Abstract
Drugs of abuse have acute and persistent effects on synapse structure and addiction-related behaviors. Trans-synaptic interactions can control synapse development, and synaptic cell adhesion molecule (SynCAM) proteins (also named nectin-like molecules) are immunoglobulin adhesion proteins that span the synaptic cleft and induce excitatory synapses. Our studies now reveal that the loss of SynCAM 1 in knockout (KO) mice reduces excitatory synapse number in nucleus accumbens (NAc). SynCAM 1 additionally contributes to the structural remodeling of NAc synapses in response to the psychostimulant cocaine. Specifically, we find that cocaine administration increases the density of stubby spines on medium spiny neurons in NAc, and that maintaining this increase requires SynCAM 1. Furthermore, mushroom-type spines on these neurons are structurally more plastic when SynCAM 1 is absent, and challenging drug-withdrawn mice with cocaine shortens these spines in SynCAM 1 KO mice. These effects are correlated with changes on the behavioral level, where SynCAM 1 contributes to the psychostimulant effects of cocaine as measured after acute and repeated administration, and in drug-withdrawn mice. Together, our results provide evidence that the loss of a synapse-organizing adhesion molecule can modulate cocaine effects on spine structures in NAc and increases vulnerability to the behavioral actions of cocaine. SynCAM-dependent pathways may therefore represent novel points of therapeutic intervention after exposure to drugs of abuse.
Keywords: synapse, cocaine, SynCAM, CADM, nectin-like molecule, MSN
INTRODUCTION
Addiction involves persistent changes in neuronal connectivity (Russo et al, 2010). Addictive drugs acutely enhance dopaminergic transmission in striatum (Berridge and Robinson, 1998; Sulzer, 2011) and exert long-lasting effects on glutamatergic transmission in the cortico−striatal system (Kalivas and O'Brien, 2008; Wolf, 1998). Excitatory inputs to the nucleus accumbens (NAc) and in the ventral−tegmental area are critical for development and expression of drug-seeking behaviors (Kalivas and Volkow, 2005; Kelley, 2004).
Addictive drugs not only alter synaptic plasticity (Kalivas and Hu, 2006; Kauer and Malenka, 2007; Ungless et al, 2001; Wolf and Ferrario, 2010) but also change synapse number (Robinson and Kolb, 2004). Long-term administration of psychostimulants increases the density of dendritic spines, the postsynaptic specializations of excitatory synapses (Jedynak et al, 2007; Lee et al, 2006; Robinson and Kolb, 1997). Select trans-synaptic molecules instruct synapse formation, including synaptic cell adhesion molecule (SynCAM) immunoglobulin proteins that act across the nascent synaptic cleft to induce excitatory synapses (Biederer et al, 2002; Fogel et al, 2007; Stagi et al, 2010). Synapse development is also guided by other trans-synaptic molecules, notably neuroligins and their neurexin ligands (Giagtzoglou et al, 2009; Missler et al, 2012). SynCAMs are distinct among trans-synaptic organizers because they not only contribute to promoting excitatory synapse formation in the brain but also are sufficient to drive this process in vivo, and subsequently maintain elevated synapse numbers (Robbins et al, 2010). SynCAM adhesion also impacts synapse ultrastructure, and presynaptic active zones and postsynaptic densities are shorter in synapses lacking SynCAM 1. This is not a simple consequence of reduced trans-synaptic adhesion as synapses lacking α−neurexins have a normal morphology (Dudanova et al, 2007). Moreover, SynCAM 1 modulates both synapse density and spatial learning in the adult hippocampus (Robbins et al, 2010).
We hypothesized that trans-synaptic interactions may contribute to the structural synaptic changes that occur in response to drugs of abuse. Although SynCAM-encoding genes have not yet been linked to addiction-related behaviors, genome-wide association studies support roles of adhesion proteins (Uhl et al, 2008). This is consistent with the association of neurexin polymorphisms with substance abuse (Hishimoto et al, 2007; Stoltenberg et al, 2011). However, functional roles of synapse-inducing adhesion molecules in drug abuse have not yet been reported.
We now address this question through studies of SynCAM 1, choosing it because of its synaptogenic role in the hippocampus and its ubiquitous expression in the adult brain, including striatum (Thomas et al, 2008). Our results show that synapse density is reduced in NAc of SynCAM 1 knockout (KO) mice. SynCAM 1 also contributes to a previously unreported, persistent increase in NAc stubby spine density following cocaine withdrawal. In addition, mice lacking SynCAM 1 are sensitized to an acute shortening of mushroom-type spines after cocaine challenge. In concert, addiction-related behavior is altered in SynCAM 1 KO mice after acute cocaine administration, repeated exposure, and withdrawal. Our results provide the first functional evidence that synapse-inducing adhesion proteins modulate synaptic and behavioral changes after psychostimulant exposure.
MATERIALS AND METHODS
Animals
The SynCAM 1 KO mouse line was generously provided by Dr T Momoi (National Institute for Neuroscience, Tokyo; Fujita et al, 2006), and mice were backcrossed for at least 11 generations onto the C57BL/6J background. Male SynCAM 1 KO mice were compared with male wild-type (WT) littermates. Animals were housed 2–4 subjects per cage, and were maintained on a 12 h light/dark cycle with free access to food and water. Cocaine was purchased from the National Institute on Drug Abuse (Baltimore, MD) and was administered by intraperitoneal injections. All experiments were approved by the Yale Animal Care and Use Committee.
Biochemistry
Mice were sacrificed by decapitation following isofluorane administration. Brains were removed rapidly, the hippocampus or NAc were punched out, frozen in liquid nitrogen, and homogenized by sonication in 8 ℳ urea. Protein concentrations were determined using BCA reagent (Pierce). Immunoblotting was performed using anti-SynCAM 1 antibody (MBL International, 3E1; 1:1000) and anti-actin antibody (MP Biomedical, clone C4 69100; 1:2000).
Imaging of Dendritic Spines
The lipophilic dye DiI (Molecular Probes, catalog no. D-282) was coated onto Tungsten particles (Bio-Rad, catalog no. 165-2267) and placed into Tefzel tubing according to the manufacturer's instructions. Mouse brains were fixed by transcardial perfusion with 4% paraformaldehyde (Electron Microscopy Sciences) 40–45 min after cocaine or saline injection. Brains remained in fixative for 1 h, were placed into PBS, and sectioned using a vibratome into 150-μm-thick sections containing the NAc (Bregma 1.70–1.18). From each brain, three slices were used for particle-based biolistic delivery of DiI using a Gene Gun system (Bio-Rad) with helium at 200 psi. Sections were mounted and imaged within 16–24 h of dye delivery to allow for dye diffusion within targeted neurons. Medium spiny neurons (MSN) in NAc were imaged without differentiating between core and shell owing to the random labeling obtained with this approach that did not yield enough dye-filled neurons for separate analysis. Imaging was performed using a Perkin Elmer UltraVIEW VoX spinning disc confocal microscope with a × 60 CFI Plan Apo objective and equipped with a Hamamatsu C9100-50 camera, with excitation at 543 nm. Z-stacks were collapsed into projection images and 2–5 neurons/brain were manually analyzed per section. Psychostimulants preferentially increase spine densities in distal dendrites (Li et al, 2003), and we therefore restricted our analysis to dendritic segments that were located 40 μm away from the cell body, choosing segments of 30–60 μm length. Mushroom, stubby, and thin spine types were distinguished using morphometric criteria (Knott et al, 2006) as originally defined in EM studies of the rat cortex (Peters and Kaiserman-Abramof, 1970). Briefly, spines with head bulb diameters much greater than their neck diameters and having thick stalks were classified as mushroom-shaped, spines that are short and thick and have similar head and neck diameters were scored as stubby, and spines with a slender stalk that expands into a small, oval or rounded end-bulb were classified as thin. For each spine type, density, head diameter, overall length, and neck length were determined.
Behavioral Experiments
Conditioned place preference (CPP) was performed as described (Narasimhaiah et al, 2009). Briefly, mice were acclimatized to the procedure room for 30 min prior to testing. CPP boxes (Med Associates, St Albans, VT) consisted of two conditioning chambers with retractable doors separated by a neutral chamber that differed in floor grid patterns. On the day prior to the first conditioning session, mice were allowed to explore the apparatus freely for 15 min to assess baseline preference. On each of the next three conditioning days, all subjects received a saline injection between 09:00 and 11:30 h and were placed in one of the two chambers for 15 min. Mice were counterbalanced for drug-paired chamber based on their baseline preference. In the afternoon between 02:00 and 04:30 h, mice were placed in the opposite chamber and were injected with cocaine (5 mg/kg) or saline. On the following mid-day between 11:30 and 02:00 h, mice were placed in the center chamber and were allowed to explore the apparatus freely for 15 min. Time spent in each chamber was detected by infrared beam breaks and recorded and quantified using Med-PC software. Mice that showed more than 80% preference for one of the three chambers during the baseline test underwent the conditioning protocol, but data obtained from them were excluded from analysis.
The same mice that were tested for CPP were later subjected to the locomotor sensitization paradigm and received either saline or cocaine injections, as they had in the place preference paradigm. Supplementary Table S1 provides a chart showing the prior injection/drug exposure for each group in the sensitization study. Locomotor sensitization was tested in a 31 cm × 35 cm cage equipped with infrared beam break detectors to record subject movement. Prior to each daily tests, animals were acclimatized in the procedure room for 30–45 min while on a cart. Mice were first habituated to the novel chamber for 3 days, receiving daily saline injections, and locomotor activity was then recorded for 30 min. On the following 9–10 days, mice received either saline or cocaine (15 mg/kg) each day and were then placed in the locomotor chamber. The difference of 9 vs 10 days was due to weather-related setbacks in the experimental procedure (hurricane Irene disrupted the last 2 days of testing for the second cohort of animals). Thus, data are shown only for the first 8 days of the test period as the analysis on the ninth day was performed by a different experimenter, leading to a deviant locomotor response on day 9 in the second cohort. During the subsequent withdrawal period of 15–17 days, animals remained in their home cages. A subset of these mice was used in a challenge experiment, where animals received on the concluding challenge day either saline or cocaine (15 mg/kg), and locomotor activity was recorded for 30 min.
Data Analysis
All quantitated analyses were performed with the researchers blind to the condition. Statistical analyses of spine measurements were performed using Student's t-test and errors correspond to the standard error of mean. CPP data were analyzed using a three-way repeated measures ANOVA (genotype × treatment × chamber). Locomotor activity across sessions was also analyzed using a three-way repeated measures ANOVA (genotype × treatment × day). To assess locomotor sensitization following withdrawal from repeated cocaine and number of stubby spines, two-way ANOVAs (treatment × challenge) were performed for each genotype. Post hoc analyses were performed using paired and unpaired t-test with Bonferroni's correction for multiple comparisons. Animals that did not survive throughout the entire test were removed from the statistical analysis.
RESULTS
Reduced Dendritic Spine Density in NAc of SynCAM 1 KO Mice
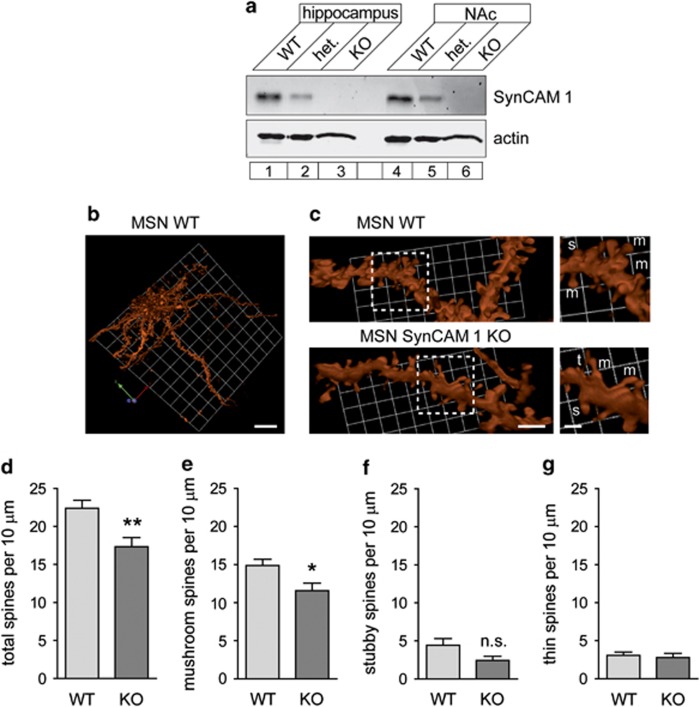
We determined that SynCAM 1 protein is expressed in NAc as high as in hippocampus, indicating potential synapse-organizing functions of this protein in this brain region (Figure 1a). To measure dendritic spines on MSN in NAc, we used particle-based biolistic delivery of the lipophilic dye DiI to dye-fill MSN in brain sections (Lee et al, 2006; Shen et al, 2009) (Figure 1b and c). Quantitation showed that MSN of male SynCAM 1 KO mice have fewer total spines than their WT littermate controls at baseline (Figure 1d; WT, 22.4±1.1 per 10 μm; KO, 17.4±1.2; P=0.008). This decrease is primarily due to a reduced mushroom-type spine density (Figure 1e; WT, 14.9±0.8 per 10 μm; KO, 11.6±1.0; P=0.014), while a trend towards fewer stubby spines in the KO did not reach significance (Figure 1f; WT, 4.4±0.9 per 10 μm; KO, 2.4±0.6; P>0.05). The comparatively rare thin spines were unaffected by SynCAM 1 loss in MSN (Figure 1g; WT, 3.1±0.4 per 10 μm; KO, 2.8±0.5; P>0.5). The lower density of mushroom-type spines in NAc parallels the reduction in excitatory synapse density in hippocampus upon loss of SynCAM 1 (Robbins et al, 2010). These data identify SynCAM 1 as the first trans-synaptic organizer controlling excitatory synapse number in NAc.
Figure 1.
SynCAM 1 is expressed in NAc and the absence of SynCAM 1 decreases synapse density. (a) SynCAM 1 expression was tested by immunoblotting of equal protein amounts of the indicated tissues from WT, heterozygotic (het.), and SynCAM 1 KO mice, which served as a negative control for the antibody. Actin was used as a loading control. (b) Three-dimensional reconstruction of a MSN in WT NAc. The neuron was visualized after biolistic transfer of the dye DiI. No gross morphological differences were observed between MSN from WT and SynCAM 1 KO mice. Scale bar, 20 μm. (c) Left, dendritic MSN segments in NAc from WT (top) and SynCAM 1 KO (bottom) male mice. Dendrites were visualized as in (b). Scale bar, 5 μm. Right, enlarged view of boxed areas. Labels mark m, mushroom; s, stubby; t, thin spines. Scale bar, 2 μm. (d–g) Lower density of total spines (d) and mushroom-type spines (e) in MSN of NAc from SynCAM 1 KO. The trend towards lower stubby spine density in the KO was not significant (f). Thin spines were unaffected (g). Data were obtained from male littermate mice imaged as in (c), with the experimenter blind to condition. (n=23 neurons from six WT mice, 10 neurons from three KO mice) *P<0.05; n.s., not significant.
MSN Spines Lacking SynCAM 1 Shorten Following Cocaine Exposure
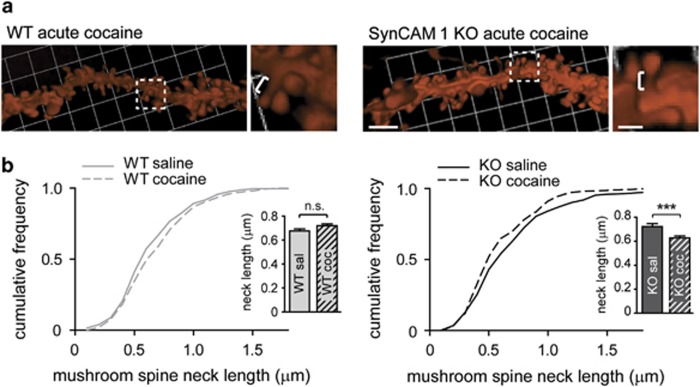
The loss of SynCAM 1 not only reduces excitatory synapse number but also shortens the postsynaptic densities of excitatory synapses in hippocampus (Robbins et al, 2010). We therefore tested whether exposure to cocaine could result in morphological synaptic changes that are SynCAM-dependent. WT and SynCAM 1 KO littermate males were habituated to handling by the experimenter twice a day for 7–10 days, and saline injections were given over 3 days to habituate them to the stress of handling, and then received a single injection of 15 mg cocaine/kg or saline as control. Mice were sacrificed 40–45 min after injection, and spines in NAc were visualized after biolistically labeling MSN with DiI (Figure 2a). This time point was chosen as transient structural changes of MSN spines in response to cocaine exposure are most pronounced within this time window (Shen et al, 2009). We measured that a single cocaine administration reduced the length of mushroom spine necks in SynCAM 1 KO but not WT MSN at 40–45 min post-injection (Figure 2b) (WT saline, 0.68±0.02 μm; WT cocaine 0.72±0.02) (KO saline, 0.72±0.02 μm; KO cocaine 0.63±0.02; P<0.0001). Total spine length was significantly shortened in KO mice as well (data not shown). A single cocaine administration also modestly reduced mushroom spine head diameter, but the extent of this effect was comparable for WT (−6.6±2.6% P<0.05) and SynCAM 1 KO mice (−8.6±2.6% P<0.05). The lack of SynCAM 1 therefore results in a shortening of MSN mushroom spines after a single cocaine exposure. This indicates that the loss of SynCAM 1 may be permissive for cocaine-induced structural remodeling, in addition to causing general effects such as a reduction in synapse number.
Figure 2.
Cocaine exposure shortens MSN spines in a SynCAM 1-dependent manner. (a) Left, dendritic MSN segments visualized after biolistic DiI transfer in NAc of WT (top) and SynCAM 1 KO (bottom) mice that had received one cocaine injection (15 mg/kg). Scale bar, 5 μm. Right, enlarged view of boxed areas. Brackets mark mushroom spine necks. Scale bar, 1 μm. (b) The neck length of MSN mushroom spines remains unchanged in WT mice (left) after a single injection of cocaine (15 mg/kg), but is reduced in SynCAM 1 KO mice (right). Results are represented as cumulative frequency distribution graphs. Insets show mean±SEM. Data were obtained from littermates with the experimenter blind to condition. (n=12 neurons from five WT saline control mice, 19 neurons from six WT cocaine-exposed mice, 11 neurons from three KO saline control mice, 12 neurons from four cocaine-exposed KO mice) ***P<0.001; NS, not significant.
Loss of SynCAM 1 Results in a Potentiated Psychostimulant Response to Cocaine
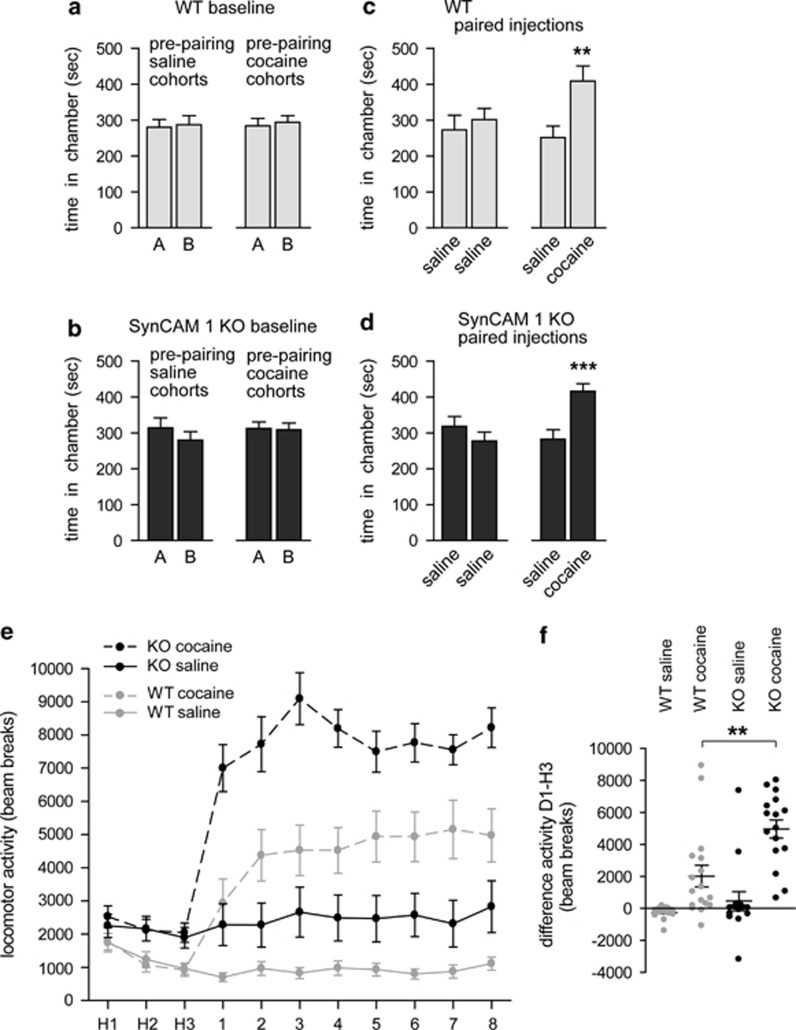
Psychostimulants change excitatory synapses in NAc as well as addiction-related behaviors. As SynCAM 1 organizes excitatory synapse number and morphology in NAc, we tested whether male SynCAM 1 KO mice exhibit altered behavioral responses to this psychostimulant compared with WT littermates. We first performed a CPP test of SynCAM 1 roles in the rewarding effect of cocaine. WT and KO exhibited no differences in time spent in either of the two chambers of the place preference apparatus at baseline (Figure 3a and b). Over three conditioning days, separate groups of mice received daily injections of saline in both chambers (control), or saline in one chamber and cocaine (5 mg/kg) in the other chamber. Following conditioning sessions, a significant interaction of treatment and paired chamber was found (three-way repeated measures ANOVA, F ratio (1,58)=6.91, P<0.01). Further analyses indicated that both WT (t-test F ratio t(15 mice)=2.26, P<0.05) and SynCAM 1 KO (t(12)=3.01, P<0.01) littermates treated with cocaine significantly preferred the cocaine-paired chamber over the saline-paired chamber, with no significant effect of genotype (Figure 3c and d).
Figure 3.
Normal cocaine place preference but increased locomotor response to cocaine in SynCAM 1 KO mice. (a, b) WT (a) and littermate SynCAM 1 KO mice (b) did not show a preference for either of two test chambers A or B prior to conditioning. (c, d) CPP was tested after three daily conditioning sessions with saline or cocaine (5 mg/kg). Mice did not show a preference for either chamber A or B following administration of saline in both chambers (left two columns), but both WT (c) and SynCAM 1 KO (d) littermate mice preferred the cocaine-paired chamber following conditioning (right two columns). (WT, n=6 mice each saline or cocaine, saline vs cocaine P=0.006; KO, n=7 mice each, P=0.0005) (e) Locomotor response to cocaine in WT and SynCAM 1 KO mice. WT (gray circles) or SynCAM 1 KO (black circles) littermate mice were habituated for 3 days to the test environment (H1−H3) and then received daily injections of saline (continuous lines) or cocaine (15 mg/kg; dashed lines). Data were obtained from littermates with the experimenter blind to condition. (WT, n=19 males saline, 17 cocaine; KO, n=15 males saline, 16 cocaine). (f) Locomotor response to an acute injection of cocaine is enhanced in KO mice compared with WT (data represent a difference score of day 1 cocaine administration minus last habituation day).
We next tested WT and KO cohorts for acute and chronic responses to cocaine in a locomotor sensitization test (Supplementary Table S1). Mice were first habituated for 3 days to the test chamber (Figure 3e). Analysis using a three-way repeated measures ANOVA indicated significant interactions of treatment and day (F (10,630)=6.91, P<0.01) and genotype and day (F (10,630)=33.18, P<0.01). WT mice exhibited a significant decrease in locomotor activity from the first to last day of habituation (saline t(18)=5.46, P<0.001; cocaine t(16)=2.54, P<0.05). In contrast, SynCAM 1 KO mice did not show progressively lowered locomotion, which may reflect an impaired ability to habituate. Alternatively, the enhanced locomotor activity we observed here in KO mice under habituation conditions could be a SynCAM 1-dependent response such as to the stress caused by injections. Note that SynCAM 1 KO mice exhibited no difference in baseline locomotor activity compared with WT controls in other behavioral tests (Robbins et al, 2010; Perez de Arce and Biederer, data not shown).
Following habituation, all mice received daily injections of 15 mg cocaine/kg or saline (Figure 3e). The response to the first cocaine injection was different between groups as indicated by a significant genotype × treatment interaction (Figure 3f; F(1,63)=4.70, P<0.05). Post hoc analysis indicated that the acute response to cocaine was significantly higher in SynCAM 1 KO mice compared with WT mice, even following subtraction of baseline locomotor activity (Figure 3f, second vs fourth column; t(31)=3.32, P<0.01). The increase in the acute response to cocaine may suggest that SynCAM 1 KO animals have already reached a peak of sensitization. This could be due to the mice reaching a ceiling in overall locomotor activity, but this does not seem likely as mice will show greater locomotion at night (Giros et al, 1996), higher than the level of activity measured in the KO mice after cocaine administration. In addition, even low doses of amphetamine will result in greater locomotor activity in C57BL/6 mice than what is reported here (Schmidt et al, 2010). Over the 8 days of the cocaine sensitization regimen, WT mice exhibited the expected increased locomotor response as indicated by a significant increase in activity between the first and eighth drug administration (t(16)=2.37, P<0.05). SynCAM 1 KO mice, however, exhibited only a non-significant trend for an increase between the first and eighth day of administration (t(15)=1.90, P=0.08). Even though the elevated locomotion in mice lacking SynCAM 1 under the conditions of this experiment needs to be considered, this result therefore indicates that KO mice show maximal response already after the first cocaine administration.
Cocaine Sensitization after Repeated Administration is Modulated by SynCAM 1
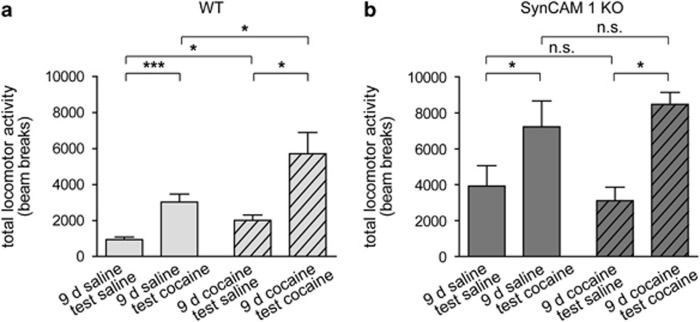
We next addressed sensitization in cocaine-withdrawn mice. As expected, WT mice that received daily injections of cocaine (15 mg/kg) over 9 days and were then withdrawn from the drug for 15–17 days showed a significantly elevated response to a saline injection compared with 9-day saline controls, consistent with contextual sensitization to the environment in which cocaine was received (Figure 4a and b, first vs third column; t(17)=3.67, P<0.01). WT mice that received nine daily injections of cocaine (15 mg/kg) and received cocaine instead of saline after the withdrawal period responded to this cocaine challenge with a similar locomotor response as observed on the last day of sensitization. This response was higher than to the saline challenge, as both contextual and pharmacological sensitization to cocaine occur under these conditions (Figure 4a, third vs fourth column; t(14)=3.01, P<0.05). Reflecting the expected difference between acute and repeat cocaine administration, the latter increased locomotor activity in WT mice to a higher extent (Figure 4a, second vs fourth column; t(20)=2.11, P<0.05).
Figure 4.
SynCAM 1 contributes to cocaine sensitization during withdrawal. Mice received daily injections of saline or cocaine (15 mg/kg) over 9–10 days, followed by 15–17 days without injections. On the test day, mice received a saline or cocaine (15 mg/kg) challenge and locomotor activity was measured. (a) WT mice injected for 9–10 days with saline showed elevated locomotor activity in response to the cocaine challenge compared with a saline challenge. In cocaine-withdrawn WT mice, a cocaine challenge further increased locomotor activity as compared with a saline challenge (n=14, 11, 5, 11 mice for cohorts left to right). *P<0.05; ***P<0.001; (b) SynCAM 1 KO mice injected for 9 days with saline showed elevated locomotor activity in response to the cocaine injection compared with a saline injection. Challenging cocaine-withdrawn SynCAM 1 KO mice with cocaine did not further increase locomotor activity as compared with mice that received only a single cocaine injection. Locomotor activities were similar to those observed following a single cocaine injection in the sensitization paradigm (see Figure 3e). Data were obtained from littermates with the experimenter blind to condition. (n=10, 9, 9, 10 mice for cohorts shown left to right) *P<0.05; NS, not significant.
Consistent with the increased response of SynCAM 1 KO mice to acute cocaine administration following 3 days of habituation, we found that 15–17 days after a 9-day period of repeated daily saline administration SynCAM 1 KO mice exhibited strongly increased locomotor activity in response to an acute cocaine injection (Figure 4b, first vs second column; t(17)=2.95, P<0.05). Although significant main effects of treatment and challenge were found for the WT mice (F(1,37)=6.25, P<0.05; F(1,37)=15.02, P<0.001), only a significant main effect for challenge was evident in KO mice (F(1,34)=17.48, P<0.001). SynCAM 1 KO mice exhibited a similar locomotor response to saline injections independent of whether they had previously received nine daily saline or cocaine injections (Figure 4b, first vs third column; t(17)=0.59, P>0.05). In the 9-day cocaine groups, the response to cocaine challenge was higher than the locomotor response to the saline challenge as expected, likely due to the concurrent contextual and pharmacological sensitization under these conditions (Figure 4b, third vs fourth column; t(17)=5.36, P<0.001). Notably, SynCAM 1 KO mice showed an identical response to a cocaine challenge (15 mg/kg), regardless of whether they had previously undergone the cocaine sensitization protocol (t(17)=0.82, P>0.05; Figure 4b, second vs fourth column). These data combined with the increased acute response to cocaine and more rapid peak in the sensitization protocol suggests that the absence of SynCAM 1 results in an acute response to cocaine similar to that observed in previously sensitized mice.
Cocaine-Induced Increases in Stubby Spine Number Require SynCAM 1 and its Loss Causes Mushroom Spines to Shorten upon Cocaine Challenge
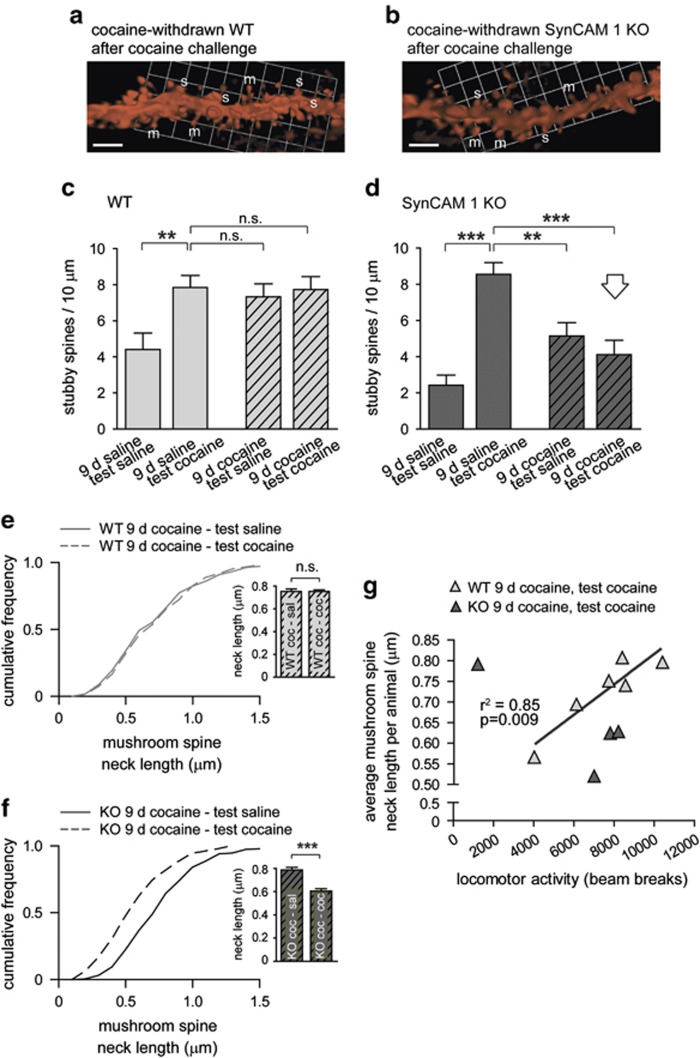
Cocaine administration results in changes in excitatory input to NAc that can be correlated with effects on addiction-related behaviors. We imaged SynCAM 1 contributions to the cocaine-induced plasticity of spine structure in mice that had undergone repeated saline or repeated cocaine exposure, followed by withdrawal and a saline or cocaine challenge (Figure 5a and b). Images were obtained from NAc sections prepared from the same cohorts whose sensitization to cocaine was measured in Figure 4. Analyses of stubby spine densities in WT groups indicated a significant main effect of challenge (F(1,84)=5.22, P<0.05) and a trend for a treatment × challenge interaction (F(1,84)=3.16, P=0.08). A single administration of cocaine (15 mg/kg) increased the density of stubby spines 1.8-fold in WT mice that had previously received repeated saline (Figure 5c, first vs second column; t(49)=3.16, P<0.01). The same increase in stubby spines was also observed in WT mice that had received repeated cocaine injections and were then withdrawn from the drug (Figure 5c, first vs third column; t(34)=2.11, P<0.05). An equivalent increase in stubby spine density was seen in mice treated for 9 days with cocaine and then challenged again with cocaine after withdrawal (Figure 5c, first vs fourth column; t(45)=2.79, P<0.01), suggesting that the sensitization regimen had a persistent effect on promoting stubby spine number that occluded further changes due to cocaine challenge.
Figure 5.
Loss of SynCAM 1 alters cocaine-induced spine structure changes. (a, b) Dendritic MSN segments visualized after biolistic DiI transfer in NAc of cocaine-withdrawn WT (a) and SynCAM 1 KO (b) mice that had received a cocaine challenge (15 mg/kg). Labels mark m, mushroom; s, stubby; t, thin spines. Scale bars, 5 μm. (c, d) SynCAM 1 KO prevents maintenance of maximal stubby spine increases during cocaine withdrawal. The density of stubby spines is elevated following cocaine challenge (15 mg/kg) in both WT (c) and SynCAM 1 KO (d) mice, but this maximal increase is only maintained during withdrawal in WT mice. Data were obtained from littermates with the experimenter blind to condition. (WT sal−sal, n=7 neurons from five mice, sal−coc 10/6, coc−sal 11/5, coc−coc 10/6; KO sal−sal 7/3, sal−coc 9/4, coc−sal 12/4, coc−coc 8/4) **P<0.01***P<0.001; NS, not significant (e, f) Absence of SynCAM 1 sensitizes MSN mushroom spines to cocaine-induced shortening. The neck length of mushroom spines in cocaine-withdrawn mice remains unchanged after a challenge injection of cocaine (15 mg/kg) in WT (e) but is reduced in SynCAM 1 KO mice (f). Results are represented as cumulative frequency distribution graphs, with insets showing mean±SEM Data were obtained from littermates with the experimenter blind to condition. (WT sal−sal, n=12 neurons from five mice, sal−coc 19/6, coc−sal 14/5, coc−coc 24/6; KO sal−sal 11/3, sal−coc 12/4, coc−sal 20/4, coc−coc 12/4) ***P<0.001; NS, not significant (g) Positive correlation between mushroom spine neck length in response to a cocaine challenge (15 mg/kg) following cocaine withdrawal and locomotor activity in WT mice (light gray triangles) but not SynCAM 1 KO mice (dark gray triangles). (WT, n=6 mice; KO, n=4).
Notably, the persistence in the maximal density of stubby spines after repeated cocaine administration requires the expression of SynCAM 1 (Figure 5d, second vs third column). Analysis indicated a significant treatment × challenge interaction (F(1,59)=22.01, P<0.001). The effect of an acute cocaine challenge following chronic saline injection on stubby spine density was significantly increased in SynCAM 1 KO mice. Although a single cocaine administration greatly increased stubby spine density in saline treated KO mice (Figure 5d, first vs second column; t(24)=6.63, P<0.01), SynCAM 1 KO mice that underwent chronic cocaine exposure and withdrawal exhibited attenuated stubby spine density following both saline (t(32)=3.47, P<0.01) and cocaine (t(33)=4.20, P<0.01) challenge.
In addition, mushroom spine densities were increased in cocaine-withdrawn WT mice 23% after 40–45 min following cocaine vs saline injection (data not shown; P<0.05), similar to a previous analysis of total spine numbers in rats (Shen et al, 2009). This effect of cocaine on mushroom spine numbers, however, likely does not depend on SynCAM 1 as KO mice showed a comparable increase in the density of mushroom spines by 14%, even though this increase did not reach significance.
Our morphometric analyses additionally demonstrated a dynamic SynCAM 1-dependent effect on mushroom spine structure after repeated cocaine exposure. Challenging cocaine-withdrawn SynCAM 1 KO mice with cocaine significantly shortened the neck of mushroom-type spines in NAc by 23% (Figure 5f; KO 9-day cocaine–test saline, 0.79±0.02 μm; KO 9-day cocaine–test cocaine, 0.61±0.02; P<0.0001) as well as their overall length (data not shown). This effect of cocaine was not observed in WT controls (Figure 5e; WT 9-day cocaine–test saline, 0.75±0.02 μm; WT 9-day cocaine–test cocaine, 0.75±0.02). Loss of SynCAM 1 therefore permits mushroom-type spines to shorten after either acute (Figure 2) or repeated cocaine administration. In addition, we observed that the average neck length of mushroom-type spines in cocaine-withdrawn WT mice following cocaine injection correlated with the locomotor activity that the individual animals exhibited (Figure 5g). This correlation was not observed in SynCAM 1 KO mice, indicating that a loss of spine structure/behavior correlation may contribute to the inability of the KO to respond to the challenge with elevated locomotor activity.
DISCUSSION
We demonstrate here that trans-synaptic interactions participate in the cellular mechanisms that organize synaptic structures in brain regions affected by drugs of abuse. This study shows that the synaptic adhesion molecule SynCAM 1 contributes to the controlling of excitatory synapse number in NAc. At the level of synaptic morphology, SynCAM 1 supports a sustained cocaine-induced increase in the number of stubby spines in withdrawn mice, and mushroom spine necks are shortened in KO mice after cocaine challenge. In addition, SynCAM 1 KO mice show impaired sensitization after acute cocaine administration and reduced maintenance of long-lasting activity changes after repeated cocaine administration.
These results reveal two previously unknown structural responses of MSN spines to cocaine that are both linked to SynCAM 1. On the one hand, we report that a single exposure to cocaine elevates stubby spine density in MSN, and our results support that SynCAM 1 is required to maintain this maximal increase upon repeat cocaine administration. This function of SynCAM 1 in NAc is consistent with its roles in maintaining excitatory synapses in hippocampus (Robbins et al, 2010). The density of mushroom-type spines in either WT or SynCAM 1 KO mice was not elevated by repeated cocaine injections over 9–10 days followed by 15–17 days of withdrawal. This was expected as protocols to measure such an increase in NAc of mice typically involve longer-term daily cocaine injections over 4 weeks (see Dobi et al (2011) and Lee et al (2006) for examples). With respect to shorter treatment regimens that include a withdrawal period, another study reported that eight daily cocaine injections followed by 14 days withdrawal resulted in a context-dependent increase of spine density in the NAc shell, but not the core (Li et al, 2004). However, our study scored spines by type, did not differentiate between MSN in core and shell, and we analyzed mice, not rats, differences that make a direct comparison of these data difficult.
The second novel structural response of MSN spines to cocaine we report here is a shortening of the necks of mushroom-type spines in SynCAM 1 KO mice within 40–45 min of cocaine challenge following repeated cocaine exposure and withdrawal. This may reflect an increased propensity of mushroom spines lacking SynCAM 1 to undergo acute, cocaine-induced structural changes. We did not observe the enlargement of spine heads upon cocaine challenge reported previously in rats (Shen et al, 2009), possibly due to different temporal profiles of this transient effect in mice analyzed here. The requirement of SynCAM 1 to prevent the cocaine-induced shortening of mushroom spine necks upon cocaine challenge may have direct functional consequences, as neck length likely controls the biochemical and electrical separation of spines from the dendritic compartment (Arellano et al, 2007; Tsay and Yuste, 2004). Notably, spine neck length is proportional to the filtering of electrical potentials, which could alter synaptic strength and contribute to synaptic plasticity (Araya et al, 2006; Rall, 1974).
Considering that synapse structure and function are tightly linked, our results indicate that dynamic changes in synapse structure in NAc may be an important factor for psychostimulant effects on neurotransmission. Such structural responses of MSN synapses to cocaine exposure may complement the effects of this psychostimulant on the ability of NAc synapses to undergo functional plasticity (Kauer and Malenka, 2007; Wolf and Ferrario, 2010). Although it remains to be determined whether SynCAM 1 affects synaptic plasticity in NAc similar to its role in regulating hippocampal LTD (Robbins et al, 2010), it can now be tested whether this adhesion molecule contributes to both structural and functional plasticity of excitatory MSN synapses. With respect to synapse structure, the effects of SynCAM 1 loss reported here may be a direct consequence of the constitutive lack of trans-synaptic interactions by this protein in the KO mice, but compensatory responses to the loss of SynCAM 1 may also contribute. Future studies using acute interference with SynCAM expression in NAc can now address this point. More generally, our findings in NAc are consistent with data in hippocampus, supporting that trans-synaptic interactions modulate structural and functional synaptic plasticity (see (Mendez et al, 2010; Okamura et al, 2004; Robbins et al, 2010) for examples).
Interestingly, SynCAM 1 KO mice show an enhanced acute locomotor response to cocaine, but no further increase upon repeated cocaine injections. Even though the nature of the link between spine density and addictive behavior remains controversial (Russo et al, 2010), these effects may suggest that the reduced baseline MSN spine density in absence of SynCAM 1 could be correlated with an enhanced response to acute cocaine. This would be consistent with the observation that the loss of spinophilin, a signaling molecule that regulates the postsynaptic cytoskeleton, decreases spine density and results in greater sensitization to cocaine reward (Allen et al, 2006; Feng et al, 2000). Further, it appears likely that long-lasting drug effects involve the remodeling and stabilization of excitatory synaptic connections in NAc. Although increases in MSN synapse density per se are not required for shorter-term effects of cocaine on locomotor sensitization (Pulipparacharuvil et al, 2008), SynCAM 1 may promote long-term changes through stabilizing stubby spines during repeated cocaine exposure. Future studies can now address to what extent such changes in stubby spines correlate with altered MSN function. Interestingly, chronic social defeat stress, which also sensitizes animals to cocaine, selectively increases stubby spines on NAc MSN and promotes excitatory transmission (Christoffel et al, 2011).
This study provides the first evidence that trans-synaptic interactions modulate the behavioral response to drugs of abuse. The finding that SynCAM 1-mediated adhesion impacts dynamic, cocaine-induced changes in spine shape provides novel insights into the mechanisms underlying remodeling of MSN synapses. It remains to be shown to what extent SynCAM 1-dependent changes in neuronal connectivity in regions other than the NAc contribute to the effects reported here. These findings expand the biomedical relevance of synapse-inducing adhesion proteins beyond developmental and neurological dysfunctions (Bill and Geschwind, 2009; Melom and Littleton, 2011; Südhof, 2008). SynCAM interactions may act in concert with integrins, extracellular matrix receptors that do not induce synapses but modulate their maturation as shown in hippocampus (Chavis and Westbrook, 2001) and undergo transient expression changes in NAc after chronic cocaine treatment (Wiggins et al, 2009). Our results further contribute to the insights gained into the transcriptional mechanism and signaling pathways that underlie cocaine-induced behavioral and synapse structural changes (Russo et al, 2010).
Together, synapse-organizing signaling pathways are potential therapeutic targets to ameliorate the long-term effects of addictive drugs on neuronal connectivity and behavior. This analysis of SynCAM 1 in addiction further supports the possibility that genetic predispositions altering synapse-organizing proteins may contribute to the striking individual differences in susceptibility to drugs of abuse.
Acknowledgments
We thank Y Lei for technical assistance. We thank the Yale CNNR program for access to its imaging core supervised by Dr S Wilson, are grateful to Dr T Momoi for providing the SynCAM 1/RA175 KO mouse line, and thank Dr A Nairn for sharing equipment and Dr G Lin for guidance. JIG performed and analyzed biochemical, imaging, and behavioral experiments, RAJ performed and analyzed biochemical and behavioral experiments, YJ supported imaging and performed behavioral experiments, NMN performed and analyzed behavioral experiments, TB and MRP conceived experimental approaches, and TB wrote the manuscript. This work was supported by NIH grants DA14241 (to MRP), R01 DA018928 (to TB), and R21 DA034492 (to TB).
JIG, YJ, RAJ, NMN and MRP declare no conflict of interest. TB is consulting for Mead Johnson Nutrition and received compensation.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Allen PB, Zachariou V, Svenningsson P, Lepore AC, Centonze D, Costa C, et al. Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience. 2006;140:897–911. doi: 10.1016/j.neuroscience.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci USA. 2006;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19:271–278. doi: 10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudanova I, Tabuchi K, Rohlmann A, Südhof TC, Missler M. Deletion of alpha-neurexins does not cause a major impairment of axonal pathfinding or synapse formation. J Comp Neurol. 2007;502:261–274. doi: 10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, et al. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E, Kouroku Y, Ozeki S, Tanabe Y, Toyama Y, Maekawa M, et al. Oligo-astheno-teratozoospermia in mice lacking RA175/TSLC1/SynCAM/IGSF4A, a cell adhesion molecule in the immunoglobulin superfamily. Mol Cell Biol. 2006;26:718–726. doi: 10.1128/MCB.26.2.718-726.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagtzoglou N, Ly CV, Bellen HJ. Cell adhesion, the backbone of the synapse: ‘vertebrate' and ‘invertebrate' perspectives. Cold Spring Harb Perspect Biol. 2009;1:a003079. doi: 10.1101/cshperspect.a003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Hishimoto A, Liu QR, Drgon T, Pletnikova O, Walther D, Zhu XG, et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Hu XT. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29:610–616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9:1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Melom JE, Littleton JT. Synapse development in health and disease. Curr Opin Genet Dev. 2011;21:256–261. doi: 10.1016/j.gde.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Mendez P, De Roo M, Poglia L, Klauser P, Muller D. N-cadherin mediates plasticity-induced long-term spine stabilization. J Cell Biol. 2010;189:589–600. doi: 10.1083/jcb.201003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Südhof TC, Biederer T. Synaptic cell adhesion. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhaiah R, Kamens HM, Picciotto MR. Effects of galanin on cocaine-mediated conditioned place preference and ERK signaling in mice. Psychopharmacology. 2009;204:95–102. doi: 10.1007/s00213-008-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K, Tanaka H, Yagita Y, Saeki Y, Taguchi A, Hiraoka Y, et al. Cadherin activity is required for activity-induced spine remodeling. J Cell Biol. 2004;167:961–972. doi: 10.1083/jcb.200406030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Studies in Neurophysiology. Cambridge University Press: Cambridge, UK; 1974. pp. 203–209p. [Google Scholar]
- Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, et al. SynCAM 1 adhesion dynamically regulates synapse number and impacts plasticity and learning. Neuron. 2010;68:894–906. doi: 10.1016/j.neuron.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb BU. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 (Supplement 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LS, Miller AD, Lester DB, Bay-Richter C, Schulein C, Frikke-Schmidt H, et al. Increased amphetamine-induced locomotor activity, sensitization, and accumbal dopamine release in M5 muscarinic receptor knockout mice. Psychopharmacology. 2010;207:547–558. doi: 10.1007/s00213-009-1685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagi M, Fogel AI, Biederer T. SynCAM 1 participates in axo-dendritic contact assembly and shapes neuronal growth cones. Proc Natl Acad Sci USA. 2010;107:7568–7573. doi: 10.1073/pnas.0911798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Lehmann MK, Christ CC, Hersrud SL, Davies GE. Associations among types of impulsivity, substance use problems and Neurexin-3 polymorphisms. Drug Alcohol Depend. 2011;119:e31–e38. doi: 10.1016/j.drugalcdep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, Akins MR, Biederer T. Expression and adhesion profiles of SynCAM molecules indicate distinct neuronal functions. J Comp Neurol. 2008;510:47–67. doi: 10.1002/cne.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay D, Yuste R. On the electrical function of dendritic spines. Trends Neurosci. 2004;27:77–83. doi: 10.1016/j.tins.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, et al. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify ‘connectivity constellation' and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Wiggins AT, Pacchioni AM, Kalivas PW. Integrin expression is altered after acute and chronic cocaine. Neurosci Lett. 2009;450:321–323. doi: 10.1016/j.neulet.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.