, stenotic kidney, and contralateral kidney of atherosclerotic renal artery stenosis patients. *P< 0.05 vs. essential hypertension, ‡P< 0.05 vs. contralateral kidney.
Inflammatory markers
Renal vein levels of G-CSF did not differ among the groups (Supplementary material online, Table S1). Renal vein levels and the net release of MCP-1, IL-6, IF-γ, TNF-α, and sTNFR-1 were higher in the STK-RV compared with normal and EH, and in the CLK compared with EH (Figure 1; Table 2), but not different between STK and CLK (Figure 1C–G). In contrast, ARAS-RV levels of IF-γ were higher in the STK compared with the CLK.
Anti-inflammatory markers
Renal vein levels of IL-10 were lower in the STK-ARAS compared with normal and EH (Table 2, P= 0.0002 and P= 0.0004, respectively), and its net release decreased (P= 0.015 vs. EH, Figure 1H). The net release of IL-10 was also lower in the CLK compared with EH, but did not differ between the STK and CLK.
Angiogenic and fibrogenic factors
Circulating and RV levels of VEGF, VEGFR-1, VEGFR-2, and PAI-1 were similar among the groups (Supplementary material online, Table S1).
Progenitor cells homing factors
Renal vein levels of SDF-1 and SCF were higher in the STK-ARAS compared with normal (both P= 0.024) and EH patients (P< 0.016 and P< 0.006, Table 2), and their net release increased (Supplementary material online, Figure S1, P< 0.009 and P= 0.012 vs. normal; P< 0.014 and P< 0.044 vs. EH).
CD34+/KDR+ progenitor cells
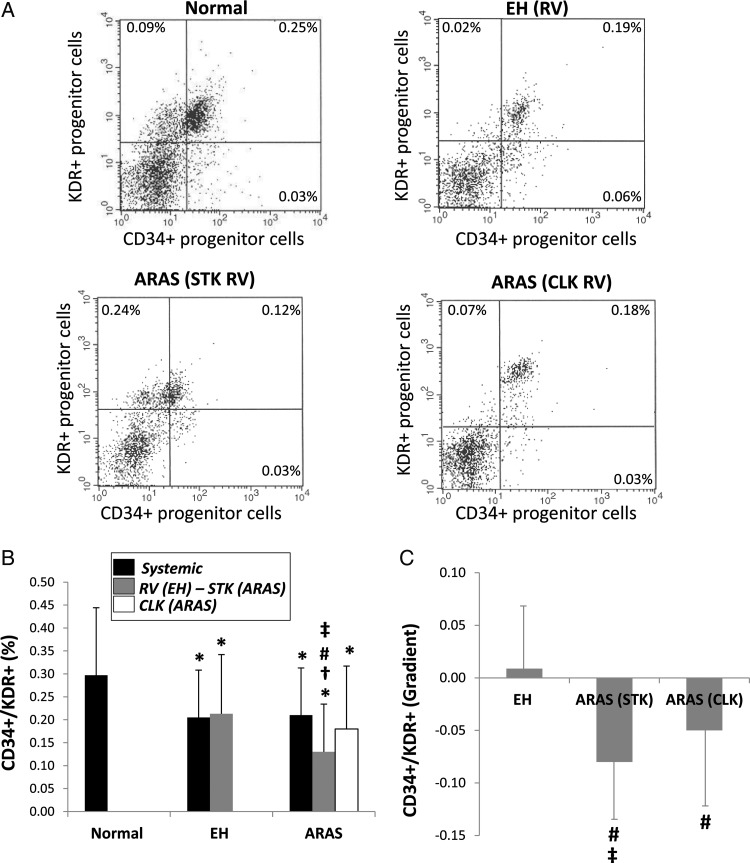
Systemic levels of CD34+/KDR+ positive progenitor cells were further decreased in ARAS and EH compared with normal (Figure 2A and B). Renal vein levels of CD34+/KDR+ progenitor cells were decreased in ARAS-STK compared with EH and ARAS-CLK, and were below systemic levels in any group. CD34+/KDR+ progenitor cells exhibited a negative gradient across the STK and CLK (Figure 2C) consistent with the net removal of progenitor cells during their passage. However, The CD34+/KDR+ progenitor cell gradient was significantly greater in the STK compared with the CLK (P= 0.017).
Figure 2.
(A) Representative flow cytometric dot plots for CD34+/KDR+ progenitor cells in study patients. Systemic and renal vein (RV) levels (B) and gradients (C) of CD34+/KDR+ progenitor cells in normal, essential hypertensive, stenotic kidney and contralateral kidney of atherosclerotic renal artery stenosis patients. Renal vein CD34+/KDR+ progenitor cell levels were reduced in the stenotic kidney-atherosclerotic renal artery stenosis kidney, leading to a negative CD34+/KDR+ progenitor cell gradient across it, greater than across the contralateral kidney. *P< 0.05 vs. normal, #P< 0.05 vs. essential hypertension, †P< 0.05 vs. systemic, ‡P< 0.05 vs. contralateral kidney.
Correlation between inflammatory biomarkers, progenitor cells, and renal haemodynamics
Renal vein levels of CD34+/KDR+ progenitor cells were weakly but significantly inversely correlated only with IF-γ, and showed a trend for correlation with TNF-α (Supplementary material online, Figure S3A and B).
Renal vein levels of sE-Selectin, sVCAM-1, TNF-α, and MCP-1 inversely correlated with STK-RBF in ARAS patients (Supplementary material online, Figure S3C–F).
Stenotic kidney-renal vein levels of sE-Selectin, TNF-α, and MCP-1 also inversely correlated with STK cortical (Supplementary material online, Figure S2A–C), but not medullary STK perfusion (Supplementary material online, Figure S2D–F) in ARAS patients. No correlation was found between the duration of the hypertension and STK-RV levels or the net release of inflammatory cytokines (data not shown).
Renal vein levels and the net release of IF-γ, MCP-1, and IL-6 were elevated in patients with severe (≥75%) vs. moderate (<75%) stenosis (Supplementary material online, Figure S4, P= 0.044, P= 0.019, and P= 0.39, respectively), while those of the anti-inflammatory cytokine IL-10 were lower (Supplementary material online, Figure S4H). Furthermore, RV levels of sE-Selectin, sVCAM-1, IF-γ, TNF-α, and MCP-1 were elevated in the severely STK compared with its CLK, while severely STK-RV levels of IL-10 were lower (Supplementary material online, Figure S5).
Finally, CLK-RV levels of inflammatory cytokines did not correlate with either CD34+/KDR+ progenitor cells or renal haemodynamics (data not shown).
Please see the Supplementary material online for extended results regarding other inflammatory biomarkers, CD34+/KDR+ progenitor cells, renal haemodynamics, etc.
Discussion
The current study demonstrates, for the first time, that the post-stenotic human kidney with a reduced GFR and single-kidney RBF releases inflammatory cytokines that portend renal injury. At the same time, the RV blood contains proportional to kidney inflammation homing signals known to recruit and retain CD34+/KDR+ progenitor cells, which were indeed sequestered in the affected STK. The elevation of pro-inflammatory mediators in the ARAS kidney despite similar blood pressure levels and renin–angiotensin system blockade might partly explain higher rates of target organ injury compared with EH.
Atherosclerotic renal artery stenosis remains an important cause of renal dysfunction, and is associated with greater cardiovascular morbidity and mortality than EH.20 Despite much research, the mechanisms underlying irreversible renal injury and progressive deterioration of the ARAS-STK have not been fully elucidated.
Accumulating experimental evidence indicates that inflammatory factors play a central role in mediating renal damage in ARAS by regulating the production and activity of growth factors, leading to collagen deposition, matrix accumulation, fibrosis, microvascular regression, and renal scarring.21–23 Studies in swine and murine STK demonstrate infiltration of macrophages and T-lymphocytes,24,25 and activation of signalling pathways related to the production of pro-inflammatory cytokines.26 Functional impairment and structural damage in ARAS are also partly mediated by inflammatory cytokines such as MCP-1, IF-γ, and IL-8.8 However, while histological analysis of atherosclerotic nephropathy shows chronic interstitial inflammatory infiltration in patients with deteriorating renal function,27 much remains to be learned about the role of inflammation in human ARAS.
The present study provides evidence demonstrating selective renal release of cell adhesion molecules (sE-Selectin, sVCAM-1) and inflammatory cytokines (TNF-α, IF-γ, IL-6, MCP-1) into the RV from the stenotic human kidney. Moreover, inverse correlations between RV levels of inflammatory mediators (TNF-α, IF-γ) and CD34+/KDR+ progenitor cells suggest a role for kidney inflammation as a central pathway to progenitor cell recruitment and retention beyond the stenotic lesion.
The cytokines identified to be released from the STK are associated with several stages of the complex interplay among cell adhesion molecules, cytokines, and chemoattractant factors. While members of the selectin family play a central role in the initial phase of adhesion (rolling), members of the immunoglobulin superfamily-like VCAM-1 regulate recruitment of mononuclear cells to inflamed tissue by interacting with leucocyte integrins (activation and firm adhesion).28 The final step involves transmigration of adherent cells through the endothelium, which requires expression of chemotactic factors (e.g. MCP-1). In addition, release of IL-6, IF-γ, and MCP-1 stimulates activation and proliferation of B-and T-lymphocytes, and recruitment of leucocytes to the inflammation site.
Tumour necrosis factor-α has been implicated in renal inflammation by inducing expression of cell adhesion molecules, MCP-1, IL-1, IL-6, and IL-8.29 Indeed, we observed elevated STK-RV levels and the net release of IF-γ, MCP-1, and IL-6 in patients with severe ARAS, as well as inverse correlations between RV levels of TNF-α, sE-Selectin, sVCAM-1, and MCP-1 and STK-RBF, implicating renal ischaemia in cytokine-mediated inflammation in ARAS patients. Interestingly, this association was mainly due to a selective decrease in cortical perfusion, reflected by the inverse correlations found between STK-cortical (but not medullary) perfusion and STK-RV levels of several inflammatory biomarkers. Taken together, these observations suggest that the severity of renal hypoperfusion (mostly in cortical regions of the kidney) and tissue ischaemia may influence renal release of inflammatory cytokines in human ARAS. Our observations are supported by a previous studies showing elevated systemic levels of TNF-α and IL-6 in patients30 and pigs31 with renovascular hypertension.
In addition, RV levels of IL-10 were decreased in ARAS patients compared with EH and healthy volunteers. This Th-2 cytokine possesses important anti-inflammatory properties including inhibition of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) production and stimulation of anti-inflammatory cells such as regulatory T-cells and M2 macrophages.32,33 Reduction of their important reparative mechanism in the STK is consistent with removal of its protective effect.34 Furthermore, myocardial infarction-induced progenitor cell mobilization and survival partly depends on IL-10.35
Progenitor cell mobilization from the bone marrow is regulated by the release of homing factors by the damaged tissue, prominent among which are SDF-1 and SCF.36 We have shown that the swine ARAS kidney releases specific homing signals that recruit progenitor cells and stimulate a reparative process.12 Likewise, RV levels and the net release of SDF-1 and SCF were elevated in the human STK. The negative CD34+/KDR+ progenitor cell gradient across the STK may suggest that progenitor cells were recruited and retained, possibly consequent to the homing signals observed. However, continued release of inflammatory biomarkers implies that the CD34+/KDR+ progenitor cell reparative effect was insufficient to completely abrogate renal injury. Possibly, intense inflammatory activity and inadequate repair may account for the higher incidence of target organ injury in ARAS compared with EH. Furthermore, reduced availability of circulating CD34+/KDR+ progenitor cells might reflect a smaller systemic pool from which the kidney can draw progenitor cells in time of injury. Although CD34+/KDR+ cells exhibited a negative gradient across the STK, the KDR ligand VEGF did not. Nevertheless, we cannot rule out up-regulation of VEGF expression in the post-ischaemic kidney tissue, as observed in some experimental models.37
Notably, this study shows higher levels of the majority of inflammatory biomarkers compared with EH in the STK-ARAS, but not in the CLK-RV, underscoring the predominant propensity for inflammation in the post-stenotic kidney. Nevertheless, comparable levels and the net release of many inflammatory biomarkers in the STK and CLK emphasize the susceptibility of the non-stenotic kidney to target organ injury in ARAS, and extend previous observations in experimental25 and clinical38 ARAS that demonstrated increased fibrosis and inflammation in the non-stenotic kidney. This may have also contributed to the decrease in GFR observed in ARAS compared with normal volunteers and EH patients. It is not unlikely that microvascular disease in the atherogenic milieu of ARAS patients contributes to CLK injury even in the absence of superimposed large vessel obstruction. Alternatively, maladaptive activation of the renin–angiotensin system in the CLK, disclosed by slightly (although not statistically significant) elevated PRA levels vs. EH might have augmented inflammation. Under these conditions, stretch-induced AT1 receptor activation39 in the CLK might have exacerbated inflammation. Nonetheless, the inverse correlation of injurious signals with RBF only in the STK supports contribution of renal ischaemia to their stimulation. Furthermore, in patients with severe ARAS (degree of stenosis ≥75%), STK-RV levels of several inflammatory biomarkers were higher compared with the CLK, underscoring the differential contribution of the STK and CLK in the pathogenesis of ARAS. Finally, sequestration of CD34+/KDR+ progenitor cells in the CLK, (although lower than in the STK-ARAS) might reflect reparative processes active in the non-stenotic kidney. These observations suggest that both kidneys are involved in the pathogenesis of ARAS, yet the stenotic kidney plays a predominant role, particularly when the degree of stenosis is high.
Limitations
This study is limited by its cross-sectional nature with a relatively small study population, which was not prospectively defined, and by the lack of data on renal outcomes or changes in levels of inflammatory biomarkers or CD34+/KDR+ progenitor cells. Hence, precise cause and effect relationships between renal haemodynamics, inflammatory biomarkers, and progenitor cell levels need to be addressed in detail in future studies. A range of event numbers per sample has been utilized for counting circulating CD34+/KDR+ progenitor cells using flow cytometry. The putative progenitor cell phenotype we quantified (CD34+/KDR+) yields high cell counts16 and thus provides adequate assessment of circulating progenitor cells using this technique.15,17,18 In addition, many patients with ARAS or EH may present with considerable elevations of serum creatinine. In our study, patients with diabetes or serum creatinine levels >1.7 mg/dL were excluded, because of the use of iodinated contrast for MDCT studies. Therefore, extrapolation of our findings to the diverse population of ARAS patients observed in clinical practice should be done with caution. Also, our hypertensive patients were all treated with renin–angiotensin system blockers, which might have masked elevation in some humoral cytokine levels, and which elevated PRA. Furthermore, these changes in cytokine levels may not be specific for ARAS, and future studies will need to determine whether similar alterations are detectable in patients with other forms of CKD. Nonetheless, the correlation between STK-RV levels of several inflammatory cytokines and STK-RBF may implicate renal hypoperfusion as the trigger for cytokine release, rather than general parenchymal damage. Finally, the contribution of STK and CLK inflammation to their dysfunction needs to be tested in further studies by the reversibility of both upon revascularization. Indeed, a recent study demonstrated that baseline levels of C-reactive protein were associated with improved renal function after renal artery stenting, supporting this paradigm.40
Conclusions
Determining the mechanisms by which ARAS leads to progressive renal injury is an important step in developing targeted treatment modalities to prevent deterioration of renal function. Our results demonstrate for the first time that CD34+/KDR+ progenitor cells are sequestered in the stenotic kidney of ARAS, in parallel with increased release of inflammatory biomarkers. These observations imply that chronic vascular occlusion contributes to inflammation in human ARAS, and its reparative process may involve progenitor cell recruitment. These processes seem to affect both kidneys even in visually unilateral disease, yet inflammation in the post-stenotic kidney pre-dominates, particularly when the stenosis is severe. Identifications of these mechanisms may provide novel therapeutic targets to attenuate loss of renal function in ARAS, as well as its sequelae.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was partly supported by the National Institutes of Health (HL085307, DK73608, DK77013, HL77131, and UL1-RR024150), the AHA, and the Mayo Clinic Center for Individualized Medicine.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
The authors are grateful to Mr Marshall Behrens, Mayo Clinic Immunology, for his technical support, and Dr Keith Knutson for the use of the Luminex machine.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443–451. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 3.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in united states patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293–301. doi: 10.1111/j.1523-1755.2005.00406.x. [DOI] [PubMed] [Google Scholar]
- 4.Losito A, Fagugli RM, Zampi I, Parente B, de Rango P, Giordano G, Cao P. Comparison of target organ damage in renovascular and essential hypertension. Am J Hypertens. 1996;9:1062–1067. doi: 10.1016/0895-7061(96)00199-9. [DOI] [PubMed] [Google Scholar]
- 5.Minuz P, Patrignani P, Gaino S, Degan M, Menapace L, Tommasoli R, Seta F, Capone ML, Tacconelli S, Palatresi S, Bencini C, Del Vecchio C, Mansueto G, Arosio E, Santonastaso CL, Lechi A, Morganti A, Patrono C. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation. 2002;106:2800–2805. doi: 10.1161/01.cir.0000039528.49161.e9. [DOI] [PubMed] [Google Scholar]
- 6.Nobuhiko A, Suganuma E, Babaev VR, Fogo A, Swift LL, Linton MF, Fazio S, Ichikawa I, Kon V. Angiotensin ii amplifies macrophage-driven atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2143–2148. doi: 10.1161/01.ATV.0000145607.03879.e0. [DOI] [PubMed] [Google Scholar]
- 7.Stouffer GA, Pathak A, Rojas M. Unilateral renal artery stenosis causes a chronic vascular inflammatory response in apoe−/− mice. Trans Am Clin Climatol Assoc. 2010;121:252–264. 264–256. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu XY, Chade AR, Krier JD, Daghini E, Lavi R, Guglielmotti A, Lerman A, Lerman LO. The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. J Hypertens. 2009;27:2063–2073. doi: 10.1097/HJH.0b013e3283300192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matavelli LC, Huang J, Siragy HM. Angiotensin at receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar A, Sun L, Klein BE, Lee KE, Muntner P, Nieto FJ, Tsai MY, Cruickshanks KJ, Schubert CR, Brazy PC, Coresh J, Klein R. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 12.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55:961–966. doi: 10.1161/HYPERTENSIONAHA.109.145227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy TP, Cooper CJ, Dworkin LD, Henrich WL, Rundback JH, Matsumoto AH, Jamerson KA, D'Agostino RB. The cardiovascular outcomes with renal atherosclerotic lesions (coral) study: rationale and methods. J Vasc Interv Radiol. 2005;16:1295–1300. doi: 10.1097/01.RVI.0000176301.69756.28. [DOI] [PubMed] [Google Scholar]
- 15.Gossl M, Modder UI, Gulati R, Rihal CS, Prasad A, Loeffler D, Lerman LO, Khosla S, Lerman A. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J. 2010;31:2909–2914. doi: 10.1093/eurheartj/ehq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Boilson BA, Kiernan TJ, Harbuzariu A, Nelson RE, Lerman A, Simari RD. Circulating cd34+ cell subsets in patients with coronary endothelial dysfunction. Nat Clin Pract Cardiovasc Med. 2008;5:489–496. doi: 10.1038/ncpcardio1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiernan TJ, Boilson BA, Tesmer L, Harbuzariu A, Simari RD, Barsness GW. Effect of enhanced external counterpulsation on circulating cd34+ progenitor cell subsets. Int J Cardiol. 2011;153:202–206. doi: 10.1016/j.ijcard.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conlon PJ, O'Riordan E, Kalra PA. New insights into the epidemiologic and clinical manifestations of atherosclerotic renovascular disease. Am J Kidney Dis. 2000;35:573–587. doi: 10.1016/s0272-6386(00)70002-3. [DOI] [PubMed] [Google Scholar]
- 21.Johns EJ. Inflammation: the underlying foe in renovascular hypertension? J Hypertens. 2009;27:1964–1965. doi: 10.1097/HJH.0b013e328331a881. [DOI] [PubMed] [Google Scholar]
- 22.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 23.Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15:958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 24.Eng E, Veniant M, Floege J, Fingerle J, Alpers CE, Menard J, Clozel JP, Johnson RJ. Renal proliferative and phenotypic changes in rats with two-kidney, one-clip goldblatt hypertension. Am J Hypertens. 1994;7:177–185. doi: 10.1093/ajh/7.2.177. [DOI] [PubMed] [Google Scholar]
- 25.Mai M, Geiger H, Hilgers KF, Veelken R, Mann JF, Dammrich J, Luft FC. Early interstitial changes in hypertension-induced renal injury. Hypertension. 1993;22:754–765. doi: 10.1161/01.hyp.22.5.754. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol. 2009;297:F1055–F1068. doi: 10.1152/ajprenal.90439.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001;16:765–770. doi: 10.1093/ndt/16.4.765. [DOI] [PubMed] [Google Scholar]
- 28.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. [PubMed] [Google Scholar]
- 29.Ernandez T, Mayadas TN. Immunoregulatory role of tnfalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- 30.Alhadad A, Guron G, Fortuna-Nowakowska E, Saeed A, Mattiasson I, Jensen G, Lindblad B, Gottsater A, Herlitz H. Renal angioplasty causes a rapid transient increase in inflammatory biomarkers, but reduced levels of interleukin-6 and endothelin-1 1 month after intervention. J Hypertens. 2007;25:1907–1914. doi: 10.1097/HJH.0b013e328244e2ca. [DOI] [PubMed] [Google Scholar]
- 31.Zhu XY, Daghini E, Chade AR, Napoli C, Ritman EL, Lerman A, Lerman LO. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephrol. 2007;18:1209–1217. doi: 10.1681/ASN.2006090976. [DOI] [PubMed] [Google Scholar]
- 32.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Harris DC. Macrophages in renal disease. J Am Soc Nephrol. 2011;22:21–27. doi: 10.1681/ASN.2010030269. [DOI] [PubMed] [Google Scholar]
- 34.George S, Ruan XZ, Navarrete C, Turner D, Reynard M, Sweny P, Hamilton G, Wheeler DC, Powis SH, Moorhead JF, Varghese Z. Renovascular disease is associated with low producer genotypes of the anti-inflammatory cytokine interleukin-10. Tissue Antigens. 2004;63:470–475. doi: 10.1111/j.0001-2815.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamurthy P, Thal M, Verma S, Hoxha E, Lambers E, Ramirez V, Qin G, Losordo D, Kishore R. Interleukin-10 deficiency impairs bone marrow-derived endothelial progenitor cell survival and function in ischemic myocardium. Circ Res. 2011;109:1280–1289. doi: 10.1161/CIRCRESAHA.111.248369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of sdf-1, vegf, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45–37. [DOI] [PubMed] [Google Scholar]
- 37.Favreau F, Zhu XY, Krier JD, Lin J, Warner L, Textor SC, Lerman LO. Revascularization of swine renal artery stenosis improves renal function but not the changes in vascular structure. Kidney Int. 2010;78:1110–1118. doi: 10.1038/ki.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tullis MJ, Zierler RE, Caps MT, Bergelin RO, Cantwell-Gab K, Strandness DE., Jr Clinical evidence of contralateral renal parenchymal injury in patients with unilateral atherosclerotic renal artery stenosis. Ann Vasc Surg. 1998;12:122–127. doi: 10.1007/s100169900127. [DOI] [PubMed] [Google Scholar]
- 39.Gobe GC, Axelsen RA, Searle JW. Cellular events in experimental unilateral ischemic renal atrophy and in regeneration after contralateral nephrectomy. Lab Invest. 1990;63:770–779. [PubMed] [Google Scholar]
- 40.Trani C, Porto I, Tommasino A, Giammarinaro M, Burzotta F, Niccoli G, Leone AM, Coroleu SF, Cautilli G, Mazzari MA, Schiavoni G, Crea F. Baseline inflammatory status and long-term changes in renal function after percutaneous renal artery stenting: a prospective study. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.03.078. Advance Access published April 12, 2012, doi:10.1016/j.ijcard.2012.03.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.