Abstract
Personality traits have been shown to interact with environmental cues to modulate biological responses including treatment responses, and potentially having a role in the formation of placebo effects. Here, we assessed psychological traits in 50 healthy controls as to their capacity to predict placebo analgesic effects, placebo-induced activation of μ-opioid neurotransmission and changes in cortisol plasma levels during a sustained experimental pain challenge (hypertonic saline infused in the masseter muscle) with and without placebo administration. Statistical analyses showed that an aggregate of scores from Ego-Resiliency, NEO Altruism, NEO Straightforwardness (positive predictors) and NEO Angry Hostility (negative predictor) scales accounted for 25% of the variance in placebo analgesic responses. Molecular imaging showed that subjects scoring above the median in a composite of those trait measures also presented greater placebo-induced activation of μ-opioid neurotransmission in the subgenual and dorsal anterior cingulate cortex (ACC), orbitofrontal cortex, insula, nucleus accumbens, amygdala and periaqueductal gray (PAG). Endogenous opioid release in the dorsal ACC and PAG was positively correlated with placebo-induced reductions in pain ratings. Significant reductions in cortisol levels were observed during placebo administration and were positively correlated with decreases in pain ratings, μ-opioid system activation in the dorsal ACC and PAG, and as a trend, negatively with NEO Angry Hostility scores. Our results show that personality traits explain a substantial proportion of the variance in placebo analgesic responses and are further associated with activations in endogenous opioid neurotransmission, and as a trend cortisol plasma levels. This initial data, if replicated in larger sample, suggest that simple trait measures easily deployable in the field could be utilized to reduce variability in clinical trials, but may also point to measures of individual resiliency in the face of aversive stimuli such as persistent pain and potentially other stressors.
Keywords: placebo, personality, resilience, stress, opioids, cortisol
INTRODUCTION
Certain personality traits are thought to influence behavioral risk/resiliency and have been further associated with treatment responses in some neuropsychiatric disorders, such as Major Depression and Bipolar Disorder (eg, Neuroticism/Extraversion) (Quilty et al, 2009) or substance use disorders (eg, Impulsivity/Deliberation) (Reske and Paulus, 2008). Furthermore, personality traits such as extraversion have been linked with placebo responses in patients diagnosed with irritable bowel syndrome, but only in the context of warm empathic interactions with the care provider (Kelley et al, 2009). These data suggest that personality variables interact with environmental (ie, social) cues to modulate behavioral and biological placebo responses.
Initial, largely inconclusive studies attempted to discover a psychological profile of placebo responders in clinical trials (Lasagna et al, 1954; Liberman, 1962). More recently, and in the context of placebo analgesia, trait optimism and trait anxiety were found to be positive and negative, reproducible predictors (Geers et al, 2010; Morton et al, 2009). Dopaminergic mechanisms have been associated with motor placebo responses in Parkinson's disease (de la Fuente-Fernandez et al, 2001) and with placebo analgesia during experimental pain (Scott et al, 2007, 2008), and has been suggested that placebo responses may represent a form of reward responding based on positive expectations. Consistent with that premise, a composite of personality traits thought to be related to dopaminergic function (novelty seeking, harm avoidance, behavioral drive, fun seeking and reward responsiveness) have been associated with both placebo analgesic effects and gray matter density in the basal ganglia and prefrontal cortex (Schweinhardt et al, 2009). In addition, neurotransmitter systems known to be involved in placebo analgesia (Scott et al, 2008), such as the dopamine D2/D3 (Buckholtz et al, 2010) and μ-opioid (Love et al, 2009) have been associated with individual differences in personality traits such as impulsivity receptor in positron emission tomography (PET) imaging studies.
There is a substantial interest in the development of simple measures that may provide predictive value for the formation of placebo effects, as such effects can be quite prominent in clinical trials. However, meta-analyses have suggested that placebo effects are mostly observed when the outcome measures utilized were continuous and subjective; this is to be expected for states for which no objective marker of disease or severity is known or is impractical to employ in large scale trials (Hrobjartsson and Gotzsche, 2001).
The present work examines a number of traits for their potential association with placebo analgesia. A partial least squares (PLS) regression approach was followed by a leave-one-out cross-validation to select the most predictive trait measures. Once selected, we examined them for their association with objective measures of placebo effects, such as placebo-induced changes in plasma cortisol and the activation of endogenous opioid neurotransmission using PET and a selective radiotracer labeling μ-opioid receptors. In addition to their potential utility in reducing response variability in clinical trials, we hypothesized that personality variables associated with the formation of placebo effects may also point to factors that promote resiliency from allostatic challenges to the organism, given the known role of the μ-opioid system and the hypothalamic–pituitary–adrenal axis in the maintenance of homeostasis during various forms of stress, including sustained pain.
MATERIALS AND METHODS
Subjects and Psychological Inventories
Fifty right-handed, non-smoking healthy volunteers were recruited via advertisement (Supplementary Material 1). Forty-seven subjects completed psychological inventories chosen to succinctly reflect various trait dimensions (data was missing in three subjects), 19 males, 28 females, 26±5 years of age, range 19–38. A written informed consent was obtained in all cases. All of the procedures used were approved by the University of Michigan Investigational Review Board for Human Subject Use and the Radioactive Drug Research Committee.
As our primary hypothesis stated that personality predictors of placebo responses would be associated with stress resiliency, the following questionnaires were included: scales assessing emotional well-being, psychological well-being and social well-being (Keyes, 1998), dispositional optimism (Life Orientation Test-Revised) (Scheier et al, 1994), satisfaction with life (Satisfaction with Life Scale) (Pavot et al, 1991) and Ego-Resiliency (ER-89) (Block and Kremen, 1996).
Personality traits related to dopaminergic function that have been associated with placebo analgesic effects (Schweinhardt et al, 2009) were also evaluated using the behavioral inhibition scale/behavioral activation scale (Reward Responsiveness and Drive) (Carver and White, 1994).
Low anticipatory responses to pain have been associated with the subsequent formation of placebo analgesia (Wager et al, 2004), therefore trait anxiety was included in the model as a potential negative predictor (State-Trait Anxiety Inventory) (Spielberg, 1983).
Finally, an overall evaluation of personality traits was included using the scores of the five dimensions of the NEO Personality Inventory-Revised (Costa and McRae, 1992a).
Quantitative Analyses
For univariate analysis, simple linear regression was used to explore the relationship between placebo analgesia responses (average reductions in pain intensity ratings acquired every 15 s during the pain challenge, over 20 min) and the 15 potential psychological predictors of placebo analgesia described above. Within the NEO Personality Inventory-Revised, Agreeableness and Neuroticism were the only dimensions significantly associated with placebo analgesic effects in the univariate analysis; therefore, these two domains were decomposed into their component facets, creating an overall initial potential predictor space of 25 variables.
For multivariate analysis, because of the presence of multi-colinearity in the predictor space, a PLS regression approach was used (Wold, 1966). For variable selection, a leave-one-out cross-validation procedure was used to extract the most significant variables from the predictor space and x-scores were computed as a function of the number of variables retained with the goal of minimizing the root mean squared error of the prediction (RMSEP) (Le Cao et al, 2008; Maitra and Yan, 2008) (Supplementary Material). In addition to R2 that measures goodness-of-fit, cross-validated R2 (or Q2) was computed to provide a measure of goodness-of-prediction, and is defined as 1 (Wold et al, 2001).
Experimental Design and Neuroimaging Methods
As previously described (Scott et al, 2008), two 90-minute [11C] carfentanil PET studies were acquired per subject, part of a larger study where four pain administrations took place (two pairs of pain and placebo studies, randomized in order and counterbalanced). Subjects were placed in the scanner gantry with needles placed in both masseter muscles ∼30 min before radiotracer administration. Each scanning session consisted of a control condition (0.9% isotonic saline, 5-25 min after start of scanning) and a painful condition (5% hypertonic saline, 45–65 min after start of scanning), infused in the masseter muscle with and without placebo administration. Once the individual infusion profiles were established for maintenance of pain during the hypertonic saline challenge, they were repeated during the pain and pain+placebo studies. An average infusion profile calculated from previous studies was utilized for the isotonic saline challenges. Volunteers were told that these two conditions would take place, but not the order in which they would take place, allowing for expectation of pain during the non-painful isotonic saline infusion (control condition). The placebo condition consisted of the introduction of 1 ml of 0.9% isotonic saline into one of the intravenous ports every 4 min starting 2 min before the pain anticipation and the pain challenges in view of the volunteer, and lasting for 15 s each time (Figure 3, Supplementary Material).
Levels of expectancy and subjectively assessed placebo effectiveness were also measured with the questions: ‘From 0 to 100 how effective do you think the treatment will be?' and ‘From 0 to 100 how effective was the treatment?'.
In order to evaluate placebo effects, subjects completed the Positive and Negative Affectivity Scale (PANAS) (Watson et al, 1988) before and after each of the challenges, a visual analog scale (VAS) of pain intensity every 15 s during the pain challenge (both during control and pain periods) and the McGill Pain Questionnaire (MPQ) (Melzack and Torgerson, 1971) at the completion of the pain challenge. Changes in the VAS measure in the absence and presence of placebo were used for the assessment of placebo responses. This variable was normally distributed and residuals from the regression models were randomly scattered, which validated the assumptions of the linear models.
Data Analysis
To examine the effects of personality traits on placebo-induced μ-opioid system activation (reductions in the receptor availability measure, nondisplaceable binding potential (BPND), from the control—when pain is expected but not received—to the control+placebo condition and pain to pain+placebo conditions), we performed a median split of the personality x-scores resulting from the PLS regression approach described above. A mixed model of analysis of variance was applied on a voxel-by-voxel basis using Statistical Parametric Mapping software (Wellcome Department of Cognitive Neurology, University College, London, England), with High and Low personality x-score groups as the between subject factor and the change in BPND as the dependent variable. Sex and age were included in the analysis as nuisance covariates. No global normalization was applied to the data, and therefore the calculations presented are based on absolute Bmax/Kd estimates. A cortical mask that excluded the cerebellum was used in the analysis. Only voxels with specific μ-opioid receptor binding were including in the analyses (BPND >0.1) to reduce statistical noise. Among these regions, which are widely distributed throughout the human brain, we hypothesized that activation would be found in the following regionsthat are associated with placebo analgesia: periaqueductal gray (PAG), rostral, dorsal and subgenual anterior cingulated cortex (ACC), prefrontal cortex, insula, thalamus, nucleus accumbens (NAc) and amygdala. These a priori-hypothesized regions were deemed significant at a P<0.001 uncorrected for multiple comparisons. For other regions, a P<0.05 family wise error-corrected was considered significant. These data were extracted for quantification of regional changes in BPND, graphing, correlational analyses and the identification of potential outliers. Further statistical analyses were performed with commercially available statistical software (SPSS for Macintosh, version 17; SPSS, Chicago, Illinois).
Cortisol Collection and Analysis
Blood samples were collected every 10 min, starting 10 min prior to tracer administration, over the 90 min scans. Sixteen subjects had missing data due to poor blood sampling and the final sample consisted of 34 volunteers.
Cortisol was assayed using the automated Immulite 1 000 chemiluminescent cortisol assay (Siemens Medical Solutions Diagnostic Division) with intra- and inter-assay variability of <5% and 7%–8%, respectively. Each pair of scans, without and with placebo) was conducted at the same time of day (starting at 8.30 a.m. or at 1.30 p.m.).
Significant changes in cortisol plasma levels were studied using a paired t-test comparison between the area under the curve (AUC) (Watamura et al, 2004) during pain and the AUC during pain+placebo. Peak reductions in cortisol plasma levels after placebo were used to correlate (Pearson correlation, two-tailed, P<0.05) with the analgesic placebo response, imaging data and personality traits using SPSS (SPSS for Macintosh, version 17). Data are expressed as the mean±1 SD.
RESULTS
Psychophysical Responses to Placebo Administration
Significant reductions in pain intensity ratings during placebo administration were observed using the average VAS acquired every 15 s (pain: 30±13; pain+placebo: 24±13; F=8.86, P=0.005), as well as the MPQ total score (pain: 23±11; pain+placebo: 21±12; F=5.76, P=0.02). Expectation of analgesia was rated as 48%±26, and the subjectively assessed effectiveness was 45%±30. Expectation of placebo analgesia was not significantly correlated with placebo response (r=0.19, P=0.19), while the difference between subjectively assessed and initially expected efficacy, which represents a measure of comparisons between expected and subjectively observed effects, was correlated with placebo response (r=0.38, P=0.008). These results suggest that positive expectations of placebo analgesia, a classical mechanism for the formation of unconditioned placebo responses (Kirsch, 1985), might not be sufficient for the development of placebo responses. No sex differences were observed in these measures.
Personality Univariate and Multivariate Models
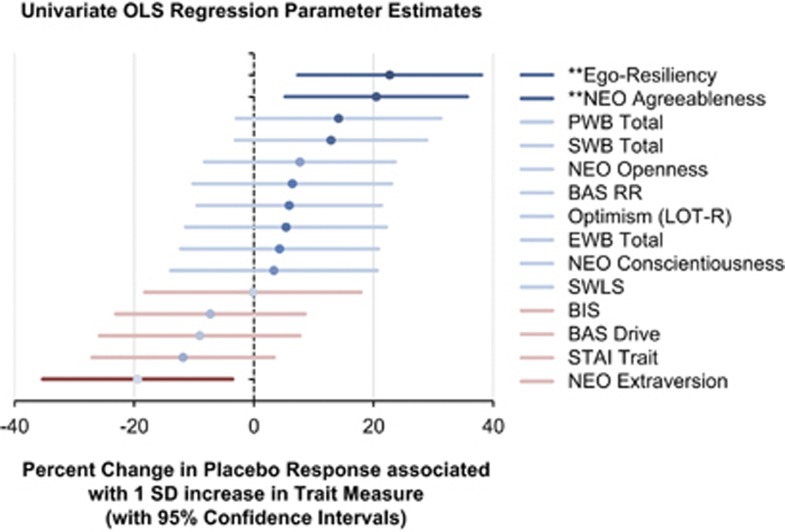
Using the change in average VAS score as dependent variable, the most predictive traits of placebo analgesia in the Univariate model were ER, NEO Agreeableness and NEO Neuroticism, which respectively explained 16%, 14% and 12% of the variance in placebo response (Figure 1, Supplementary Table S1). The former two were positive predictors, whereas the latter was a negative predictor. The three variables were highly intercorrelated (P<0.0001 for all pairs): ER and NEO Agreeableness, r=0.56; ER and NEO Neuroticism, r=−0.61; and NEO Agreeableness and NEO Neuroticism, r=−0.53.
Figure 1.
Simple linear regression representing percent change in Placebo Response associated with 1 SD increase in Trait Measure (with 95% confidence intervals). (** indicates P<0.01; * indicates P<0.05).
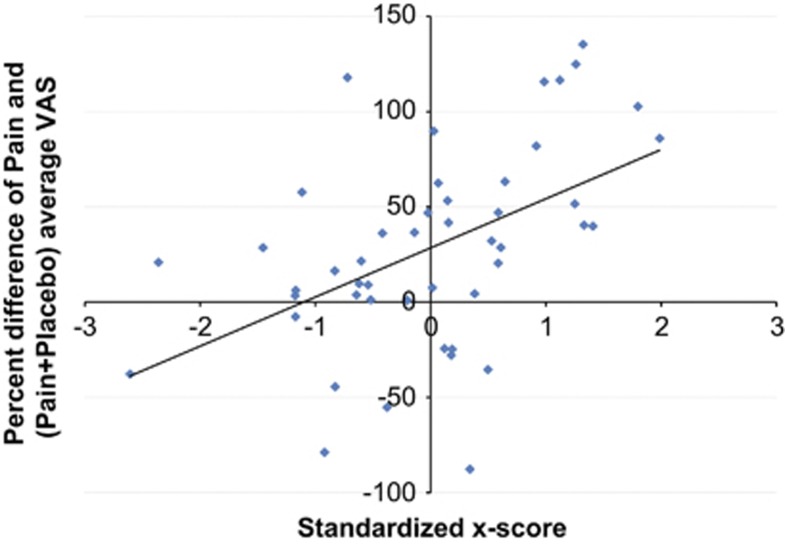
After decomposing NEO Agreeableness and NEO Neuroticism dimensions into their facets, the multivariate model with ER, NEO Altruism, NEO Straightforwardness and NEO Angry Hostility (negative predictor) accounted for 25% of the variation in placebo analgesic responses (R2) and had a predictive ability of 18% (Q2). The leave-one-out cross-validation showed that the retention of these four variables yielded to global minimum prediction error (RMSEP=46.95). Prediction values were reported as an x-score that estimated how well the model explained existing data and how well it could be expected to predict new data. A 1 SD increase in the resulting x-score was associated with a 25% increase in placebo response (95% confidence interval: 11.74–38.31; F=14.40, P=0.0004) (Figure 2).
Figure 2.
Scatterplot and least squares regression line: Percent placebo response (change in pain intensity ratings) and standardized x-score from the optimal PLS model: scatterplot and least squares regression line.
Personality Traits and Placebo-Induced Changes in μ-Opioid Receptor Availability
The x-score dimension resulting from the multivariate model was then related to an objective measure of placebo response, namely placebo-induced changes in μ-opioid receptor BPND, measured during the pain expectation (control) and during sustained pain challenge. A median split value of the x-score was used in order to get Low and High predictor trait groups.
During the anticipation of pain, the mixed model of variance revealed a significant effect of personality traits (High>Low x-score) on placebo-induced endogenous opioid system activation in the ventral striatum, including the NAc (x, y, z coordinates, −20, 18, −5, extent 65 mm3, Z=3.5). Activation of μ-opioid neurotransmission (reductions in μ-opioid BPND) in this region were, at trend level, inversely correlated with increases in PANAS negative affect ratings (r=−0.25, P=0.07).
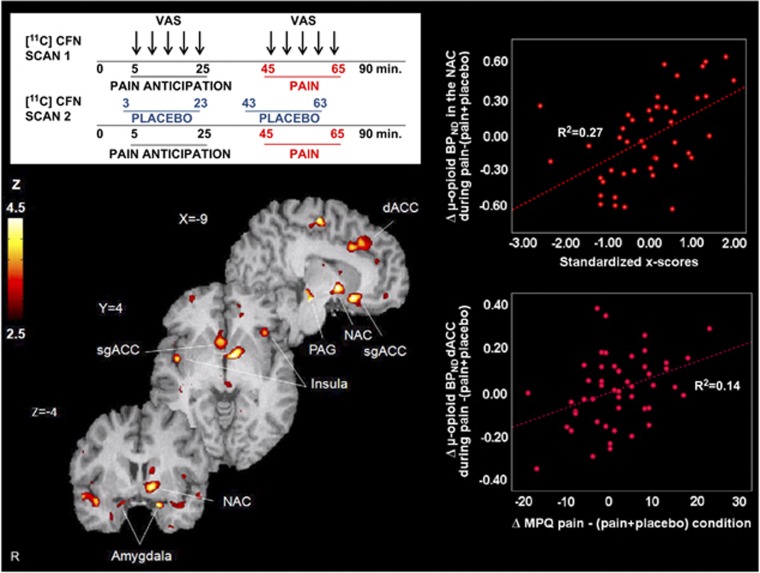
During sustained pain, a significant effect of traits (High>Low x-scores) on placebo-induced μ-opioid system activation was found in several a priori-hypothesized regions (for all regions: Z>3.5, P<0.001, k⩾ 10 mm3): the subgenual ACC bilaterally ((MNI coordinates x, y, z, cluster size mm3, Z score), (left: −9, 16,−14, 307 mm3, Z=4.33; right: 4, 14,−6, 492 mm3, Z=3.81)), left mid-ACC (−11, −15, 58, 197 mm3, Z=4.02), the left dorsal ACC (−19, 29, 28, 710 mm3, Z=4.08), the left orbitofrontal cortex (OFC) (−17, 53,−19, 101 mm3, Z=4.08), bilaterally the anterior (left: −43, 13,−19, 113 mm3, Z=3.5; right: 42, 6,−18, 184 mm3, Z=3.89) and posterior insula (left: −48, −11, 5, 483 mm3, Z=4.33; right: 46,−2,−1, 215 mm3, Z=4.29), the left NAc (−8, 1, −4, 664 mm3, Z=4.33), the amygdala bilaterally (left:−19, 5, −21, 98 mm3, Z=3.95; right: 14, 0, −20, 88 mm3, Z=3.85) and a brainstem region that included the PAG (−8,−24,−16, 193 mm3, Z=4.6) (Figure 3). Greater placebo-induced μ-opioid system activation in the high-scoring group was also noted at a lower threshold in the thalamus (−12, −13, 15, Z=2.88, P=0.002) and the dorsolateral prefrontal cortex (43, 23, 14, Z=2.96, p=0.002). No significant differences in opioid function were observed for the opposite contrast (Low>High x-scores).
Figure 3.
Upper left: experimental design. Two [11C] carfentanil scans were obtained in each subject, with and without administration of a placebo. Lower left: regions of greater μ-opioid release during placebo administration in subjects with High x-scores vs Low x-score. Upper right: x-scores correlations with μ-opioid system activation (change in μ-opioid BPND) in the NAc after placebo administration. Lower right: correlations between μ-opioid system activation in the NAc during placebo and the change in pain ratings as measured with the MPQ. BPND, binding potential nondisplaceable; MPQ, McGill Pain Questionnaire; NAc, Nucleus accumbens; PAG, Periaqueductal gray; sgACC/dACC, subgenual/dorsal Anterior Cingulate Cortex; VAS, Visual analog scale.
Confirmatory analysis on extracted data showed that endogenous opioid system activation in these regions was significantly correlated with not only the composite measure, but also the individual personality traits that were most significant in the simple linear regression and PLS regression model (Supplementary Table S2). Consistent with the central role of the NAc in placebo analgesia (Scott et al, 2008), the x-score dimension predicted up to 27% of the magnitude of endogenous opioid release in the left NAc.
Regional activations of μ-opioid neurotransmission during placebo administration were significantly correlated with reductions in experimental pain ratings as measured with the MPQ in the dorsal ACC (MPQ total: r=0.31, P=0.036; MPQ sensory: r=0.31, P=0.034) and PAG (MPQ affect: r=0.29, P=0.048). Ratings of expectations of analgesia were significantly correlated with the activation of μ-opioid neurotransmission in the OFC (r=0.31, P=0.038).
Cortisol Plasma Levels During Placebo Administration
Ten cortisol repeated measures were used to define the AUC during each condition. Paired t-tests comparing the AUC during pain without placebo (115.63±11.9 μg/dl) and with placebo (92.59±11.64 μg/dl) were found significant (t=18.75, df=8, P<0.001).
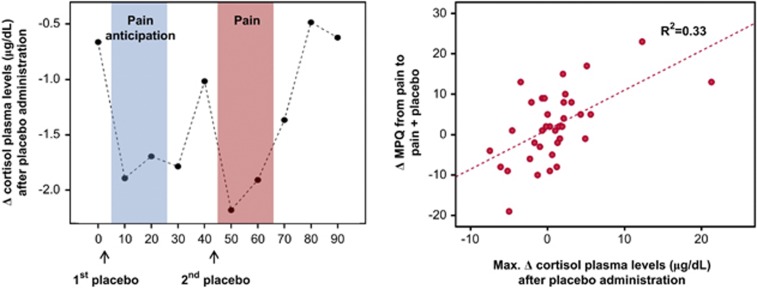
Placebo-induced reductions in cortisol plasma levels were particularly pronounced early after its administration, from min 0 to 10 and from min 40 to 50 (Figure 4). Based on these results, the peak reduction in cortisol plasma levels at those times was used to correlate with placebo analgesic effects, imaging data and traits. Significant correlations were observed between placebo-induced reductions in cortisol from 40 min to 50 min, and the average change in VAS acquired every 15 s during the pain challenge (r=0.52, P<0.001), MPQ total (r=0.58, P<0.001) (Figure 4), MPQ sensory subscale (r=0.51, P<0.001), and MPQ pain affect subscale (r=0.60, P<0.001). Reductions in cortisol were also associated with μ-opioid system activation in the dorsal ACC (r=0.35, P<0.04) and in the PAG (r=0.33, P<0.05). No significant relationships were observed between placebo-induced changes in cortisol and trait predictor variables, with exception of a trend negative correlation with NEO Angry Hostility trait (r=−0.30; P=0.07). No significant relationships were observed between changes in cortisol and PANAS ratings, imaging data or trait measures during the control (pain expectation) condition.
Figure 4.
Left: reductions in cortisol plasma levels (μg/dl) after placebo administration. The sustained pain challenge was administered during 20 min, starting at 45 min scan time. Right: Pearson correlations between total MPQ scores and decreases in cortisol plasma levels (μgr/dl) during (pain+placebo)−pain condition 5 min after pain starts. Decreases in cortisol plasma levels within the first 10 min of the pain challenge during placebo administration predicted 33% of the variance of the MPQ scores.
DISCUSSION
The present study shows that a composite of four stable personality traits (high ER, NEO Altruism, NEO Straightforwardness and low NEO Angry Hostility) predicted up to 25% of placebo analgesic responses and 27% of the NAc μ-opioid system activation during placebo administration. Additional regions where endogenous opioid responses to placebo administration were predicted by these personality traits largely overlap with those identified in previous reports as responsive to placebo administration and involved in the regulation of the pain experience (Scott et al, 2008; Wager et al, 2007; Zubieta et al, 2005): subgenual ACC, dorsal ACC, OFC, anterior and posterior insula, amygdala and, at lower thresholds, PAG, thalamus and dorsolateral prefrontal cortex. As previously observed, activation of μ-opioid receptor mediated neurotransmission in some of these regions were associated with reductions in individual pain report. In addition, we found significant reductions in cortisol plasma levels during placebo administration, which were correlated with reductions in subjective pain report and μ-opioid system activation in the dorsal ACC and PAG. Although no significant relationships were observed between the composite x-score scale and reductions in cortisol, these were negatively correlated with NEO Angry Hostility scale scores at trend levels.
One of the positive predictors of placebo effects, resilience, as defined by the ER-89 scale, refers to a person's ability to adapt successfully to acute stress, trauma or more chronic forms of adversity (Feder et al, 2009). This trait is thought to be mediated by positive emotions (Fredrickson et al, 2003) and adaptive changes in reward and emotional processing circuits (Feder et al, 2009), which may include interactions with the endogenous opioid system (Zhou et al, 2008). Animal models of stress resiliency have linked this trait to lower levels of ventral tegmental area dopamine neuron excitability (Krishnan et al, 2007). In humans, lower levels of activation during expectation of reward in the NAc and in the subgenual ACC have been observed in a sample of resilient Special Forces soldiers, compared with a matched control group using functional magnetic resonance imaging (Vythilingam et al, 2009). Lower levels of tonic synaptic dopamine have been further associated with a greater capacity to activate endogenous μ-opioid neurotransmission during a sustained painful stressor (Zubieta et al, 2003), providing a mechanism by which stress resiliency can be associated with greater effectiveness of the placebo through μ-opioid receptor mediated neurotransmission.
Trait agreeableness, as structured in the NEO Personality Inventory-Revised (Costa and McCrae, 1992b), includes the personality facets trust, straightforwardness, altruism, compliance, modesty and tender-mindedness; straightforwardness and altruism were the two facets of NEO Agreeableness that were more closely linked with placebo responsiveness in the analyses conducted. Some authors have related Agreeableness to prosocial motivation, dispositional empathy, and situational induced empathy (Graziano et al, 2007). In the patient doctor relationship, Agreeableness appears likely to contribute to a strong therapeutic alliance, as well as to frank, collaborative feedback throughout the therapeutic process. Thus, it appears that individuals high upon this trait are particularly well equipped to fully engage in therapeutic efforts, (Quilty et al, 2008) and in this sense, be a good responder to treatment, even if it is placebo (Mackenbach, 2005). Interestingly, research in the mechanisms of empathy in the context of pain have shown responses to observed pain in mid-ACC and anterior insula, two key regions of the so-called ‘shared circuits' for pain in self and that observed in others (Danziger et al, 2009). Given the overlapping neurobiology of pain, reward and placebo analgesia circuitry (Leknes and Tracey, 2008), it would not be surprising that regions engaged in empathic responses to others would also be involved in placebo responses. In our data, this was reflected by positive correlations between agreeableness and μ-opioid system activation in several midline regions (subgenual ACC, dorsal ACC and OFC) as well as the anterior and posterior insular cortex, NAc, amygdala and PAG. In this respect, agreeableness has also been linked to greater effectiveness of placebo acupuncture (Kelley et al, 2009) and empathic traits to placebo analgesia mediated through social learning (Colloca and Benedetti, 2009). Moreover, reciprocal altruism has been related to resilience (Charney, 2004) and the activation of brain areas linked to reward processing (Rilling et al, 2002).
Neuroticism was, on the other hand, a negative predictor for the formation of placebo effects. Neuroticism is associated with the tendency to experience negative emotions (Costa and McCrae, 1992b), and includes the facets anxiety, angry hostility, depression, self-consciousness, impulsivity and vulnerability. In our analyses, the facet angry hostility showed the greatest negative predictability. The negative association between angry hostility scores and the formation of biological placebo effects is perhaps not surprising, given the literature linking anger to lower endogenous μ-opioid receptor system function (Burns et al, 2009; Martin del Campo et al, 1994). Neuroimaging studies have also shown that endogenous opioid activity in the OFC, rostral ACC, amygdala, insula and PAG regions contribute to modulation of both pain and negative affect, supporting the possibility that stable variations in the function of this system may have a relationship with trait anger–hostility and its behavioral manifestations during stress (eg, sustained pain) (Bingel et al, 2006; Zubieta et al, 2003). In fact, it has been hypothesized that inadequate endogenous opioid inhibitory activity in the rostral limbic system might simultaneously lead to elevated pain sensitivity and reduce ability to modulate anger when it is experienced (Bruehl et al, 2009). This is consistent with our findings, whereby anger–hostility was negatively associated with the capacity of a placebo to activate μ-opioid receptor mediated neurotransmission within the PAG, anterior and posterior insula, OFC, dorsal ACC, NAc and amygdala.
Previous studies have suggested that dispositional optimism, as defined by the Life Orientation Test-Revised scale, might be a predictor of placebo analgesia using experimental models of phasic heat and a preconditioning procedure to increase expectations of analgesia (Morton et al, 2009), or cold pain (cold pressor test) without preconditioning, where an interaction between Life Orientation Test-Revised scores and experimental condition was reported (Geers et al, 2010; Morton et al, 2009). However, this finding was not replicated in the present study using a sustained pain model and no preconditioning procedures.
Finally, we observed significant placebo-induced reductions in cortisol plasma levels during both, anticipation of pain and pain. Reductions in cortisol plasma levels during placebo administration in the context of pain anticipation were not associated with increases in endogenous opioid neurotransmission. In the absence of pain, lowering of cortisol plasma levels may be secondary to reductions in anxiety (Wager et al, 2004), potentially engaging different neurocircuitry. During the pain challenge, reductions in cortisol plasma levels during placebo administration were positively correlated with both placebo analgesic responses and the activation of regional μ-opioid neurotransmission in the dorsal ACC and PAG. At a trend level, they were also negatively correlated with the trait angry hostility. Decreases in cortisol levels during placebo administration could be a consequence of pain relief or part of the endogenous opioid modulation of the hypothalamic–pituitary–adrenal axis. Hypothalamic corticotrophin-releasing hormone neurons receive direct inhibitory input from β-endorphin producing neurons in the arcuate nucleus, but also regulate corticotrophin-releasing hormone neurons indirectly through noradrenergic mechanisms (Jackson et al, 1990). Naloxone, a non-selective opioid receptor antagonist, induces increases in adrenocorticotropic hormone and cortisol levels, an effect thought to be mediated through the blockade of β-endorphin and enkephalinergic input to corticotrophin-releasing hormone neurons (Bujdoso et al, 2001). In addition, it has also been shown that the arcuate nucleus is a part of an endogenous opioid descending pain suppressing system impacting on the ventrolateral PAG (Sim and Joseph, 1991).
The results presented in this report suggest that a composite of stable personality traits can be predictive of placebo responses, explaining one-fourth of the variance in the formation of placebo analgesic effects in healthy volunteers. These effects were observed using subjective pain report scales, but also objective measures, namely the activation of endogenous opioid neurotransmission after placebo administration. Positive predictors related to resiliency and interpersonal function (ER, NEO Altruism, NEO Straightforwardness) and a negative predictor (NEO Angry Hostility) were obtained. If replicated in clinical samples, these simple to administer measures may aid in the interpretation of clinical trials and the stratification of clinical research volunteers to reduce variability in therapeutic responses. In addition, they may represent traits that impact on individual variations in resiliency to physical and emotional stressors, such as pain, and potentially other stress-related disorders.
Acknowledgments
Work was supported by R01 AT 001415, R01 DA 016423, R01 DA 022520 (JKZ) and the Phil F. Jenkins Foundation. We would also like to acknowledge the contribution of the technologists of the PET Center at the University of Michigan.
JKZ received compensation as consultant from Eli Lilly, Johnson & Johnson, Abbot and Merck pharmaceutical during the 3 years prior to manuscript submission for work unrelated to the content of the manuscript. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Block J, Kremen AM. IQ and ego-resiliency: conceptual and empirical connections and separateness. J Pers Soc Psycol. 1996;70:349–361. doi: 10.1037//0022-3514.70.2.349. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Chont M. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neurosci Biobehav Rev. 2009;33:475–491. doi: 10.1016/j.neubiorev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujdoso E, Jaszberenyi M, Tomboly C, Toth G, Telegdy G. Behavioral and neuroendocrine actions of endomorphin-2. Peptides. 2001;22:1459–1463. doi: 10.1016/s0196-9781(01)00466-1. [DOI] [PubMed] [Google Scholar]
- Burns JW, Bruehl S, Chung OY, Magid E, Chont M, Goodlad JK, et al. Endogenous opioids may buffer effects of anger arousal on sensitivity to subsequent pain. Pain. 2009;146:276–282. doi: 10.1016/j.pain.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS Scales. J Pers Soc Psycol. 1994;67:319–333. [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144:28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Costa P, McRae R. Normal personality assessment in clinical plactice: the NEO Personality Inventory. Psychol Assessment. 1992a;4:5–13. [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory professional manual. Psychological Assessment Resources. Odessa, FL; 1992b. [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. J Pers Soc Psychol. 2003;84:365–376. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional optimism predicts placebo analgesia. J Pain. 2010;11:1165–1171. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano WG, Habashi MM, Sheese BE, Tobin RM. Agreeableness, empathy, and helping: a person x situation perspective. J Pers Soc Psychol. 2007;93:583–599. doi: 10.1037/0022-3514.93.4.583. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Jackson RV, Grice JE, Jackson AJ, Hockings GI. Naloxone-induced ACTH release in man is inhibited by clonidine. Clin Exp Pharmacol Physiol. 1990;17:179–184. doi: 10.1111/j.1440-1681.1990.tb01302.x. [DOI] [PubMed] [Google Scholar]
- Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, et al. Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med. 2009;71:789–797. doi: 10.1097/PSY.0b013e3181acee12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes CLM. Social well-being. Soc Psychol Quart. 1998;61:121–140. [Google Scholar]
- Kirsch I. Response expectancy as a determinant of experience and behavior. Am Psychol. 1985;40:1189–1202. [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lasagna L, Mosteller F, Von Felsinger JM, Beecher HK. A study of the placebo response. Am J Med. 1954;16:770–779. doi: 10.1016/0002-9343(54)90441-6. [DOI] [PubMed] [Google Scholar]
- Le Cao KA, Rossouw D, Robert-Granie C, Besse P. A sparse PLS for variable selection when integrating omics data. Stat Appl Genet Mol Biol. 2008;7:Article 35. doi: 10.2202/1544-6115.1390. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Liberman R. An analysis of the placebo phenomenon. J Chronic Dis. 1962;15:761–783. doi: 10.1016/0021-9681(62)90048-6. [DOI] [PubMed] [Google Scholar]
- Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–1134. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbach JP. On the survival of the altruistic trait in medicine: is there a link with the placebo effect. J Clin Epidemiol. 2005;58:433–435. doi: 10.1016/j.jclinepi.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Maitra S, Yan Y. Principle component analysis and partial least squares: two dimension reduction techniques for regression. CAS Casualty Actuarial Society 2008 Discussion Paper Program. 2008.
- Martin del Campo AF, Dowson JH, Herbert J, Paykel ES. Effects of naloxone on diurnal rhythms in mood and endocrine function: a dose-response study in man. Psychopharmacology. 1994;114:583–590. doi: 10.1007/BF02244988. [DOI] [PubMed] [Google Scholar]
- Melzack R, Torgerson WS. On the language of pain. Anesthesiology. 1971;34:50–59. doi: 10.1097/00000542-197101000-00017. [DOI] [PubMed] [Google Scholar]
- Morton DL, Watson A, El-Deredy W, Jones AK. Reproducibility of placebo analgesia: Effect of dispositional optimism. Pain. 2009;146:194–198. doi: 10.1016/j.pain.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Pavot W, Diener E, Colvin CR, Sandvik E. Further validation of the satisfaction with life scale: evidence for the cross-method convergence of well-being measures. J Pers Assess. 1991;57:149–161. doi: 10.1207/s15327752jpa5701_17. [DOI] [PubMed] [Google Scholar]
- Quilty LC, De Fruyt F, Rolland JP, Kennedy SH, Rouillon PF, Bagby RM. Dimensional personality traits and treatment outcome in patients with major depressive disorder. J Affect Disord. 2008;108:241–250. doi: 10.1016/j.jad.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Quilty LC, Sellbom M, Tackett JL, Bagby RM. Personality trait predictors of bipolar disorder symptoms. Psychiatry Res. 2009;169:159–163. doi: 10.1016/j.psychres.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Reske M, Paulus MP. Predicting treatment outcome in stimulant dependence. Ann N Y Acad Sci. 2008;1141:270–283. doi: 10.1196/annals.1441.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J Neurosci. 2009;29:4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Joseph SA. Arcuate nucleus projections to brainstem regions which modulate nociception. J Chem Neuroanat. 1991;4:97–109. doi: 10.1016/0891-0618(91)90034-a. [DOI] [PubMed] [Google Scholar]
- Spielberg CD. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologist Press, Inc: Palo Alto; 1983. [Google Scholar]
- Vythilingam M, Nelson EE, Scaramozza M, Waldeck T, Hazlett G, Southwick SM, et al. Reward circuitry in resilience to severe trauma: an fMRI investigation of resilient special forces soldiers. Psychiatry Res. 2009;172:75–77. doi: 10.1016/j.pscychresns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wold H.1966Estimation fo principal components and related models by iterative least squaresIn: Krishnaiah PR, (Ed)Mulitvariate Analysis Academic Press: New York; 391–420. [Google Scholar]
- Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometr Intell Lab Syst. 2001;58:109–130. [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.