Abstract
Plastic changes in the nucleus accumbens (NAcc), a structure occupying a key position in the neural circuitry related to motivation, are among the critical cellular processes responsible for drug addiction. During the last decade, it has been shown that memory formation and related neuronal plasticity may rely not only on protein synthesis but also on protein degradation by the ubiquitin proteasome system (UPS). In this study, we assess the role of protein degradation in the NAcc in opiate-related behaviors. For this purpose, we coupled behavioral experiments to intra-accumbens injections of lactacystin, an inhibitor of the UPS. We show that protein degradation in the NAcc is mandatory for a full range of animal models of opiate addiction including morphine locomotor sensitization, morphine conditioned place preference, intra-ventral tegmental area morphine self-administration and intra-venous heroin self-administration but not for discrimination learning rewarded by highly palatable food. This study provides the first evidence of a specific role of protein degradation by the UPS in addiction.
Keywords: nucleus accumbens, proteasome, protein degradation, opiate, behavior, reinforcement
INTRODUCTION
Addiction is defined as a compulsive use of a substance despite negative consequences. The high reinforcing properties of drug of abuse lead to pathological usurpation of neural processes that normally serve reward-related learning (Conrad et al, 2008). In recent years, it was shown that the transition to addiction depends on plastic changes in the reward system (Conrad et al, 2008; Kasanetz et al, 2010; McClung and Nestler, 2008). These neuro-adaptations, such as synaptic plasticity and changes in neuronal morphology, share many similarities with mechanisms involved in long-term memory (Hyman et al, 2006; Kauer and Malenka, 2007; Kelley, 2004; Robbins et al, 2008).
In rodents, contextual memory involves a reorganization of hippocampal neuronal networks that requires protein synthesis (Alberini, 2005). Although it has attracted less attention than gene transcription and protein synthesis, protein degradation by the ubiquitin proteasome system (UPS) is now believed to have a crucial role in synaptic plasticity and memory (Fioravante and Byrne, 2011). The UPS function is based on the enzymatic linkage of a chain of ubiquitins (poly-ubiquitination), which directs the protein to degradation in a large proteolytic complex called the proteasome (Hegde, 2010). UPS-dependent protein degradation in the hippocampus of rodents is necessary for synaptic plasticity (Dong et al, 2008; Fonseca et al, 2006; Karpova et al, 2006) and long-term memory consolidation and reconsolidation in spatial and contextual learning (Artinian et al, 2008; Kaang and Choi, 2011; Lee et al, 2008; Lopez-Salon et al, 2001).
UPS activity is also involved in cellular adaptations induced by long-term morphine treatments. We reported that cellular sensitization produced by morphine involves the degradation of G proteins by the UPS in neuroblastoma cells (Mouledous et al, 2005). The UPS has also been implicated in morphine-induced downregulation of the glutamate transporter EAAC1 (Yang et al, 2008a, 2008b). An important question is, therefore, whether the UPS is necessary for the reinforcing properties of drugs of abuse.
The nucleus accumbens (NAcc) is an important point of convergence in the reward circuitry (Robbins and Everitt, 1996) and has a major role in the reinforcing properties of drugs of abuse (Cardinal and Everitt, 2004). Pathological plasticity in the NAcc is thought to participate in the transition from recreational to compulsive drug use (Belin et al, 2009). The purpose of the present study was to evaluate the necessity of UPS in the NAcc for the development of opiate-induced behaviors in rodents. First, we investigated whether injection of morphine in vivo could induce poly-ubiquitination in the NAcc. Then, our strategy was to determine the effects of protein degradation blockade by using lactacystin, a proteasome inhibitor, on a wide range of behavioral paradigms commonly used to test morphine reinforcement: morphine conditioned place preference (morphine CPP), morphine-induced behavioral sensitization, and two operant paradigms, intra-ventral tegmental area (VTA) morphine self-administration in the Y-maze and free access intra-venous heroin self-administration. This strategy allowed us to provide compelling evidence for a role of protein degradation in the NAcc in opiate-related behaviors.
MATERIALS AND METHODS
Subjects
Three-month-old C57BL/6J male mice obtained from Charles River (l'Arbresle, France) were used in all experiments except for intra-venous self-administration of heroin, which was conducted on 10- to 12-week-old male Sprague-Dawley rats obtained from Janvier (Le Genest Saint Isle, France). They were subjected to a 12-h light–dark cycle, with lights on at 0800 h with food and water ad libitum. Animal surgery and experimentation are authorized by the French Direction of Veterinary Service to BF, J-MZ, LM, MS, PR, and VD and were approved by the French Animal Care and Use Committee (MP 02/02/02/06). Experiments were performed in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (NRC, 2003) and the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Surgery
NAcc guide cannulae implantations were performed as described in Marchand et al (2006). Briefly, mice and rats were anesthetized with a mixture of Ketamine and Xylazine (respectively 100 and 15 mg/kg for mice and 60 and 10 mg/kg for rats) and placed in a stereotaxic apparatus. For mice, the guide cannulae (9 mm long, 0.3 mm in diameter) were lowered vertically at the following coordinates measured from bregma: A, +1.6, L, ±1, and V, −3 mm from skull. VTA guide cannulae implantation was performed during the intra-NAcc implantation procedure and was positioned 1.5 mm above the VTA under the following coordinates: 0.40 mm anterior to the interaural line, 0.30 mm lateral to the sagittal line, and 3.30 mm vertically below the surface of the skull as described in Baudonnat et al (2011). Rats were implanted with catheters in the right jugular vein (Chauvet et al, 2009) and bilateral guide cannulae (24GA; Plastic One, Roanoke, VA) aimed at the NAcc shell following coordinates measured from bregma: A, +1.8; L, ±3.2; V, −6.6 mm from the skull, angle: 16°.
Drugs
Saline solution refers to 0.9% NaCl dissolved in sterile water. Lactacystin (Sigma) was dissolved in 2% DMSO, MG132 (ZLeu-Leu-Leu-al, Sigma) was dissolved in saline solution, both at a final concentration of 200 μM. Morphine hydrochloride (10 mg/kg, i.p.) was obtained from Francopia (Gentilly, France). Heroin hydrochloride was obtained from Research Triangle Institute (Research Triangle Park, North Carolina) and dissolved in sterile water.
Intracerebral Infusion
At least 1 week after surgery, the injector tip was inserted into the guide cannula and protruded 1 mm for mice and 2 mm for rats. Proteasome inhibitor solutions were delivered in a volume of 0.5 μl per side at a speed of 0.125 μl/min (animals were single housed and freely moving during the injection).
Test of Proteasome Activity
Mice (n=8) received an injection of lactacystin in one side of the NAcc and 2% DMSO in the other. NAcc was dissected out under binocular microscope from slices of 1 mm thickness obtained as described (Artinian et al, 2008) and homogenized in Tris 100 mM, pH 8. Protein concentrations were equilibrated and proteasome activity was measured as described in Artinian et al (2008).
Place Conditioning
Apparatus and pre-conditioning session were described in Marchand et al (2006). During conditioning, drug treatment (intra-peritoneal saline or morphine) and side presentation (black or white) were randomly assigned. Morphine and saline were given alternatively in the morning and in the afternoon during 3 days, half of the mice receiving morphine in the morning and the other half receiving morphine in the afternoon. One hour before receiving i.p. morphine, animals were pre-injected with either intra-NAcc proteasome inhibitors (lacta or MG132) or its vehicle (DMSO or saline, respectively). One hour before i.p. saline, mice received intra-Nacc vehicle. Ten minutes after the i.p. injection of morphine or saline, mice were confined to the associated compartment during 20 min. Twenty-four hours after the last conditioning session, post-conditioning test was performed in the same conditions as in the pre-conditioning test without habituation session (both test sessions lasted 20 min).
Behavioral Sensitization
Locomotor activity was measured in an apparatus composed of six Plexiglas boxes (25 × 21.5 × 9.5 cm) equipped with infrared beams to measure mice horizontal activity (Apelex, Evry, France). Mice were first exposed 10 min to the apparatus for habituation. Then, animals were injected i.p. with morphine solution (10 mg/kg) 10 min before being placed again in the box. On the following 4 days, the same procedure was repeated and bilateral injection in the NAcc with lactacystin or 2% DMSO took place 1 h before i.p. treatment. On days 6 and 7, mice were left in their home cages without any treatment. On the eighth day, animals were challenged with an injection of morphine (10 mg/kg) and were placed in the box 10 min later. Two control groups with no injection in the NAcc were added: the first group (Saline Control) received saline injections during the induction protocol (days 1–5) and on day 8. The second one (Saline/Mor Control) received saline injections during the induction protocol and morphine on day 8. Locomotion was measured during 1 h. Comparison between morphine-induced locomotion on days 1 and day 8 was used to evidence behavioral sensitization.
Egocentric Y-maze Procedure
Egocentric Y-maze procedure rewarded by intra-VTA morphine injections was described in Baudonnat et al (2011). Mice were placed in a fixed starting box of the Y-Maze apparatus. By interrupting the photocell beam in one of the two target arms, mice could obtain a 5-mm2 crisp (Vico) placed in a 10-mm diameter plastic cup or trigger an intra-VTA microinjection of morphine sulfate (50 ng) dissolved in artificial cerebrospinal fluid (aCSF 50 nl). The other arm was neutral. Mice were maintained for 30 s in the chosen arm. Intracranial injections were performed using an automatic computer-controlled apparatus and lasted 8 s. One hour before each daily session mice received intra-NAcc lactacystin or DMSO injections.
Heroin Self-Administration Procedure under Fixed-Ratio 1 Schedule in Rats
Behavioral testing was performed in Imetronic experimental chambers equipped with nose-pokes as operanda and controlled by Imetronic interfaces and software (Imetronic, Pessac, France). Fifteen 3 h self-administration sessions were conducted using a Fixed-Ratio 1 (FR1) schedule of reinforcement after 10 days of recovery from surgery and were preceded by injections of lactacystin or 2% DMSO 1 h before each session. A single response in the active nose-poke hole immediately delivered an i.v. injection of heroin (or saline for control animals) and caused the house light to pulse for 5 s followed by a 5-s timeout. Duration of the injection varied between 1, 2, or 4 s to determine the dose of heroin injected (12.5, 25, and 50 μg, respectively). Responses in the inactive nose-poke hole were recorded but had no programmed consequences.
Histology
At the end of behavioral experiments, mice were killed by cervical dislocation and their brains removed for the histological verification of cannula placement as described in Artinian et al (2008). Rats were euthanized with an overdose of chloral hydrate. Brains were removed and placed in 4% formaldehyde for a day and stored in 18% sucrose PBS for 1–5 days until sectioning. Coronal sections (30 μm) were cut with a cryostat, mounted on gelatin-treated slides and examined with a light microscope. Animals with incorrect cannula placements were excluded from the analysis.
Western Blot Analysis
Mice were treated with morphine or saline and confined 20 min in a compartment of the CPP apparatus. Directly or 40 min after the end of the confinement, animals were euthanized and NAcc were dissected as described for proteasome activity test. All samples were flash frozen in liquid nitrogen and later homogenized in Tris 50 mM, EDTA 5 mM, Sucrose 320 mM, and a protease inhibitor cocktail (Roche, Neuilly-Sur-Seine, France). Bilateral NAcc structures from four mice were pooled in a single sample and crude synaptosomal fractions were prepared as described by Dunah and Standaert (2001). Western blot procedure was performed as described in Mouledous et al (2008) using ubiquitin monoclonal antibody (1/1000) (P4D1, Santa Cruz Biotechnology, Santa Cruz) and actin antibody (1/5000) (Sigma). Ubiquitination levels above 55 kDa were quantified and normalized with actin using Quantity One Software (Bio-Rad, UK).
Immunohistochemistry of Activated Caspase 3
Mice were injected intra-NAcc twice daily with lactacystin or 2% DMSO as described for place preference conditioning. One hour after the last injection of proteasome inhibitor, animals were deeply anesthetized with an overdose of sodium pentobarbital. Perfusion and immunohistochemistry were performed as described in Mouledous et al (2010) using anti-activated caspase-3 rabbit IgG (Ozyme, Saint-Quentin, France) at a concentration of 1/400 overnight and biotinylated secondary antibody (1/250; AB Cam, Paris, France). For each mouse, the lesion area inside the NAcc (lesion+immunopositive areas) was calculated from three slices adjacent to the injection site and expressed as a percentage of the total area of the NAcc.
Statistics
All results are presented as group means±SEM. Differences between groups were assessed using appropriate t-test or ANOVA followed, when necessary, by a Bonferonni post hoc test. Tests were performed using GraphPad Prism or Statview software.
RESULTS
Morphine Exposure Increases Protein Poly-Ubiquitination in NAcc Synaptosomal Fractions
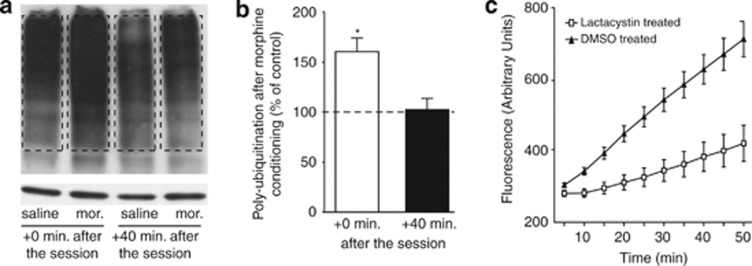
We first investigated whether the UPS was activated in the NAcc of mice treated with morphine. Mice were injected with morphine (10 mg/kg, i.p.) or saline and placed, 10 min after injection, into a compartment of the place preference apparatus for 20 min. Immediately after this session, or 40 min after replacing the mice in their home cages, NAcc was dissected. Considering growing evidence of a crucial role of proteasome activity in this cellular compartment, NAcc was processed to obtain crude synaptosomal fractions. The rate of poly-ubiquitination, reflecting the amount of proteins addressed to the proteasome for degradation, was measured by western blotting (Figure 1). Protein poly-ubiquitination in the synaptosomal fractions of the NAcc in morphine-treated mice was increased immediately after the session (160.6±13.54% of control; p<0.021) and returned to control level after 40 min (102.6±11.07% of control; p>0.832), suggesting that ubiquitin-tagged proteins were degraded shortly after the session (Figure 1). Morphine exposure in a novel environment thus appears to induce a transient protein ubiquitination.
Figure 1.
A single morphine conditioning increases synaptosomal poly-ubiquitination rate in the NAcc. (a, b) Mice were injected i.p. with 10 mg/kg morphine or saline and placed in the CPP apparatus, as in a conditioning session. Protein poly-ubiquitination was evaluated in NAcc synaptosomes isolated immediately or 40 min after the session. (a) Representative western blot. Upper panel: ubiquitin bands over 55 kDa (dotted lines) were compared; lower panel: actin. (b) Data quantification. Morphine-treated mice showed an increase in synaptic proteins poly-ubiquitination (n=4) immediately, but not 40 min after the session (n=4). Data are expressed as the mean±SEM of percent change in poly-ubiquitination compared with saline-treated mice. (c) Mice were injected in the NAcc with lactacystin (100 pmol) in one side and 2% DMSO in the other side. One hour later, NAcc proteasome activity was measured by the rate of degradation of the fluorogenic substrate Leu-Leu-Val-Tyr-7-amino-4 methylcoumarin. A comparison between slopes of the linear part of the two curves reveals a significant inhibition of proteasome catalytic activity in lactacystin-injected NAcc (n=6, Mann Whitney test, p<0.041) of 59.28±17.01%. Data are expressed in counts of fluorescence (arbitrary unit) over time. *p<0.05.
Injections of Lactacystin in Mice NAcc Are Effective Without Noticeable Toxicity
To test the role of UPS in the NAcc, we used local injections of the proteasome inhibitor lactacystin (Kisselev and Goldberg, 2001). To verify the efficacy of this treatment, we quantified the inhibition of UPS 1 h after intra-NAcc injections using a fluorogenic proteasome substrate. Injection of 100 pmol lactacystin reduced proteasome activity in ex vivo NAcc homogenates by 59.3±17.01% (p<0.035; Figure 1c). In addition, to test for toxicity induced by intra-Nacc injections, we measured activated caspase-3 activity in eight mice. Caspase 3 immunoreactivity was restricted to the vicinity of the injection site. Lactacystin and DMSO produced similar damage in the NAcc (lesion+caspase immunoreactivity=14.7±8.3 and 7.4±5.0% of total NAcc area, respectively, for DMSO and lactacystin; p>0.48; Supplementary Figure S1). This dose of lactacystin was thus considered as both effective and non-toxic, and was used to study the role of the UPS in behavioral procedures.
Development of Morphine CPP Is Prevented by UPS Inhibition in the NAcc
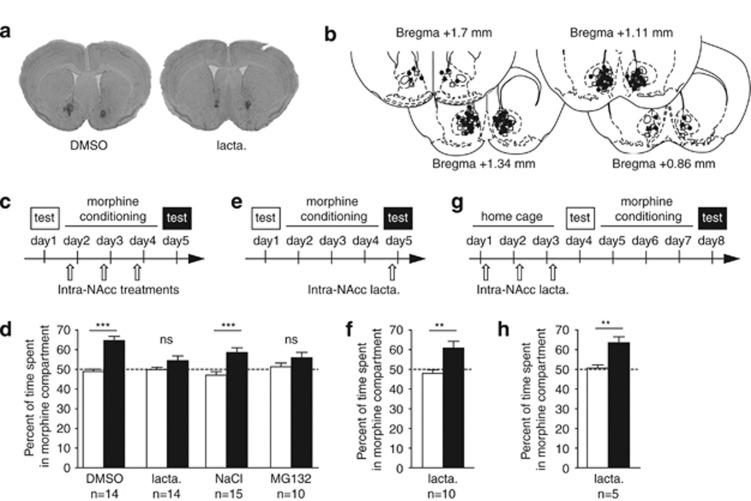
The rewarding effects of morphine were measured by morphine CPP (Tzschentke, 1998). During the pre-conditioning test, mice displayed no natural preference for a particular compartment. They were then conditioned for 3 consecutive days in a counterbalanced manner. Each day, mice were submitted to a morphine (10 mg/kg, i.p.) and a saline conditioning session preceded by intra-NAcc infusion of lactacystin or DMSO. To rule out any non-specific effect of lactacystin, we also used MG132 (or NaCl for control group), an UPS inhibitor with a different mechanism of inhibition and a shorter duration of action (Kisselev et al, 2001). One day after conditioning, mice were allowed to explore the entire apparatus and time spent in the morphine-paired compartment was recorded. A two-way ANOVA revealed an effect of conditioning (F1,51=36.9, p<0.0001) and an interaction between conditioning and intra-NAcc treatments (F3,51=4.22, p<0.01). Post hoc analysis confirmed that mice treated intra-NAcc with DMSO or NaCl spent more time in the drug-associated compartment after conditioning than before (Figure 2d, DMSO: p<0.001; NaCl: p<0.001). In sharp contrast, mice treated with lactacystin or MG132 did not show any preference for the morphine-associated compartment (Figure 2d, lactacystin: p>0.05; MG132: p>0.05). DMSO and lactacystin injections alone did not produce any appetitive or aversive effect (pre-conditioning: DMSO-saline (n=7), 49.98±1.19% and lactacystin-saline (n=9), 48.35±1.77% post-conditioning: DMSO-saline, 54.00±4.27% (p>0.05) and lactacystin-saline, 47.62±3.73% (p>0.05)).
Figure 2.
Development, but not expression, of morphine CPP is prevented by intra-NAcc injections of proteasome inhibitors. (a) Representative microphotographic view of injection sites in animals treated with DMSO (left) and lactacystin (right). (b) Schematic localization of injection sites of DMSO (squares), lactacystin (circles), and MG132 (stars) in coronal sections ranging from +1.7 to +0.86 mm from Bregma. (c, e, g) Schematic representations of procedures. (d, f, h) Pre-conditioning (empty bars) and post-conditioning (filled bars) test results. (c) Mice were injected with vehicle or proteasome inhibitors 1 h before each conditioning session. (d) Lactacystin- and MG132-injected mice (n=14 and n=10, respectively) did not develop any morphine place preference, whereas control animals (DMSO: n=14 and NaCl: n=15) showed a preference for the drug-paired side. (e) Lactacystin was injected 1 h before the post-conditioning test. (f) Proteasome inhibition did not affect the expression of morphine place preference on test day (n=10). (g) Mice were injected during 3 days with lactacystin before a classical conditioning procedure. (h) Repeated intra-NAcc lactacystin injections did not alter the acquisition of a preference for the drug compartment (n=5). All results are expressed as percentage of time spent in the drug-associated compartment±SEM during pre-conditioning tests (empty bars) and post-conditioning tests (filled bars). NS, p>0.05; **p<0.01; ***p<0.001.
Because of its long lasting effects, lactacystin could have interfered with the expression of morphine CPP 24 h after the last intra-Nacc injection. We treated mice with a single intra-NAcc injection of lactacystin 1 h before the post-conditioning test (Figure 2f). Lactacystin-injected mice exhibited a preference for the drug-associated compartment (p<0.01) demonstrating that protein degradation by the UPS is not necessary for CPP expression. Finally, we were also able to exclude a possible irreversible toxic effect of lactacystin by showing that intra-NAcc lactacystin injections performed once daily during 3 days before submitting mice to a conditioning process did not disrupt drug-induced place preference (p<0.01; Figure 2h).
Taken together, these results demonstrate the involvement of protein degradation via the UPS in the NAcc in the establishment of morphine CPP but not in its expression.
Behavioral Sensitization Depends on UPS Activity in the NAcc
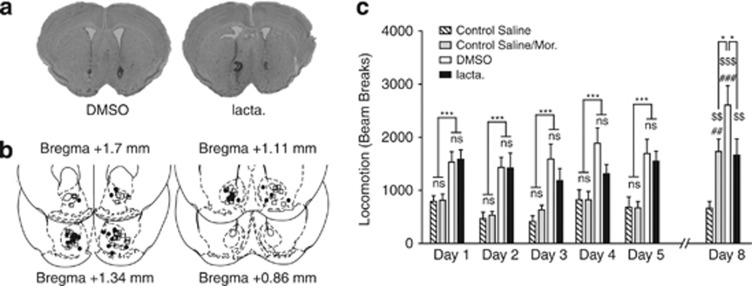
In mice, morphine induces an increase in locomotion, and repeated injections followed by a withdrawal period enhance this response (Contet et al, 2008). This effect, called behavioral sensitization, depends on neuro-adaptations in the NAcc triggered by the exposure to the drug and developing during cessation of drug administration (Vanderschuren and Kalivas, 2000). To test the effects of intra-NAcc injections of lactacystin on locomotion induced by morphine exposure and on context-dependent behavioral sensitization paradigm, we used a sensitization protocol that consists of two phases: an induction phase, consisting of five daily injection of morphine (10 mg/kg, i.p.), and an expression test, consisting of the injection of the same dose of morphine after 3 days of withdrawal (Figure 3). During the induction protocol, morphine-treated mice exhibited a higher locomotion than saline-treated ones. A two-way ANOVA for repeated measures (days 1–5) shows an effect of i.p. treatment (F1,148=35.43, p<0.001), a main effect of time (F4,148=3.35, p=0.0117) and no interaction effect (F4,148=0.36, p>0.83). Post hoc test did not show any difference between any pair of days (p>0.05). Thus, mice did not exhibit sensitization during the induction phase. There was no difference between the two groups that received daily morphine injection (DMSO and lacta). A two-way ANOVA for repeated values reported no effect of intra-NAcc treatment (F1,68=0.69, p=0.4), no effect of the test day (F4,68=0.70, p>0.59), and no time × intra-NAcc interaction (F4,68=1.10, p>0.37). Thus, intra-NAcc injection of lactacystin did not impair the acute locomotor effect of morphine.
Figure 3.
Behavioral sensitization depends on UPS functionality. Control Saline mice (n=11) received daily i.p. saline injections during the induction protocol (days 1–5) and the sensitization test at day 8. Control Saline/Mor mice (n=9) received daily i.p. saline injections during the induction protocol and one i.p. injection of morphine on day 8. Mice included in the lacta. and DMSO groups, respectively, received bilateral injections of lactacystin (n=8) or DMSO (n=11) in the NAcc 1 h before morphine exposure during the induction protocol and a morphine challenge on day8. (a) Representative microphotographic view of injection sites in animals treated with DMSO (left) and lactacystin (right). (b) Schematic localization of injection sites of DMSO (squares) and lactacystin (circles) on coronal sections ranging from +1.7 to +0.86 mm from Bregma. (c) Morphine injection induces hyperlocomotion as compared with i.p. saline injection (two-way ANOVA: p<0.0001 for treatment independently of the day). Lactacystin had no significant effect during the induction protocol (p>0.40). DMSO-treated mice exhibited sensitization to morphine on day 8, whereas lactacystin-treated ones did not. Furthermore, lactacystin-treated animals show hyperlocomotion similar to the one observed after a first morphine exposure in the Control Saline/Mor group on day 8. Data are expressed as number of beam breaks±SEM measured during 1 h sessions following morphine injections (10 mg/kg). NS, p>0.05, *p<0.05 and ***p<0.001; ##p<0.01 and ###p<0.001 compared with day 1; $$p<0.01 and $$$p<0.001 compared with the Saline Control group.
Comparing locomotion observed on day 1 and day 8 allows evaluating sensitization. A two-way ANOVA for repeated measures reveals an effect of treatment (F3,35=10.60, p<0.0001), an effect of day (F1,35=14.40, p<0.0006), and a treatment × day interaction (F3,35=5.82, p<0.0025). Post hoc tests show a clear difference between day 1 and day 8 for the DMSO group (p<0.001) and for the Saline/Mor control group injected with morphine for the first time on day 8 (p<0.01), but not for the two other groups (p>0.44 for the saline control and p>0.77 for the lacta. group). At day 8, the locomotion of the lacta. group was significantly lower than the locomotion of the DMSO group (p<0.05) but not different from the locomotion of mice that received their first morphine injection on day 8 (p>0.05). Thus, protein degradation by the UPS in the NAcc is necessary for the development of behavioral sensitization.
Lactacystin Infusions into the NAcc Disrupt Intra-VTA Morphine Self-Administration
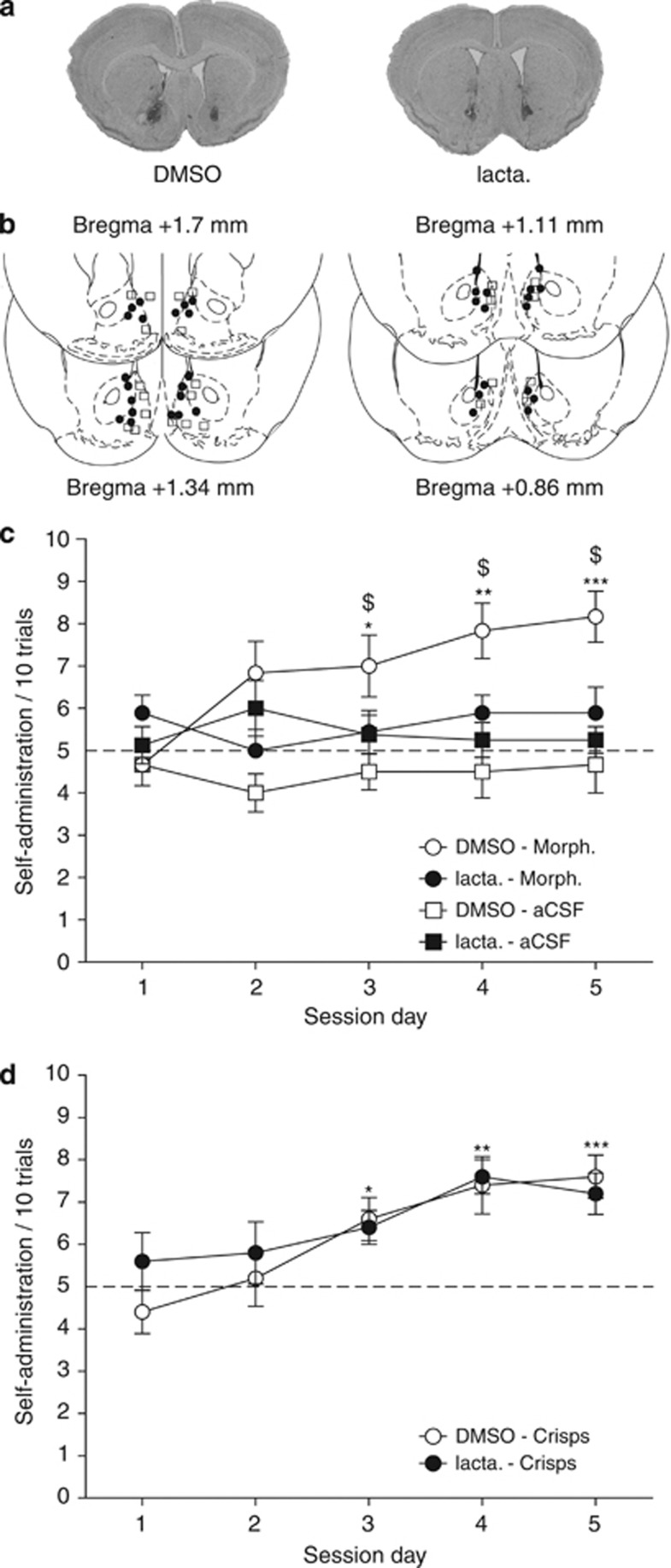
To test whether the UPS is involved in the dopamine-dependent reinforcing effects of morphine, we used intra-VTA morphine self-administration (David et al, 2002). Mice had to choose between two arms of the Y-maze, one only being rewarded. In a first group of mice, the reward consisted in intra-VTA morphine injection. In the control group, intra-VTA injection of morphine was replaced by aCSF. In a third group, the arm was rewarded by a small piece of crisps. For all groups, half of mice were infused with lactacystin and the other half was injected with DMSO into the NAcc 1 h before each session. For each of the five daily sessions, performance was measured by the number of rewarded-arm choices over 10 trials. A three-way ANOVA with one repeated factor (session) revealed a main effect of reward (F2,33=11.053, p<0.002), of session (F4,132=11.278, p<0.0001), a reward × session interaction (F8,132=2.859, p<0.006), a treatment × session interaction (F4,132=2.89, p<0.024), and a reward × treatment × session interaction (F8,132=2.034, p<0.047). Post hoc analyses showed that DMSO-treated animals receiving intra-VTA morphine injections increased their number of self-administrations (p<0.05 in day 3, p<0.01 in day 4, and p<0.001 in day 5 as compared with the control aCSF group) (Figure 4c). Lactacystin-treated mice did not develop such a preference for the morphine-reinforced arm (p>0.61). On the third, fourth, and fifth day, their number of self-injections was significantly different from DMSO-treated animals (p<0.05, Figure 4c). Interestingly, lactacystin treatment did not impair the task acquisition when highly palatable food (crisps) was used as reward (p>0.57, Figure 4d). Together, these results demonstrate the involvement of proteasome-dependent protein degradation in the NAcc in operant responding reinforced by intra-VTA morphine injections but not by highly palatable food.
Figure 4.
Protein degradation by the UPS in the NAcc is involved in intra-VTA morphine self-administration. Mice received intra-NAcc injections of lactacystin or DMSO before each session of intra-VTA morphine or highly palatable food (crisps) self-administration in the Y-maze. (a) Representative microphotographic view of injection sites in animals treated with DMSO (left) and lactacystin (right). (b) Schematic localization of injection sites of DMSO (squares) and lactacystin (circles) in coronal sections ranging from +1.7 to +0.86 mm from Bregma. (c) Mice were exposed to intra-VTA morphine or aCSF self-administration. DMSO-treated animals (open circles, n=6) increased gradually their number of choice of the rewarded arm, whereas lactacystin animals did not (full circles, n=9). No preference was observed for the control group receiving aCSF into the VTA (squares, DMSO: n=6 and lactacystin: n=8). (d) Mice receiving either lactacystin (n=6) or DMSO (n=6) infusions into the NAcc developed a preference for the highly palatable food-rewarded arm. Data are presented as mean number of entries in the rewarded arm over 10 successive trails±SEM for each daily session.*p<0.05, **p<0.01, ***p<0.001 for morphine vs aCSF or crisps vs chance (5 over 10 trials). $p<0.05 for lactacystin vs vehicle (DMSO).
Inhibition of Protein Degradation by the UPS in the NAcc Shell Impairs Heroin Self-Administration in Rats
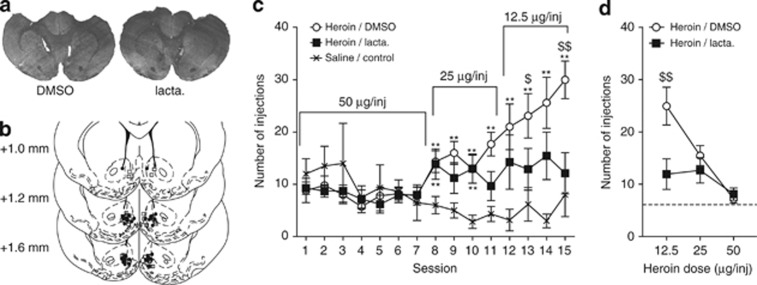
Finally, we studied the impact of proteasome inhibition on opiate reinforcement in a self-administration procedure in which rats could freely obtain intra-venous heroin infusion during 3-h daily sessions. Rats were injected bilaterally in the NAcc shell with lactacystin 1 h before each self-administration session. Animals were allowed to self-administer three decreasing doses of heroin (50 μg/inj, 25 μg/inj, and 12.5 μg/inj) to establish a dose–response curve, whereas control rats could self-administer only saline (Figure 5). At the dose of 50 μg/inj, rates of self-administration did not differ from saline. When the dose was decreased to 25 μg/inj, both lactacystin and DMSO rats self-administered significantly more than saline control rats. One-way ANOVA revealed that intra-NAcc treatment induced modifications in self-administration response rate during the session (F2,24=3.71, p<0.05). When the dose was finally lowered to 12.5 μg/inj, DMSO-treated rats further increased the number of injections whereas lactacystin-treated rats did not. One-way ANOVA revealed that intra-NAcc treatment modified drug intake (F2,23=8.08, p<0.01). Actually, the self-administration rate of lactacystin-treated rats did not differ from saline rats (Figure 5c). A dose–response curve was obtained by grouping data by heroin dose (Figure 5d). A two-way ANOVA for repeated values revealed an effect of dose (F2,18=53.73, p<0.0001), treatment (F1,18=9.486, p<0.0001), and an interaction between these two factors (F2,18=12.49, p<0.0001). A Bonferroni post hoc test showed that, at the heroin dose of 12.5 μg/inj, lactacystin-treated animals self-administered significant less opiate than controls (p<0.01; Figure 5d). Therefore, administration of lactacystin produces a vertical downshift (Zernig et al, 2004) in the heroin dose–response curve, which is consistent with a role of protein degradation in the reinforcing effects of heroin.
Figure 5.
Protein degradation in the NAcc shell is necessary for the development of an adaptive addictive behavior to a decrease in reward delivery in self-administration paradigm. Rats were allowed to self-administer intra-venous heroin according to an FR1 schedule 1 h after intra-NAcc injections of lactacystin (heroin/lacta. group, n=9) or vehicle (heroin/DMSO group, n=10). Saline/control rats (n=6) were allowed to self-administer saline under the same schedule. (a) Representative microphotographic view of injection sites in animals treated with DMSO (left) and lactacystin (right). (b) Schematic localization of injection sites of DMSO (squares) and lactacystin (circles) in coronal sections ranging from +1.6 to +1.0 mm from Bregma. (c) Number of injections during daily sessions indicates that heroin/DMSO rats developed adaptations to decreasing dose of heroin, whereas heroin/lacta.-treated animals did not. (d) Number of injections per session as a function of the dose of heroin. The dotted line indicates the average number of injection in the saline/control group. The heroin/DMSO group exhibits a strong increase in number of injections for the lower dose compared with the heroin/lacta. group. Data are presented as mean number of injections±SEM. **p<0.01 different from saline control group, $p<0.05 and $$p<0.01 compared with lactacystin-treated group.
DISCUSSION
This study used several complementary models to evidence the role of protein degradation by the UPS in the reinforcing effects of opiates in rodents. We demonstrated that inhibition of protein degradation by UPS in the NAcc blocks the establishment of all behaviors tested in this study, involving either passive (place preference, behavioral sensitization) or active (self-administration) exposure to the drug.
Our lactacystin treatments did not appear to be toxic or to have an overall detrimental effect on mice behavior. Indeed, our data show that lactacystin injections do not affect choice latencies in the egocentric task of the Y-maze experiment and do not block CPP expression (i.e., when injected only before the test, Figure 2f) nor CPP acquisition when injected during 3 days before the beginning of the conditioning (Figure 2h). It also spares acute motor hyperactivity induced by morphine. Altogether, this suggests that the effects of lactacystin are unlikely to rely on a blockade of the acute, unconditioned, effects of morphine exposure.
An important aspect in the tests that we used to evaluate morphine-related behaviors is their context dependency. In place preference and in the Y-maze experiments, mice have to associate drug exposure with a location using environmental cues. The behavioral sensitization paradigm we used is also known to depend on the context (Badiani and Robinson, 2004). It has been suggested that drugs of abuse lead to abnormally strong association between the context and the drug by potentiating glutamate-mediated inputs that provide contextual information to the NAcc (Girault et al, 2007; McClung et al, 2008). Here we show that, although UPS blockade does not impair acute effects of morphine, it blocks sensitization and place preference, which require long lasting plastic changes. Thus, our data strongly suggest a requirement for UPS-dependent protein degradation in drug-induced plasticity in the NAcc, which is necessary for the development of the conditioned effects of the drug.
Several lines of evidence support this conclusion. First, protein degradation has been shown to participate in synaptic plasticity in the hippocampus (Citri et al, 2009; Dong et al, 2008; Karpova et al, 2006; Sun and Wolf, 2009) and the processing of episodic-like memory requires, in that structure, the degradation of proteins associated with synaptic plasticity such as Shank, GKAP, and IκB (Lee et al, 2008; Lopez-Salon et al, 2001). Second, UPS has previously been associated with synaptic plasticity in cultured NAcc neurons (Sun and Wolf, 2009). Furthermore, recent studies showed that behavioral sensitization (Pascoli et al, 2011) and drug craving rely on the potentiation of glutamate inputs to the NAcc (Conrad et al, 2008; Mameli et al, 2009) and that vulnerability to addiction is due to an impairment of NAcc long-term depression of these inputs (Kasanetz et al, 2010). In the light of these results and on the basis of our observations, we propose that exposure to drugs of abuse triggers, in the NAcc, the degradation of a specific set of proteins that is necessary for synaptic plasticity underlying the development of addiction. Several known direct or indirect targets of the UPS, such as synaptic anchoring proteins Shank and GKAP (Lee et al, 2008) or transcription factors CREB and deltaFosB (Carle et al, 2007; Dong et al, 2008), could be regulated by this process. Future work will help identifying the NAcc UPS targets whose degradation participates in the development of morphine-related behaviors described in this study.
Finally, one of the interesting results of the present study is the differential effect of lactacystin on food and morphine reinforcement. Using the same experimental conditions, we have recently reported that food-reinforced mice use a spatial learning strategy involving the hippocampus, whereas drug-reinforced subjects will use instead an associative, stimulus-response process depending on the striatum to learn the same task (Baudonnat et al, 2011). Therefore, although interfering with UPS activity within the NAcc is likely to decrease both food and drug reinforcing properties, this would disrupt learning more efficiently in drug-reinforced animals than in food-reinforced mice which rely on a more distributed circuit to learn the task.
In conclusion, this study constitutes the first demonstration of a behavioral role of protein degradation in the NAcc. This knowledge is crucial for the understanding of the molecular adaptations required for drug addiction. However, targeting proteasome activity in the brain by peripheral drug injections would probably be accompanied by too many detrimental effects (Chen et al, 2011). E3 ubiquitin ligases that allow the fixation of the ubiquitin chain on specific proteins and present a very large diversity in humans (Ande et al, 2009) could represent more selective pharmacological targets. The identification of these E3 ubiquitin ligases as well as their target proteins is thus warranted to fully exploit the potency of the UPS for the control of addiction-related behaviors.
Acknowledgments
We thank Julien Familiades, Fanny Botreau, Ingrid Waldschmidt, and Claudia Chauvet for help in behavioral procedures (place preference, behavioral sensitization, and intra-venous self-administration); Claire Rampon and Magaly Alonso for help with the immunohistochemistry; Helene Halley for technical assistance and animal care; Jean-Pierre Changeux, Peter Redgrave, Nasser Haddjeri, Manuel Mameli, and Fred Ambroggi for their useful comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes. Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Ande SR, Chen J, Maddika S. The ubiquitin pathway: an emerging drug target in cancer therapy. Eur J Pharmacol. 2009;625:199–205. doi: 10.1016/j.ejphar.2009.08.042. [DOI] [PubMed] [Google Scholar]
- Artinian J, McGauran AM, De Jaeger X, Mouledous L, Frances B, Roullet P. Protein degradation, as with protein synthesis, is required during not only long-term spatial memory consolidation but also reconsolidation. Eur J Neurosci. 2008;27:3009–3019. doi: 10.1111/j.1460-9568.2008.06262.x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Baudonnat M, Guillou JL, Husson M, Vandesquille M, Corio M, Decorte L, et al. Disrupting effect of drug-induced reward on spatial but not cue-guided learning: implication of the striatal protein kinase A/cAMP response element-binding protein pathway. J Neurosci. 2011;31:16517–16528. doi: 10.1523/JNEUROSCI.1787-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, et al. Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur J Neurosci. 2007;25:3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Frezza M, Schmitt S, Kanwar J, Ping DQ. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11:239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Soler-Llavina G, Bhattacharyya S, Malenka RC. N-methyl-D-aspartate receptor- and metabotropic glutamate receptor-dependent long-term depression are differentially regulated by the ubiquitin-proteasome system. Eur J Neurosci. 2009;30:1443–1450. doi: 10.1111/j.1460-9568.2009.06950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Filliol D, Matifas A, Kieffer BL. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology. 2008;54:475–486. doi: 10.1016/j.neuropharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology. 2002;160:307–317. doi: 10.1007/s00213-001-0981-2. [DOI] [PubMed] [Google Scholar]
- Dong C, Upadhya SC, Ding L, Smith TK, Hegde AN. Proteasome inhibition enhances the induction and impairs the maintenance of late-phase long-term potentiation. Learn Mem. 2008;15:335–347. doi: 10.1101/lm.984508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Byrne JH. Protein degradation and memory formation. Brain Res Bull. 2011;85:14–20. doi: 10.1016/j.brainresbull.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nagerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity. Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Hegde AN. The ubiquitin-proteasome pathway and synaptic plasticity. Learn Mem. 2010;17:314–327. doi: 10.1101/lm.1504010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kaang BK, Choi JH. Protein degradation during reconsolidation as a mechanism for memory reorganization. Front Behav Neurosci. 2011;5:2. doi: 10.3389/fnbeh.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Thomas U, Knopfel T, Behnisch T. Involvement of protein synthesis and degradation in long-term potentiation of Schaffer collateral CA1 synapses. J Neurosci. 2006;26:4949–4955. doi: 10.1523/JNEUROSCI.4573-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction; shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza T, Izquierdo I, et al. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14:1820–1826. doi: 10.1046/j.0953-816x.2001.01806.x. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Marchand S, Betourne A, Marty V, Daumas S, Halley H, Lassalle JM, et al. A neuropeptide FF agonist blocks the acquisition of conditioned place preference to morphine in C57Bl/6J mice. Peptides. 2006;27:964–972. doi: 10.1016/j.peptides.2005.07.023. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- Mouledous L, Neasta J, Uttenweiler-Joseph S, Stella A, Matondo M, Corbani M, et al. Long-term morphine treatment enhances proteasome-dependent degradation of G beta in human neuroblastoma SH-SY5Y cells: correlation with onset of adenylate cyclase sensitization. Mol Pharmacol. 2005;68:467–476. doi: 10.1124/mol.105.013391. [DOI] [PubMed] [Google Scholar]
- Mouledous L, Frances B, Zajac JM. Modulation of basal and morphine-induced neuronal activity by a NPFF(2) selective agonist measured by c-Fos mapping of the mouse brain. Synapse. 2010;64:672–681. doi: 10.1002/syn.20774. [DOI] [PubMed] [Google Scholar]
- Moulédous L, Merker S, Neasta J, Roux B, Zajac JM, Mollereau C. Neuropeptide FF-sensitive confinement of mu opioid receptor does not involve lipid rafts in SH-SY5Y cells. Biochem Biophys Res Commun. 2008;373:80–84. doi: 10.1016/j.bbrc.2008.05.174. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann NY Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Sun X, Wolf ME. Nucleus accumbens neurons exhibit synaptic scaling that is occluded by repeated dopamine pre-exposure. Eur J Neurosci. 2009;30:539–550. doi: 10.1111/j.1460-9568.2009.06852.x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang S, Lim G, Sung B, Zeng Q, Mao J. Inhibition of the ubiquitin-proteasome activity prevents glutamate transporter degradation and morphine tolerance. Pain. 2008a;140:472–478. doi: 10.1016/j.pain.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang S, Sung B, Lim G, Mao J. Morphine induces ubiquitin-proteasome activity and glutamate transporter degradation. J Biol Chem. 2008b;283:21703–21713. doi: 10.1074/jbc.M800809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A.2004Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate "sensitization" to "drug wanting" Psychopharmacology (Berl) 171349–351.author reply 352–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.