Abstract
Skin wounds comprise a serious medical issue for which few pharmacological interventions are available. Moreover, the inflammatory, angiogenic, and proliferative facets of a typical response to a wound each have broader relevance in other pathological conditions. Here we describe a genomics-driven approach to identify secreted proteins that modulate wound healing in a mouse ear punch model. We show that adiponectin, when injected into the wound edge, accelerates wound healing. Notably, adiponectin injection causes upregulation of keratin gene transcripts within hours of treatment, and subsequently promotes collagen organization, formation of pilosebaceous units, and proliferation of cells in the basal epithelial cell layer and pilosebaceous units of healing tissue. The globular domain of adiponectin is sufficient to mediate accelerated dorsal skin wound closure, and the effects are lost in mice that are homozygous null for the adiponectin receptor 1 gene. These findings extend recent observations of a protective role of adiponectin in other tissue injury settings, suggest modulation of AdipoR1 for the clinical management of wounds, and demonstrate a new approach to the identification of regulators of a wound healing response.
Introduction
The repair of most tissue injury in mammals is associated with a fibrotic response, resulting in scar tissue formation that often compromises the function of the affected organ. However, regeneration of adult mammalian liver and wounded mammalian fetal tissue are some of the few examples in which healing occurs without scar formation. Identification of regulators of the response to tissue injury would have major implications for the treatment of dermal lesions and may translate into novel strategies for the management of fibrotic conditions such as cirrhosis of the liver, fibrotic lung disease, and myocardial infarction (Gurtner et al., 2008). Thus far, only a few promising therapeutic candidates have been shown to promote wound healing in murine models, and these belong primarily to members of growth factor families such as fibroblast growth factor, transforming growth factor, and platelet-derived growth factor (Ortega et al., 1998; Keswani et al., 2004; Ferguson et al., 2009). In some cases, these findings have translated to clinical practice: topical platelet-derived growth factor administration for the treatment of diabetic ulcers and intravenous KGF injection to enhance mucosal epithelialization in the treatment of patients undergoing stem cell transplant for hematological malignancies (Smiell et al., 1999; Beaven and Shea, 2007).
Healing after an ear punch wound in the mouse ear is an experimentally tractable system that recapitulates much of the biology of tissue injury, including hemostasis, inflammation, and tissue remodeling. Wide variation in the quality and speed of the response to this injury has been noted across inbred mouse strains (Metcalfe et al., 2006), suggesting that experimental manipulation of the model might uncover new pathways with the potential for clinical modulation of tissue injury.
Here, we use the same model to identify several genes in which messenger RNA (mRNA) expression was highly regulated during the ear and dorsal punch wound response in MRL/MpJ (fast ear healing) or SJL/J (slow ear healing) mice. We used an automated mammalian protein production system (described in Gonzalez et al., 2010) to generate a subset of these proteins predicted to be secreted or single-pass transmembrane. Finally, we tested the effect of these proteins on the wound healing process by direct injection into the skin surrounding the wound and identified adiponectin as a novel regulator of cutaneous wound healing in mice. Molecular and histological effects of adiponectin on promoting epidermal reconstitution indicate this protein to be of potential use in human diseases of compromised epithelial barrier function.
Results
Selection of proteins to test using in vivo wound healing models
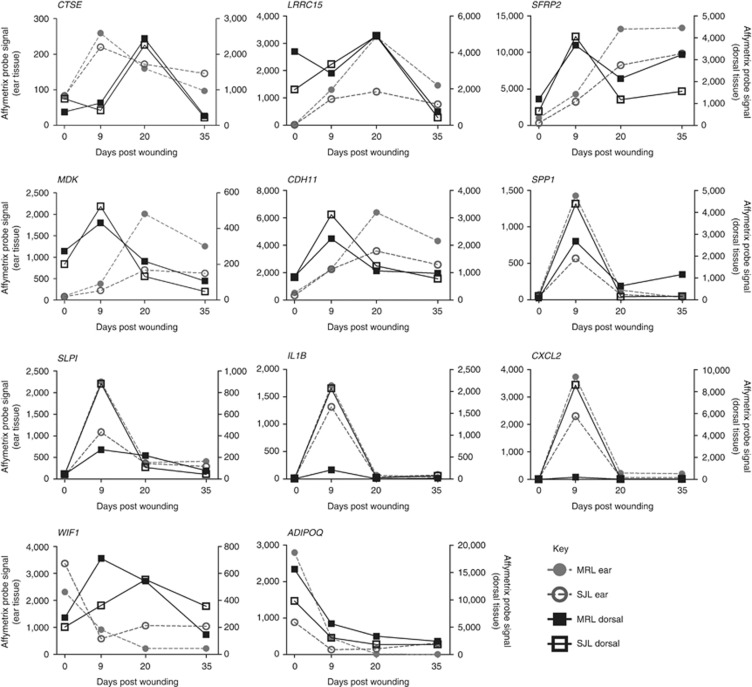
To identify genes with strongly regulated expression during cutaneous wound healing, we used expression arrays to analyze skin samples 9, 20, and 36 days after ear and dorsal skin wounding in two inbred mouse strains (MRL/MpJ and SJL/J). These data confirmed the upregulation of gene-expression modules known to be regulated by wounding, such as an inflammation signature at day 9 in ear (P=7.4 E−4) and dorsal tissue (P=0.03) and a keratinization signature at day 20 in dorsal tissue (P=9.5 E−21) (Supplementary Table S1 online, Supplementary Figure S1 online). A total of 250 mRNAs were at least 10-fold regulated during the wound healing process in at least one of the four data sets: dorsal or ear wounds in MRL/MpJ or SJL/J mice. These 250 mRNAs included 22 predicted single-pass membrane or secreted proteins, of which we were able to express 11 proteins for in vivo wound healing studies. Ten of these secreted proteins represent genes with increased gene expression after wound healing, whereas only one gene, adiponectin, had a sustained reduction in gene-expression levels after wounding in both tissue types and in both mouse strains (Figure 1).
Figure 1.
Temporal gene-expression profiles of 11 maximally altered secreted proteins tested in the ear punch wound healing model. Gene expression from MRL/MpJ and SJL/J ear (left y-axis) and dorsal (right y-axis) wound tissue is shown.
Identification of adiponectin as a regulator of murine wound healing
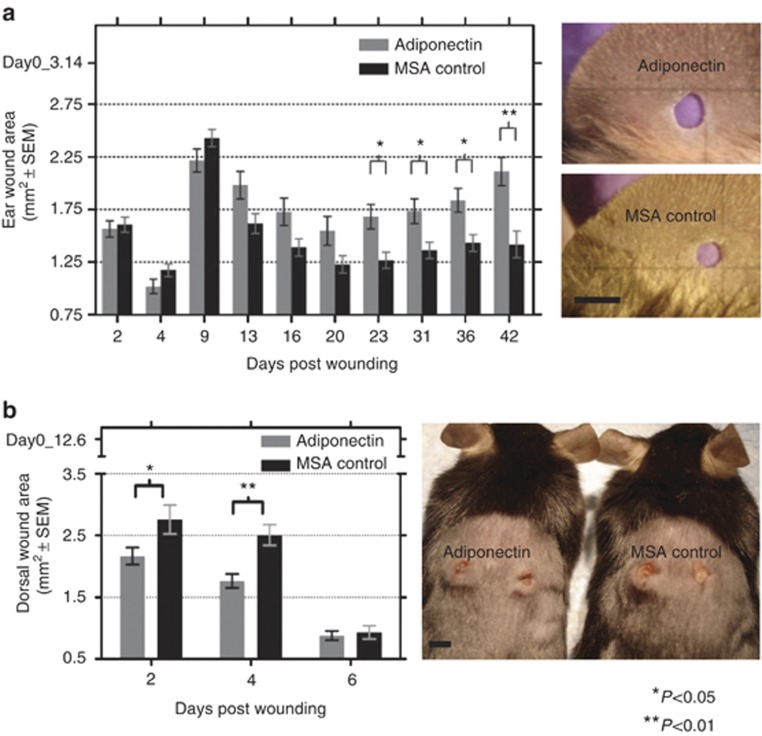
We injected 1 μg of recombinant purified protein into the wound edge area of 2-mm-diameter ear wounds of C57BL/6J mice on days 0, 1, 2, and 3 post wounding (Supplementary Figure S2 online). Subsequently, the wound area was measured over a period of 5–6 weeks (see Materials and Methods section for description of the in vivo ear wound model). In all, 10 of the 11 proteins tested showed no significant or reproducible effect on the rate of hole closure after an ear punch wound. However, adiponectin injection caused a significant healing phenotype when compared with mouse serum albumin (MSA) control injections in repeated experiments (Figure 2a). The hole diameter in mice from the adiponectin-treated group decreased less in the first 3 weeks after wounding than in the MSA control group. Between 3 and 6 weeks after wounding, the holes in the adiponectin-treated animals became larger, whereas those in the control groups remained of approximately constant size during this period. The ability of 1 μg adiponectin to affect ear hole closure was also confirmed in the SJL/J strain of mice (Supplementary Figure S3 online), but a lower dose of 400 ng adiponectin had no effect (Supplementary Figure S4 online).
Figure 2.
Direct injection of adiponectin at the wound site promotes in vivo wound healing. (a) Effects of adiponectin injection on hole closure following ear punch in C57Bl/6J mice. Photographs of representative mice ear wounds (day 42 post wounding). Bar=2 mm. (b) Effects of adiponectin injection on wound closure following dorsal punch in C57Bl/6J mice. Photograph of representative mice dorsal wounds (day 2 post wounding). Bar=4 mm. MSA, mouse serum albumin.
Wound healing in dorsal skin is distinguished from the ear punch model by a more prominent vasculature component and the presence of a migratory surface for attachment of the healing epithelial tongue. Biopsy punches were administered on either side of the mid-dorsum region excising the dermal and epidermal skin layers. A measure of 4 μg of adiponectin protein was injected at the wound site by inserting the needle intradermally adjacent to the wound punch (see Materials and Methods section for description of the in vivo dorsal wound model). In adiponectin-treated mice, we noticed significantly faster healing of dorsal wounds compared with MSA-treated controls (P=0.034 and P=0.006 at days 2 and 4 post wounding, respectively) (Figure 2b).
Adiponectin causes enhanced expression of keratin genes in undamaged skin, and enhanced epithelialization at the wound margin
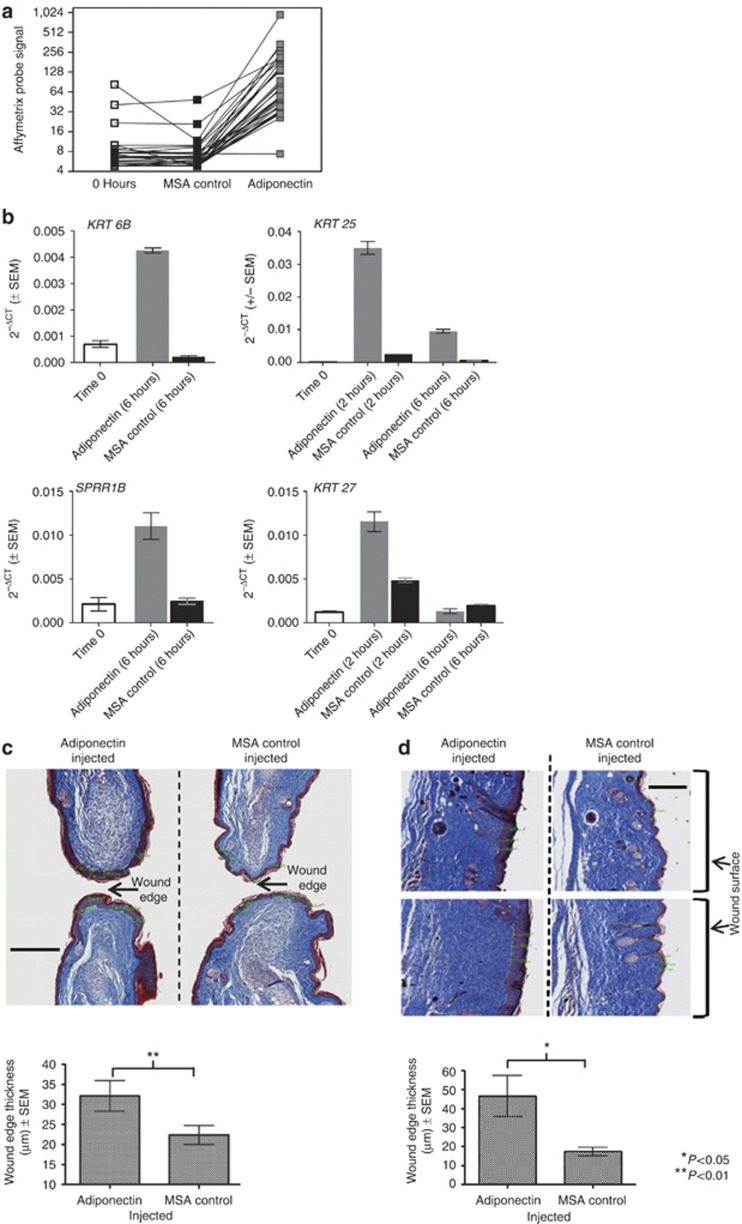
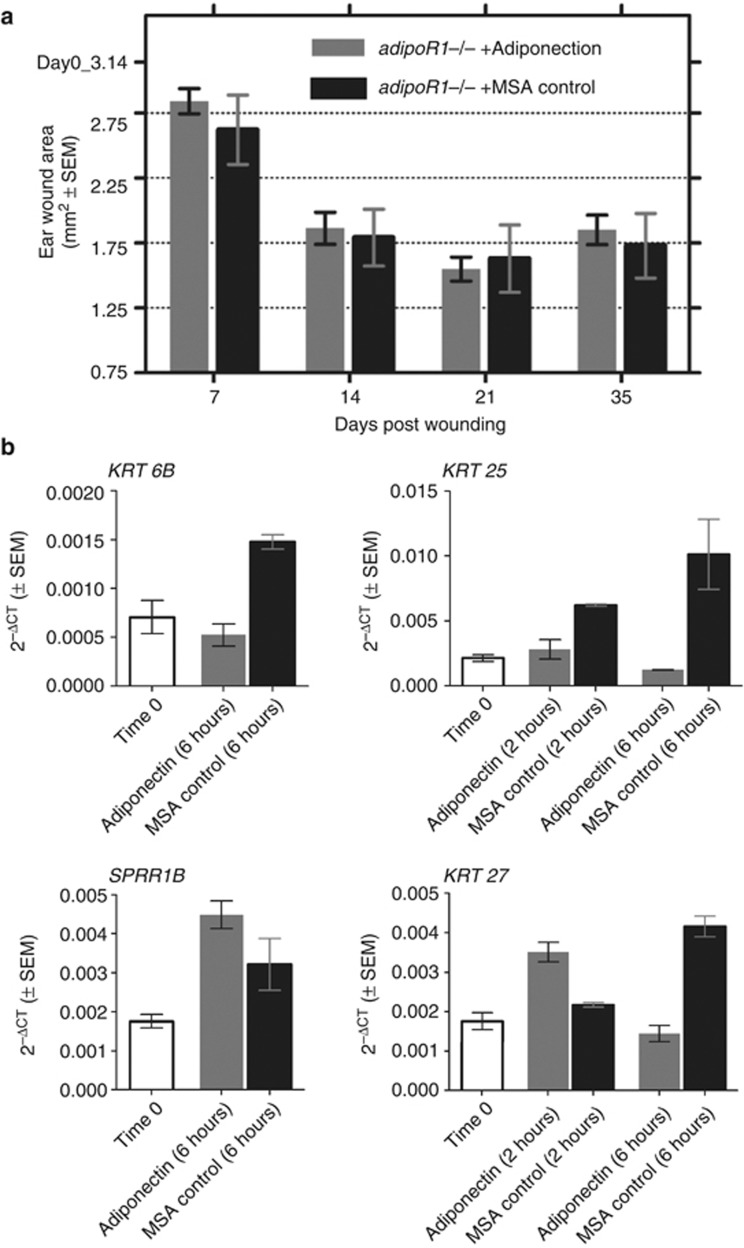
To detect changes in gene expression directly attributable to adiponectin injection, we tested the effects of adiponectin injection on gene expression in vivo in unwounded mouse skin, using whole-genome Affymetrix expression arrays. These data indicated a striking and transient upregulation in the expression of genes associated with keratinization at 6 hours following adiponectin injection when compared with control MSA-injected mouse ears (Figure 3a) (P=9.9 E−29, Bonferroni-corrected significance test for “keratin” keyword—Swiss Prot & Protein Information Resource Keyword Search, DAVID Bioinformatics Resource). We measured the expression of a select group of genes involved in keratinization (Krt6b, Sprr1b, Krt25, Krt27) in an independent in vivo experiment. Krt6b and Sprr1b are canonical markers of a wound healing response (Mansbridge and Knapp, 1987; Wong and Coulombe, 2003; Vermeij and Backendorf, 2010), whereas Krt25 and Krt27 are structural proteins of the proliferative hair follicular unit (Langbein et al., 2006). By using quantitative real-time reverse-transcriptase–PCR (qRT-PCR), we confirmed the ability of adiponectin to directly upregulate the expression of these genes as early as 2 hours after injection of adiponectin (Figure 3b). However, adiponectin had no effect on keratin gene expression or on cell migration using in vitro assays in primary human keratinocyte NHEK cells (Supplementary Figure S5 online).
Figure 3.
In vivo effects of adiponectin on epithelialization. (a) Adiponectin injection causes upregulation of “keratin” genes 6 hours post injection in ear tissue in C57Bl/6J mice (Affymetrix gene-expression data). (b) Adiponectin injection causes upregulation of select genes involved in keratinization of the epidermis in C57Bl/6J wild-type mice (quantitative real-time reverse-transcriptase PCR data). (c) Adiponectin injection causes enhanced epithelialization at ear wound edge (day 20 post wounding). Bar=200 μm. (d) Adiponectin injection causes enhanced epithelialization at the dorsal wound surface (day 14 post wounding). Bar=200 μm. Masson's trichome stain used in c and d; the epidermis is stained purple.
Histological analysis of adiponectin-injected ear wounds revealed that at day 20 post wounding adiponectin-injected ears displayed a significant thickening of the epidermal layer at the leading edge of the wound (P=0.001) (Figure 3c). This enhanced epithelialization was not evident in ear tissue peripheral to the wound site (data not shown). Adiponectin treatment was also associated with a significantly thicker epidermal layer compared with the MSA-treated control mice at 14 days after a dorsal wound (P=0.037) (Figure 3d).
Assessment of histological effects of adiponectin on wound healing parameters
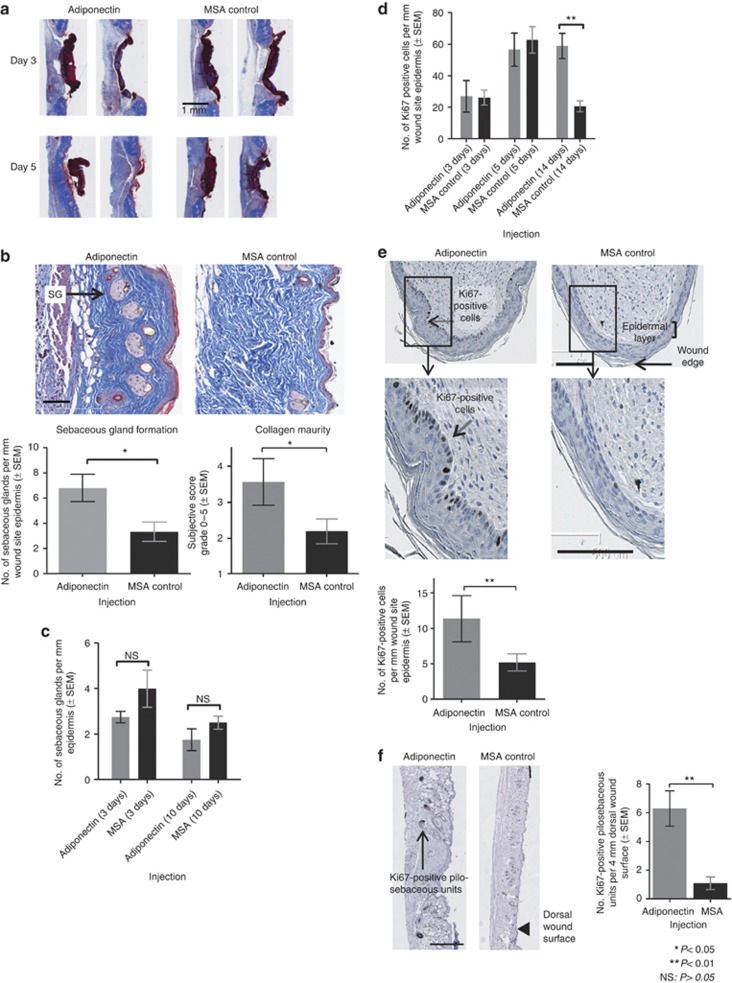
Adiponectin was injected in dorsal wounds and mice were killed at days 3, 5, 10, and 14 post wounding for analysis by Masson's trichome staining. No differences in tissue architecture could be identified at day 3 post wounding. However, analysis of day 5 post-wounding sections clearly showed earlier resolution of surface scab tissue in adiponectin-injected samples (Figure 4a). By day 10, collagen fibers were thicker and deposition was more orderly in adiponectin-treated wounds than in MSA controls, with a parallel arrangement of collagen fibrils in the adiponectin-treated mice (P=0.02, blind scoring for collagen fiber thickness and organization) (Figure 4b). We also noted a small, yet reproducible, transient upregulation of col3a1 (collagen type III), but not of col1a1 (collagen type I), gene expression, 6 hours after adiponectin injection in vivo (Supplementary Figure S6 online). A greater number of sebaceous glands were present in adiponectin-treated dorsal skin at day 10 post wounding (P=0.013) (Figure 4b). Notably, adiponectin injection in vivo did not cause an increase in the number of sebaceous glands compared with controls in unwounded dorsal skin (Figure 4c). We did not observe any changes in staining of CD31, a marker of angiogenesis, between adiponectin-injected and MSA-injected mice at 3, 10, or 20 days after an ear wound (data not shown), nor did we see any effect on neutrophil influx. Histological and gene-expression analyses of macrophages at dorsal wound sites indicated that adiponectin did not affect macrophage infiltration (Supplementary Figure S7 online). Collectively, these data highlight the expedited wound healing caused by adiponectin at the tissue level.
Figure 4.
In vivo effects of adiponectin on tissue reorganization and cellular proliferation. (a) Adiponectin injection in dorsal wounds causes faster restoration of normal tissue architecture at the wound surface (notable at day 5 post wounding; Masson's trichome stain). (b) Adiponectin injection causes enhanced collagen maturity and increased sebaceous glands formation (denoted as “SG” in figure) in dorsal wounds (day 10 post wounding). Collagen scoring based on subjective blind scoring assessing collagen fiber thickness and organization (0=poor score, 5=excellent score) (Masson's trichome stain: the epidermis is stained purple, collagen is stained blue, and sebaceous glands are stained pink). Bar=100 μm. (c) Adiponectin injection does not cause an increase in sebaceous glands at day 3 or day 10 post injection in unwounded dorsal skin. (d) Adiponectin injection causes increased Ki67 staining in the basal epidermal layer at the wound site in dorsal wounds (day 14 post wounding). (e) Adiponectin injection causes increased Ki67 staining in the basal epidermal layer at wound edge in ear wounds (day 20 post wounding). Bar=100 μm. (f) Adiponectin injection causes increased Ki67 staining in pilosebaceous units near the wound site in dorsal wounds (day 14 post wounding). Bar=500 μm. MSA, mouse serum albumin; NS, nonsignificant.
We then asked whether adiponectin caused greater proliferation at the wound site. Adiponectin did not affect epidermal proliferation at days 3 and 5 post wounding in dorsal skin, as assessed by Ki67 staining (a marker for cell proliferation) (Figure 4d). However, in tissues in which wounds had re-epithelialized, we noticed that adiponectin caused more frequent expression of Ki67 in both dorsal and ear wounds (Figure 4d and e). Notably, Ki67 expression was only observed in the basal layer of healed epidermis. These data indicate that the effects of adiponectin on epidermal proliferation are distinct from psoriatic skin conditions, in which general hyperplasia is observed in all epidermal layers (Staiano-Coico et al., 1993).
We also noticed significantly enhanced Ki67 staining in the pilosebaceous units of dorsal wounds in healed wounds (Figure 4f). The pilosebaceous units are a seat for a stem cell population that is known to be important in both tissue homeostasis and cutaneous wound healing (Blanpain, 2010).
Effects of adiponectin on wound healing are mediated via the AdipoR1 receptor
The biological effects of adiponectin are known to be regulated via two transmembrane receptors: AdipoR1 and AdipoR2, as well as the cell membrane–anchored T-cadherin receptor (Takeuchi et al., 2007). AdipoR1, the best-characterized adiponectin receptor, is ubiquitously expressed and is present at high levels in skeletal tissue and cardiac myocytes (Guerre-Millo, 2008). We noted that adipoR1 homozygous knockout mice (Bjursell et al., 2007) had no ear wound healing phenotype compared with wild-type C57BL/6J mice (Supplementary Figure S8 online). These data suggest that the AdipoR1 receptor itself does not regulate wound healing. Importantly, injection of adiponectin protein in wounded ears of adipoR1 knockout mice did not affect wound healing rates in these mice compared with control injections (Figure 5a).
Figure 5.
Effects of adiponectin on wound healing are mediated via AdipoR1. (a) Adiponectin injection does not alter ear wound resolution in adipoR1 knockout mice. (b) Adiponectin injection does not cause the upregulation of genes involved in keratinization in adipoR1 knockout mice (quantitative real-time reverse-transcriptase–PCR data). MSA, mouse serum albumin.
To test whether the effects of adiponectin on keratin gene expression were also dependent on signaling via the AdipoR1 receptor, we conducted a time-course study of adipoR1 knockout mice injected with adiponectin in ear tissue. Unlike in the case of C57BL/6J mice, adiponectin injection had no effect on keratin gene expression in adipoR1 knockout mice (Figure 5b). These data prove that the functions of exogenous adiponectin in regulating wound healing are acting via the AdipoR1 receptor. These studies also indicate that the effects of wound healing are mediated by exogenous adiponectin injection rather than contaminants in the adiponectin protein preparation.
The role of g-Ad in dorsal wound healing
Adiponectin is known to exist in various multimeric aggregates formed by homodimerization of the basic trimeric form, each with different biological properties (Simpson and Whitehead, 2010). Western blot analysis revealed that our recombinant adiponectin protein samples included trimeric, multimeric low-molecular-weight, and high-molecular-weight forms of the protein (Supplementary Figure S9 online). Adiponectin is a 247-amino-acid protein comprising a collagen N-terminal domain and a globular C-terminal domain. Enzymatic cleavage of the collagenous tails by leukocyte elastase enzymes releases the globular domain of globular-adiponectin (g-AD) species into circulation (Fruebis et al., 2001; Waki et al., 2005). In vivo administration of g-AD has been shown to increase free fatty acid oxidation and decrease body weight in mice, with greater potency than full-length adiponectin (f-AD) (Fruebis et al., 2001). All forms of adiponectin are thought to be biologically active in skeletal muscle, although only f-AD or high-molecular-weight complexes are thought to be active in the liver (Guerre-Millo, 2008).
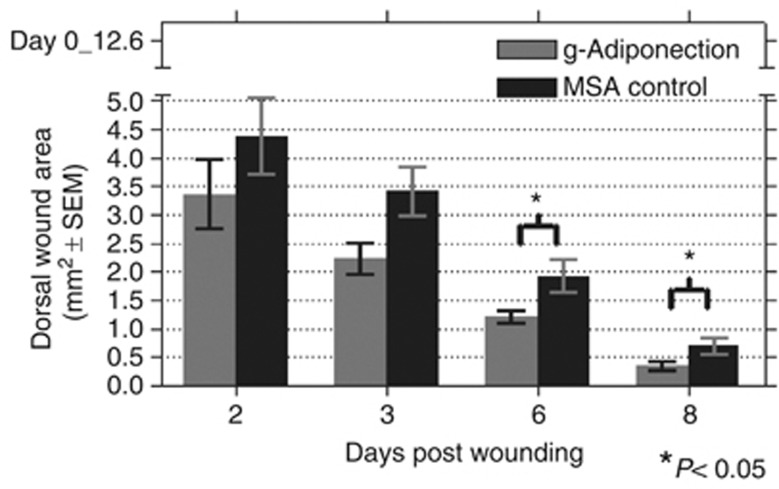
Given the potent biological activities of g-AD on in vivo metabolism, we tested the ability of g-AD to affect wound healing in dorsal wounds. Similar to the effects of full-length adiponectin, g-AD significantly increased the rate of dorsal wound healing in C57BL/6J mice (P=0.037, P=0.026 on days 6 and 8 post wounding, respectively) (Figure 6).
Figure 6.
Globular-adiponectin (g-Adiponectin) injection causes expedited dorsal wound closure in wild-type C57Bl/6J mice. MSA, mouse serum albumin.
Discussion
In this report, we describe a generalizable approach to the identification of modulators of wound healing. First, we identified mRNA expression changes after ear and dorsal skin wounding in two strains of mice with different wound healing phenotypes. We then generated recombinant proteins for a subset of these genes predicted to encode secreted or single-pass transmembrane proteins. Finally, we injected these candidate proteins directly around the healing wound for several days after injury. Gross observation of the wound diameter identified adiponectin as a regulator of wound healing. Further characterization of this process showed that adiponectin injection causes an immediate increase in mRNA of genes encoding keratin proteins, a thicker epithelial cell layer in the epidermis, and a late proliferative response of the basal layer of the epithelium and within the pilosebaceous structures. We propose that adiponectin injection into an ear wound promotes re-epithelialization of the cut edge, and this resolution likely halts or slows further hole closure. By contrast, adiponectin injection around a dorsal wound accelerates re-epithelialization across the basement membrane and consequent healing of the wound. Histological characterization of both the ear and dorsal wound models demonstrates the consistent effects of adiponectin in promoting re-epithelialization and increasing proliferation in the basal epidermal layer. Common cellular processes thus appear to underlie both healing phenotypes. A similar phenomenon has been described for inbred mouse strains, in that Balb/C mice have a slower ear hole closing phenotype than MRL/MpJ mice, yet exhibit faster dorsal wound closure in response to burn wounds (Davis et al., 2007).
Adiponectin injection caused wounds to heal with a more organized collagen deposition and promoted the formation of differentiated sebaceous gland structures, with skin architecture akin to normal skin (Lakshmi et al., 1989; Oxlund et al., 1996). At the molecular level, the effects of adiponectin appear to be mediated through the globular domain and are dependent on signaling through the AdipoR1 receptor. Recent studies have reported biologically active g-AD molecules with improved pharmacokinetic properties (Ge et al., 2010), and an adiponectin-mimetic peptide has also been developed (Otvos et al., 2011). These molecules offer great potential as wound healing therapeutics or for prophylaxis before surgical incision.
Adiponectin, an adipokine secreted from adipocytes, is a pleiotropic regulator of metabolism, exerting insulin-sensitizing effects and regulating glucose and lipid metabolism (Yamauchi et al., 2003, 2007; Holland et al., 2010). Leptin, also an adipokine with profound effects on metabolism, has similarly been shown to promote wound healing and has a proliferative effect on epithelial cells at the wound edge (Frank et al., 2000). In addition to the effects of adiponectin on dermal injury described in this report, a protective role for adiponectin has been demonstrated in multiple models of visceral tissue injury. For example, adiponectin knockout mice are prone to larger infarct sizes after myocardial ischemia–reperfusion injury, an effect rescued by the addition of exogenous adiponectin (Shibata et al., 2005). The effect of mechanical trauma in potentiating cardiac reperfusion injury is also mitigated by adiponectin treatment (Liu et al., 2011), and protective effects of adiponectin on cerebral and renal reperfusion injuries have also been noted (Nishimura et al., 2008) (Cheng et al., 2012). Finally, adiponectin knockout mice are more prone to intratracheal LPS-induced lung injury, an effect attributable to well-described anti-inflammatory properties of adiponectin (Konter et al., 2012), and adiponectin has also been reported to protect against liver fibrosis (Buechler et al., 2011). However, the role of adiponectin in wound healing described herein, and its role in other tissue injury models, is poorly understood at a mechanistic level. Our data suggest that adiponectin treatment causes rapid cellular differentiation, manifested by an abrupt increase in keratin gene expression immediately after adiponectin injection, and subsequent restoration of a thickened epithelial layer and pilosebaceous units. Proliferation of cells in the pilosebaceous units and basal epithelial layer, noted 2 weeks after adiponectin treatment, might then reflect restoration of the depleted stem cell niche. However, we did not notice any effects of adiponectin on the induction of keratin gene expression, cell proliferation, or migration in mesenchymal skin stem cells or the sebaceous gland cell line SZ95 in vitro (data not shown).
Indeed, further study of the role of adiponectin in wound healing might benefit in the understanding of the protective role of adiponectin in more complex settings of tissue injury, such as reperfusion injury, lung inflammation, and fibrosis. We queried publicly available microarray gene-expression data sets (Kupershmidt et al., 2010) and identified 14 epidermal disease states showing a reduction in adiponectin mRNA expression, whereas symptom-alleviating therapies were consistently associated with an upregulation of adiponectin (Supplementary Figure S10 online). Collectively, these data indicate that agonizing adiponectin signaling may be beneficial in a variety of epidermal disease settings. If warranted, the development of a therapeutic biologic would be enabled by our observation that monomeric g-Ad facilitates expedited wound healing, thus circumventing the difficulties inherent in developing an otherwise heterogeneous mix of multimers present in preparations of full-length adiponectin.
Materials and methods
Gene-expression profiling
For Affymetrix gene-expression profiling, total RNA was prepared from wound tissues using Trizol (Invitrogen, Carlsbad, CA), followed by RNeasy (Qiagen, Valencia, CA) cleanup. RNA was quantified, and the quality was subjectively examined on a BioRad Experion (Hercules, CA). A measure of 500 ng of total RNA was used to amplify cRNA using Affymetrix 3′ IVT Express kits. Microarray hybridization and scanning were performed using standard Affymetrix protocols. CEL files were processed using GC-RMA (Wu and Irizarry, 2005). Hierarchical clustering of gene-expression profiling was done using Spotfire DecisionSite 9.1.1 (Tibco, Somerville, MA). Clustering of normalized gene-expression values was conducted using the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) method with a Euclidean distance similarity measure. For qRT-PCR, 500 ng of total RNA was used for reverse transcription and complementary DNA synthesis (Qiagen) and to analyze mRNA levels using SYBR green amplification (Applied Biosystems, Carlsbad, CA) on the Applied Biosystems 7900HT RT-PCR machine. qRT-PCR data were analyzed using the comparative CT method.
Protein preparation for in vivo wound healing studies
Human Adiponectin (24–244), including human IgG signal sequence (1–16) and a C-terminal FLAG6His (DYKDDDDKHHHHHH) tag, was cloned in pRSmut vector and expressed in FreeStyle 293-F cells. Conditional medium containing secreted adiponectin was harvested 96 hours after DNA/PEI transfection by centrifugation at 2,000 g for 10 min. A volume of 600 ml of cultured medium was filtered through a 0.22-μm low protein–binding membrane filter and loaded onto an Ni-pentadentate (Etendx, San Diego, CA) column at a rate of 1 ml min−1. Adiponectin was eluted in 20 mℳ Tris (pH 7.4), 150 mℳ NaCl, and 250 mℳ Imidazole. The fractions containing adiponectin were pooled and buffer exchanged in 20 mℳ Tris (pH 7.4) and 150 mℳ NaCl. For additional details of adiponectin protein production, see Supplementary Figure S9 online and Supplementary Figure S11 online. Further information regarding PEPP protein purification platform can be found in Gonzalez et al., 2010.
In vivo studies
The Institutional Animal Care and Use Committee of The Genomics Institute of the Novartis Research Foundation approved all described animal procedures. Five- to six-week-old male mice were used for in vivo wound healing studies. Inbred mouse strains were purchased from The Jackson Laboratory (Bar Harbor, ME): C57BL/6J (000664), MRL/MpJ (000486), and SJL/J (000686). adipoR1 knockout mice (Deltagen, San Mateo, CA, T345) (Bjursell et al., 2007) were backcrossed mutant line to C57BL/6J mice for six generations to obtain mutant mice with a pure genetic background. For ear punch experiments, a 2-mm-diameter hole-punch, a through-and-through wound, was made in the center of the ear pinnae. Adiponectin or MSA control protein was diluted in sterile Tris-buffered saline to a concentration of 50 μg μl−1, and 20 μl of protein was injected in each ear of mice, for a total of 1 μg of protein per injection. Ear wound size was measured using a loupe with a reticle scale (Edmund Optics, Barrington, NJ). Width and height of each wound were measured, from which wound area was calculated. For dorsal punches, 2 × 4 mm diameter biopsy punches were made on either side of the shaved mid-dorsum region Injections. Adiponectin or MSA control protein was diluted in sterile Tris-buffered saline to a concentration of 80 μg μl−1, and 50 μl of protein was injected in each dorsal wound, for a total of 4 μg of protein per injection. Injections were administered to the ear and dorsal wounds for 4 consecutive days starting from the day of wounding. For dorsal wound measurement, a loupe with a reticle scale (Edmund Optics) was placed on the wound and photographed with a digital camera. Digital image sizes were calibrated using the reticle scale and wound area was measured with the Image J software (NIH, version 1.44p).
Histology protocols
Wound tissue was fixed in 10% neutral buffer formalin for at least 48 hours. Wounds were then bisected and processed into paraffin and embedded. Five-micron serial sections were mounted onto slides. Slides were stained with Masson's trichrome. For Ki67, staining was done using a Ventana Discovery XT platform using CC1 Standard Heat-Induced Epitope Retrieval (Ventana Medical Systems, Tucson, AZ, 950-124), followed by Avidin/Biotin blocking (Ventana Medical systems, endogenous biotin blocking kit, 760-050). Serum blocking with 1:20 normal goat serum (Jackson Immuno labs, 005-000-121) was followed by 1 hour of 1:200 diluted primary antibody incubation at 37 °C (Ki67 (Thermo-RM9106-s)). Secondary antibody was added and incubated for 32 minutes (Goat anti Rabbit, (Jackson immuno labs 711-065-152) at 1:500 dilution). Secondary antibody was labeled with the Ventana DABMap kit (Ventana Medical systems, 760-124). Slides were scanned on an Olympus Nanozoomer (Olympus, Tokyo, Japan). Histological measurements were recorded with the Olympus NDPI viewing software. F4/80 chromagen staining was carried out on skin tissue by IHC Tech (http://ihctech.net), using F4/80 primary antibody (Serotec, Raleigh, NC, MCA497R), followed by AP Polymer detection (Thermo Scientific, Waltham, MA, TL-125-AP), and stained with Warp RedTM (BioCare Medical, Concord, CA, WR806).
In vitro scratch migration assay
A density of 0.25 × 106 NHEK primary human keratinocyte cells (ATCC, Manassas, VA) was plated in 24-well tissue culture plates (Greiner, Monroe, NC) in complete medium and scratched after 12 hours, using a P1000 pipette tip (Molecular Bio-Products, San Diego, CA) to make two perpendicular scratches (to orient scratches and enable measuring of migration rates from the same location over the time course). Cells were then washed and kept in serum-free medium with relevant exogenous protein added. Scratches were photographed over a time course using a light microscope with an integrated digital camera (Nikon, Tokyo, Japan). Migration across the scratch wound was measured using Image J (version 1.44p, National Institutes of Health, Bethesda, MD).
Sebaceous gland cell line
The sebaceous gland cell line SZ95 (Zouboulis et al., 1999) was a generous gift from Dr Anton Stuetz, Novartis Institute of Biomedical Research, Austria.
Statistical testing
Statistical tests for treatment effects were conducted using R for Statistical Computing v2.5.1 (http://cran.r-project.org) and Microsoft Excel 2007. Functional enrichment studies were conducted using DAVID Bioinformatics Resources version 6.7 (http://david.abcc.ncifcrf.gov). We used probes that were regulated >3-fold in either mouse strain, in ear, or dorsal tissue during the wound healing time course, as background probe sets for functional enrichment of the hierarchical clusters shown in Supplementary Table S1 online and Supplementary Figure S1 online.
Acknowledgments
We are grateful to Evelyn Rodrigo for assistance in conducting in vivo wound healing assays. We also thank Anthony Munday and the Genomics Institute of the Novartis Research Foundation vivarium staff for managing mouse colonies, as well as Rita Moran, James Watson, and Teri Johnson for histology support.
Glossary
- g-AD
globular-adiponectin
- MSA
mouse serum albumin
- qRT-PCR
quantitative real-time reverse-transcriptase–PCR
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Beaven AW, Shea TC. Recombinant human keratinocyte growth factor palifermin reduces oral mucositis and improves patient outcomes after stem cell transplant. Drugs Today (Barc) 2007;43:461–473. doi: 10.1358/dot.2007.43.7.1119723. [DOI] [PubMed] [Google Scholar]
- Bjursell M, Ahnmark A, Bohlooly YM, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56:583–593. doi: 10.2337/db06-1432. [DOI] [PubMed] [Google Scholar]
- Blanpain C. Stem cells: skin regeneration and repair. Nature. 2010;464:686–687. doi: 10.1038/464686a. [DOI] [PubMed] [Google Scholar]
- Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17:2801–2811. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CF, Lian WS, Chen SH, et al. Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin-PPARalpha-heme oxygenase-1 signaling pathway. J Cell Physiol. 2012;227:239–249. doi: 10.1002/jcp.22726. [DOI] [PubMed] [Google Scholar]
- Davis TA, Amare M, Naik S, et al. Differential cutaneous wound healing in thermally injured MRL/MPJ mice. Wound Repair Regen. 2007;15:577–588. doi: 10.1111/j.1524-475X.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- Ferguson MW, Duncan J, Bond J, et al. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet. 2009;373:1264–1274. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kampfer H, et al. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest. 2000;106:501–509. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Xiong Y, Lemon B, et al. Generation of novel long-acting globular adiponectin molecules. J Mol Biol. 2010;399:113–119. doi: 10.1016/j.jmb.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Jennings LL, Knuth M, et al. Screening the mammalian extracellular proteome for regulators of embryonic human stem cell pluripotency. Proc Natl Acad Sci USA. 2010;107:3552–3557. doi: 10.1073/pnas.0914019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34:12–18. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2010;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SG, Katz AB, Lim FY, et al. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12:497–504. doi: 10.1111/j.1067-1927.2004.12501.x. [DOI] [PubMed] [Google Scholar]
- Konter JM, Parker JL, Baez E, et al. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol. 2012;188:854–863. doi: 10.4049/jimmunol.1100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupershmidt I, Su QJ, Grewal A, et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS One. 2010;5:pii: e13066. doi: 10.1371/journal.pone.0013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi R, Lakshmi AV, Bamji MS. Skin wound healing in riboflavin deficiency. Biochem Med Metab Biol. 1989;42:185–191. doi: 10.1016/0885-4505(89)90054-6. [DOI] [PubMed] [Google Scholar]
- Langbein L, Rogers MA, Praetzel-Wunder S, et al. K25 (K25irs1), K26 (K25irs2), K27 (K25irs3), and K28 (K25irs4) represent the type I inner root sheath keratins of the human hair follicle. J Invest Dermatol. 2006;126:2377–2386. doi: 10.1038/sj.jid.5700494. [DOI] [PubMed] [Google Scholar]
- Liu S, Yin T, Wei X, et al. Downregulation of adiponectin induced by tumor necrosis factor alpha is involved in the aggravation of posttraumatic myocardial ischemia/reperfusion injury. Crit Care Med. 2011;39:1935–1943. doi: 10.1097/CCM.0b013e31821b85db. [DOI] [PubMed] [Google Scholar]
- Mansbridge JN, Knapp AM. Changes in keratinocyte maturation during wound healing. J Invest Dermatol. 1987;89:253–263. doi: 10.1111/1523-1747.ep12471216. [DOI] [PubMed] [Google Scholar]
- Metcalfe AD, Willis H, Beare A, et al. Characterizing regeneration in the vertebrate ear. J Anat. 2006;209:439–446. doi: 10.1111/j.1469-7580.2006.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, et al. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L, Haspinger E, La Russa F, et al. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxlund H, Christensen H, Seyer-Hansen M, et al. Collagen deposition and mechanical strength of colon anastomoses and skin incisional wounds of rats. J Surg Res. 1996;66:25–30. doi: 10.1006/jsre.1996.0367. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F, Whitehead JP. Adiponectin—it's all about the modifications. Int J Biochem Cell Biol. 2010;42:785–788. doi: 10.1016/j.biocel.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Smiell JM, Wieman TJ, Steed DL, et al. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen. 1999;7:335–346. doi: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- Staiano-Coico L, Krueger JG, Rubin JS, et al. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993;178:865–878. doi: 10.1084/jem.178.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Ohtsuki Y, et al. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol. 2007;40:115–120. doi: 10.1007/s00795-007-0364-9. [DOI] [PubMed] [Google Scholar]
- Vermeij WP, Backendorf C. Skin cornification proteins provide global link between ROS detoxification and cell migration during wound healing. PLoS One. 2010;5:e11957. doi: 10.1371/journal.pone.0011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Yamauchi T, Kamon J, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790–796. doi: 10.1210/en.2004-1096. [DOI] [PubMed] [Google Scholar]
- Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003;163:327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA. Stochastic models inspired by hybridization theory for short oligonucleotide arrays. J Comput Biol. 2005;12:882–893. doi: 10.1089/cmb.2005.12.882. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Seltmann H, Neitzel H, et al. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113:1011–1020. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.