Abstract
Negative affect promotes dysregulated alcohol consumption in non-dependent and alcohol-dependent animals, and cues associated with negative affective states induce withdrawal-like symptoms in rats. This study was designed to test the hypotheses that: (1) the kappa-opioid receptor (KOR) system mediates phenotypes related to alcohol withdrawal and withdrawal-like negative affective states and (2) cues associated with negative affective states would result in dysregulated alcohol consumption when subsequently presented alone. To accomplish these goals, intracerebroventricular infusion of the KOR antagonist nor-binaltorphimine (nor-BNI) was assessed for the ability to attenuate the increase in 22-kHz ultrasonic vocalizations (USVs) associated with alcohol withdrawal and KOR activation in adult male wistar rats. Furthermore, cues associated with a KOR agonist-induced negative affective state were assessed for the ability to dysregulate alcohol consumption and the efficacy of intracerebroventricular KOR antagonism to reduce such dysregulation was evaluated. KOR antagonism blocked the increased number of 22-kHz USVs observed during acute alcohol withdrawal and a KOR agonist (U50,488) resulted in a nor-BNI reversible increase in 22-kHz USVs (mimicking an alcohol-dependent state). Additionally, cues associated with negative affective states resulted in escalated alcohol self-administration, an effect that was nor-BNI sensitive. Taken together, this study implicates negative affective states induced by both alcohol withdrawal and conditioned stimuli as being produced, in part, by activity of the DYN/KOR system.
Keywords: alcohol and alcoholism, cues, kappa-opioid receptor, negative affect, operant self-administration, ultrasonic vocalizations
INTRODUCTION
Alcoholism is a major problem in the United States and abroad, with an estimated 7.0% of the US population 12 or older dependent on alcohol (Substance Abuse and Mental Health Services Administration, 2010). Recent research suggests early alcohol use as being a significant predictor of future licit and illicit drug use (Kirby and Barry, 2012), and, furthermore, that alcohol is the most harmful of all abused drugs (Nutt et al, 2010). Alcohol use disorders (AUDs; comprised of alcohol abuse and alcohol dependence) are commonly comorbid with affective disorders such as depression (Boden and Fergusson, 2011) and negative affective states that are present during withdrawal can promote excessive alcohol use (Koob and Le Moal, 1997). However, there are currently no pharmacotherapies for the treatment of AUDs that focus on this aspect of dysregulated alcohol consumption.
Negative affect has been implicated in the increased consumption of alcohol in non-dependent (Liu and Weiss, 2002; Sperling et al, 2010) and dependent animals (Liu and Weiss, 2002). Foot-shock stress has been shown to facilitate conditioned place preferences for alcohol and operant alcohol self-administration (Sperling et al, 2010). Furthermore, withdrawal from chronic alcohol exposure increases thresholds for intracranial self-stimulation (Schulteis et al, 1995) that are indicative of decreased brain reward function (Koob and Bloom, 1988), and alcohol-dependent animals in acute withdrawal show escalated alcohol consumption that is positively correlated with negative affective-like behaviors, as discussed in the previous work (Williams et al, 2012). Collectively, the evidence suggests that negative affective states influence alcohol consumption both before and after the development of dependence.
The dynorphin/kappa-opioid receptor (DYN/KOR) system has been implicated in alcohol abuse and dependence, as well as neuropsychiatric disorders including depression (for reviews see Walker et al (2012) and Knoll and Carlezon (2010)). Selective KOR antagonists attenuate escalated alcohol self-administration in alcohol-dependent animals (Walker and Koob, 2008), anhedonic- and anxiety-like behavior associated with alcohol and drug withdrawal (Chartoff et al, 2012; Valdez and Harshberger, 2012), and anxiety-like phenotypes (Knoll et al, 2007). KOR agonists produce conditioned place aversions in rodents (Bals-Kubik et al, 1989) and dysphoria in humans (Pfeiffer et al, 1986). Furthermore, activation of KORs potentiates the rewarding effect of drugs of abuse, such as nicotine (Smith et al, 2012) and cocaine (McLaughlin et al, 2006), while mice lacking the prodynorphin gene do not exhibit stress-induced facilitation of cocaine place preferences (McLaughlin et al, 2003).
Cues associated with negative affective states that accompany withdrawal produce withdrawal-like symptoms themselves (Kenny et al, 2006; Wikler and Pescor, 1967). Thus, negative affective cues may pose as risk factors for the development of AUDs and promote relapse; however, little research has been conducted on the neurobiological basis for this phenomenon. Emerging evidence for the role of the DYN/KOR system in alcohol dependence, fear conditioning (Knoll et al, 2007), and relapse to drugs of abuse (Land et al, 2009) point to the DYN/KOR system as a possible mediator of cue-induced negative affective-like states.
Previous work has established that measurements of 22-kHz ultrasonic vocalizations (USVs) are an ethologically valid measure of negative affective states (Williams et al, 2012; Knapp and Pohorecky, 1995). To further investigate the role of the DYN/KOR system in the production of negative affective states, we evaluated whether the increased 22-kHz USVs observed during acute withdrawal in alcohol-dependent rats could be altered by intracerebroventricular (ICV) infusions of a KOR antagonist. In a complementary experiment, we assessed whether ICV administration of the KOR agonist U50,488 would result in similar increases in the production of USVs as seen in dependent animals. Finally, as evidence suggests that both negative affective states and cues induce escalated consumption of drugs, we assessed whether cues paired with KOR agonism would result in dysregulated alcohol consumption when presented alone and whether such a response was sensitive to KOR antagonism.
MATERIALS AND METHODS
Animals
In total, 90 male wistar rats at least 70 days of age (bred from Charles River Laboratory (Hollister, CA) breeding pairs) were used for all experiments. Animals were group housed (2–3 rats per cage) in a temperature-controlled (21±2 °C) vivarium kept on a 12 h reverse light cycle with ad libitum food and water, and were handled for at least 5 days before experimentation. Animal care adhered to the National Research Council's Guide for the Care and Use of Laboratory Animals (1996) and Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003), with all procedures approved by the Washington State University Institutional Animal Care and Use Committee.
Surgeries
All animals were anesthetized with isoflurane gas (∼2%) and bilateral guide cannulae were implanted into the lateral ventricles using stereotaxic coordinates (from bregma: DV −3.7; AP −0.8; and ML ±1.5) (Paxinos and Watson, 2005). ICV guide cannulae were secured in place using acrylic dental cement and three machine screws were used to anchor the acrylic. The open ends of the guide cannulae were sealed with bilateral obturators (PlasticsOne, Roanoke, VA) to reduce risk of infection and maintain guide cannulae patency. Obturators and cannula guides were protected with stainless-steel cap nuts. Postoperative analgesics (flunixin; MWI Veterinary Supply, Meridian, ID) and antibiotics (Baytril; MWI Veterinary Supply) were administered daily for a total of 5 days following surgery. Animals were allowed to recover for at least 1 week before behavioral testing began. Cannula patency was verified throughout the experiment by ensuring that the proper volume of solution had been infused into each subject during pharmacological testing (with infusions occurring at a rate of 1 μl/min) and by histology at the conclusion of the experiment by injecting 1 μl 0.6% cresyl violet over 1 min, extracting the brain, and confirming intraventricular dye penetration had occurred.
Intermittent Alcohol Vapor Exposure
Intermittent alcohol vapor exposure (14 h on/10 h off; 7 days/week) induces increased motivational and negative affective states symptomatic of alcohol dependence as evidenced by escalated alcohol self-administration and affective indices such as increased 22-kHz USVs (Nealey et al, 2011; Walker and Koob, 2008; Walker et al, 2010; Williams et al, 2012). The apparatus (La Jolla Alcohol Research, La Jolla, CA) allows blood alcohol levels (BALs) to be titrated by the experimenter by adjusting the rate of 95% alcohol that is vaporized and introduced into the air flow to the sealed environmental chambers in which the animals are housed. This allowed the animal's BALs to be kept within a desired range (175–225 mg%), which was determined semiweekly by collecting blood from the tails (∼50 μl). After centrifugation, plasma samples were assayed for alcohol content using the Analox AM1 (Analox Instruments, Lunenburg, MA).
Behavioral Measures
Ultrasonic vocalizations
Production of 22-kHz USVs were assessed in a quiet room with dim (15 lx) lighting by administering an air-puff (∼60 psi) to the nape of the animal's neck. Air-puffs have been validated as a non-painful method of inducing 22-kHz USVs in rodents (Knapp and Pohorecky, 1995). Vocalizations were recorded by a microphone affixed 15 cm above the animal's head. Each test consisted of two trials, and each trial consisted of 15 air-puffs, or until the animal vocalized. Air-puffs were separated by 15 s and once an animal vocalized, the experimenter waited until the animal stopped vocalizing for 1 min before beginning a new trial. If an animal vocalized for 10 min, the trial was stopped and a new trial began. Vocalizations were recorded with a P48 Electret Ultrasound Microphone (Avisoft Bioacoustics, Germany), E-MU Systems Audio/MIDI Interface (Scotts Valley, CA), and Avisoft Bioacoustics software (created by AEST, Italy). The number and duration of 22-kHz USVs from the second trial were counted with Avisoft Bioacoustics software (Berlin, Germany) and used for all data analyses.
Operant alcohol self-administration
Animals were trained to self-administer a 10% alcohol solution with a sweetener-fade technique (Samson, 1986) during 30 min operant self-administration sessions, as described previously (Nealey et al, 2011). Operant training took place in standard operant conditioning chambers (Med Associates, St Albans, VT) with custom drinking wells (Behavioral Pharma, La Jolla, CA) using a continuous schedule of reinforcement. Acquisition of the operant response occurred using a sweetened fluid as the reinforcer (0.125% saccharin and 3% glucose), which is preferred to sucrose (Valenstein et al, 1967) and does not require food or water deprivation. A measure of 10% alcohol (w/v) was added to the sweetened fluid, and over 3 weeks, sweetener was slowly removed until only 10% alcohol (w/v) was left in the solution. After responding for alcohol stabilized (<10% deviation in lever-presses over three sessions), the animals underwent ICV cannulation. After recovery from surgery, animals were allowed access to 30 min operant alcohol self-administer sessions that occurred 5 days a week until responding for alcohol re-stabilized.
Experiment 1
In all, 30 alcohol vapor-exposed animals were used to evaluate the effect of the KOR antagonist nor-binaltorphimine (nor-BNI) on air-puff-induced 22-kHz USVs. After ICV cannulation and recovery, animals were randomly assigned to one of four doses of nor-BNI administration (0, 4, 8, or 16 μg) and placed into alcohol vapor chambers for a 2-week exposure regimen. Following the 2-week vapor exposure duration, animals were infused with nor-BNI or vehicle 24 h before testing that occurred during acute withdrawal (6 h into withdrawal).
Experiment 2
A total of 24 animals were used to evaluate the effect of the KOR agonist U50,488 on air-puff-induced 22-kHz USVs. After ICV cannulation and recovery, animals were assigned to one of four doses of U50,488 (0.0, 0.25, 2.5, and 25 μg), and 15 min after ICV infusion of U50,488 or vehicle, 22-kHz USVs were measured. Separate groups of animals (n=7/group) were used to assess whether the effect of U50,488 on 22-kHz USVs was sensitive to KOR antagonism by infusing nor-BNI (8 μg) or vehicle 24 h before U50,488 (25 μg) pre-treatment that occurred 15 min before 22-kHz USV measurement.
Experiment 3
Five animals were used initially to evaluate whether cues associated with the KOR agonist U50,488 could affect alcohol drinking during a 30-min operant self-administration session. After recovery from surgery, animals were allowed to self-administer alcohol until individual stability was achieved. Before conditioning, almond scent, a neutral stimulus (NS), was presented before self-administration sessions to ensure that the NS alone did not affect alcohol consumption. The almond scent was presented using a gauze pad saturated with 0.5 ml almond extract (McCormick & Company, Hunt Valley, MD) around the upper perimeter of a cage used specifically for conditioning and then leaving the animal in the cage for 15 min. Then, on two consecutive non-self-administration days, an ICV infusion of 25 μg U50,488 was paired with the almond scent for 15 min. Once the individual animals displayed stable alcohol self-administration rates without the presentation of the cue (up to 12 additional sessions), the almond scent, now considered a conditioned stimulus (CS), was presented alone before a final alcohol self-administration session. Additional groups of animals (n=5–8/group) were used to assess whether KOR antagonism could reverse the cue-induced escalation of operant responding. Operant self-administration and conditioning were conducted as described above. Following the conditioning trials and once stable self-administration was achieved, infusions of artificial cerebrospinal fluid (aCSF) or nor-BNI (1.6 or 16 μg) occurred 12 h before the final test session. On test day, the CS was presented alone and alcohol self-administration was measured.
Drugs
The KOR agonist U50,488 and the KOR antagonist nor-BNI (both of which have previously been used to modulate the DYN/KOR system; Walker et al, 2011; Nealey et al, 2011; Land et al, 2009) were purchased from Tocris Bioscience (Ellisville, MI) and dissolved in sterile aCSF. Both compounds were prepared daily and infused at a rate of 1 μl/min ICV.
Statistics
The effects of both nor-BNI in dependent animals and U50,488 in non-dependent animals on number of USVs were analyzed with a one-way univariate analysis of variance (ANOVA). If a main effect of dose was identified, post-hoc least significant difference (LSD) tests were conducted. The effect of nor-BNI vs vehicle infusion on U50,488-mediated 22-kHz USV production was analyzed with a univariate ANOVA. To protect against both type I and type II errors, both the significance (α=0.05) and power (1−β, with β=0.2) were assessed. To avoid the unnecessary use of animals, subjects were added to each experiment in a counterbalanced manner until both appropriate significance and power were achieved.
A univariate ANOVA was conducted on the cue-induced alcohol consumption with a dose of U50,488 or nor-BNI as the between-group factors. All values were expressed as percent change between test day and the previous self-administration session. When a main effect of dose was found, post-hoc LSD tests were conducted to compare the experimental treatments to the vehicle condition. If assumptions of normality were not met, nonparametric statistics were employed.
RESULTS
Because of post-surgical complications, four animals were removed from the study. Thus, of the 94 animals that began the study, 90 successfully completed the study and were included in the data analysis.
Figure 1 shows a main effect of nor-BNI dose on the number of 22-kHz USVs emitted in alcohol-dependent animals. The number of USVs decreased in a dose-dependent manner (F(3,30)=4.814, p<0.01) and post-hoc LSD tests identified that the 8 and 16 μg doses of nor-BNI significantly reduced (p<0.01) the number of USVs emitted.
Figure 1.
Mean (±S.E.M.) number of 22-kHz USVs in dependent animals (n=6–9/group) after 24 h pre-treatment with nor-binaltorphimine (nor-BNI) (0, 4, 8, or 16 μg). The two highest doses of nor-BNI decreased the number of USVs (*p<0.01 and †p<0.05).
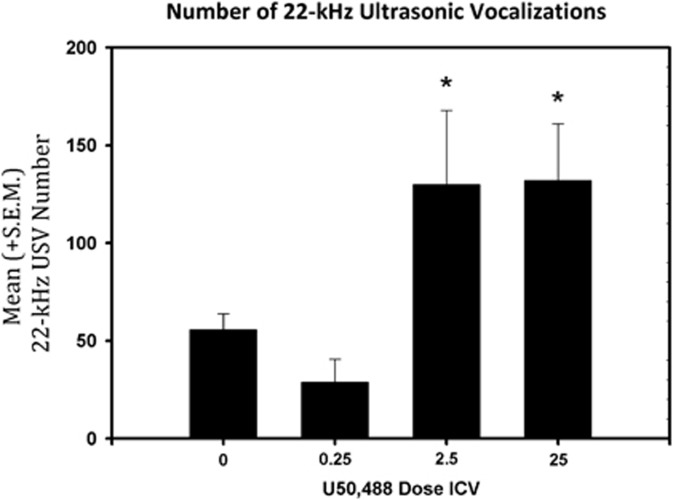
As seen in Figure 2, a main effect of dose (F(3,23)=4.446, p<0.05) was identified for the number of 22-kHz USVs emitted following ICV U50,488 pre-treatment. Post-hoc LSD tests identified the 2.5 and 25 μg dose of U50,488 significantly increased (p<0.05) the number of 22-kHz USVs emitted. Similarly, a main effect of nor-BNI dose (F(1,12)=6.530, p<0.05) was identified (Figure 3) and illustrated that nor-BNI reduced the number of 22-kHz USVs produced by U50,488.
Figure 2.
Mean (±S.E.M.) number of 22-kHz (n=6/group) 15 min after intracerebroventricular (ICV infusion of U50,488 (0, 0.25, 2.5, and 25 μg). U50,488 dose-dependently increased the number of USVs (*p<0.05).
Figure 3.
Mean (±S.E.M.) number of 22-kHz USVs (n=7/group) after 24 h pre-treatment with nor-binaltorphimine (nor-BNI) (8 μg) and 25 μg of U50,488 15 min before USV measurement. Nor-BNI decreased the number of USVs (*p<0.05).
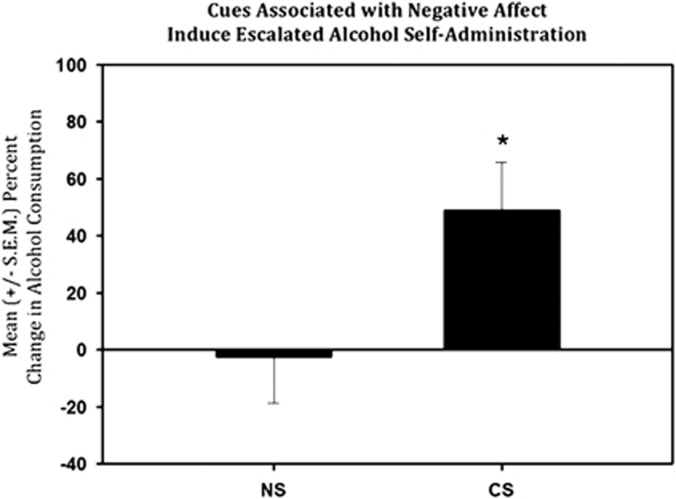
The effect of a cue associated with U50,488 administration can be seen in Figure 4. As the data from this sample was found to be skewed (Zg1=2.847) and leptokurtic (Zg2=1.679), indicating a lack of normal distribution (p=9.210, α<0.01), nonparametric tests were used. The Wilcoxon's signed-rank test found that the percent change between baseline and test day was significantly different from the percent change between baseline and presentation of the NS (W=18.00, p<0.05).
Figure 4.
Mean (±S.E.M.) percent change in alcohol consumption in non-dependent animals (n=5) following presentation of the neutral stimulus (NS), almond scent, or the conditioned stimulus (CS) as compared with baseline responding. Presentation of the CS alone resulted in an increase in alcohol self-administration compared with the stimulus before it being paired with kappa-opioid receptor agonism; *p<0.05 when compared with NS.
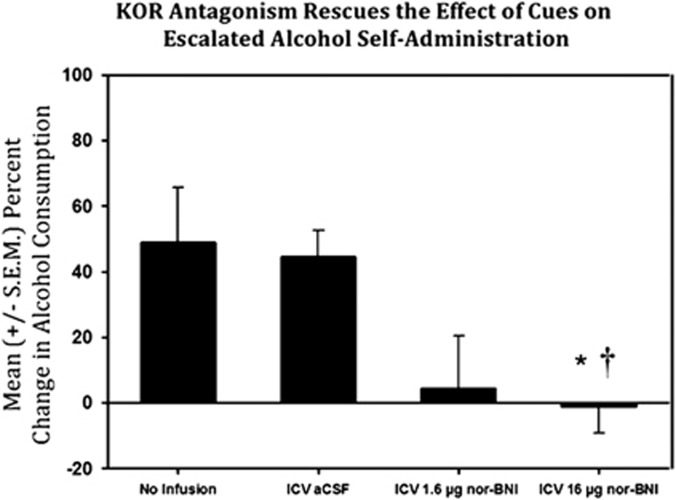
The analysis of affective cue-induced self-administration following ICV KOR antagonism (see Figure 5) revealed a significant main effect of dose (F(2,19)=6.774, p<0.01). Post-hoc LSD tests indicated that the 16 μg nor-BNI pre-treatment group differed significantly (p<0.01) from the no infusion group as well as the aCSF pre-treatment group. The 1.6 μg nor-BNI pre-treatment group, however, was not significantly different from the no infusion group or the aCSF pre-treatment group (both p>0.05).
Figure 5.
Mean (±S.E.M.) percent change in alcohol consumption in non-dependent animals (n=5–8/group) following presentation of the conditioned stimulus (neutral stimulus (NS)), almond scent, as compared with baseline responding in the no infusion group, intracerebroventricular (ICV) artificial cerebrospinal fluid (aCSF) group, 1.6 μg nor-binaltorphimine (nor-BNI), and 16 μg nor-BNI. This figure indicates that the escalated self-administration produced by the cue could be blocked by pre-treatment with 16 μg nor-BNI (a selective kappa-opioid receptor antagonist; *p<0.05 when compared with no infusion group and †p<0.05 when compared with the aCSF group).
DISCUSSION
Consistent with previous research reporting increased 22-kHz USVs during acute withdrawal in alcohol-dependent rats following 2 weeks of vapor exposure (Williams et al, 2012), the alcohol-dependent rats in this study robustly produced 22-kHz USVs during acute withdrawal. For the first time, dependence-induced 22-kHz USVs were shown to be dose-dependently reduced by the KOR antagonist nor-BNI. These data support earlier assertions that the negative affect present during withdrawal involves neuroadaptations in the DYN/KOR system that can drive excessive alcohol consumption according to a ‘self-medication' hypothesis (Walker et al, 2012; Walker and Koob, 2008). Of interest is the previous observation that 2 weeks of vapor exposure seemed to be pro-depressive, rather than anxiety-promoting, and that the increased 22-kHz USVs during withdrawal from 2-week vapor exposure corresponded to a depressive-like phenotype. It follows that since this study used the same 2-week exposure duration, one could posit that the effects of nor-BNI in this study were rescuing the depressive-like phenotype produced during acute withdrawal.
The number of 22-kHz USVs was dose-dependently increased by an ICV infusion of the KOR agonist U50,488, indicating that the KOR agonist can effectively induce a negative affective state in rodents. Specifically, the two highest doses of ICV U50,488 (2.5 and 25 μg) produced significantly higher numbers of 22-kHz USVs compared with vehicle. Furthermore, the effect of the highest dose of U50,488 (25 μg) on USVs was significantly attenuated by nor-BNI, affirming that the negative affective state was specific to KORs. Therefore, based on experiments 1 and 2, KOR agonist infusions successfully mimic an alcohol withdrawal-like state, which is reversible by blockade of the KOR.
This is also the first published evidence that a cue paired with a negative affective state can result in the dsyregulation of alcohol self-administration. While previously ineffective at altering alcohol consumption, once the NS was paired with the negative affective state produced by U50,488, the CS was able to induce a robust increase in alcohol self-administration. Furthermore, infusion of the 16 μg dose of nor-BNI before presentation of the CS prevented the ability of the CS to increase alcohol self-administration. The no-infusion group and the aCSF groups did not differ significantly from each other, although they both differed significantly from the highest nor-BNI dose. This indicates that the potentiated drinking produced by affective cues was sensitive to nor-BNI, and thus mediated by the DYN/KOR system, this effect was not due to different basal alcohol consumption between the four groups (data not shown).
Other research has also examined the relationship between negative affect and the DYN/KOR system. The KOR antagonist nor-BNI reverses depressive-like phenotypes in the forced swim test (FST) for cocaine withdrawn (Chartoff et al, 2012) and has anxiolytic effects in the elevated plus maze (EPM; Knoll et al, 2007). Both the EPM and FST are commonly used models of anxiety- and depressive-like behavior, respectively, as they have good predictive validity for anxiolytic and antidepressant drug efficacy (Duman, 2010). Another study showed that while nor-BNI decreases immobility in the FST, the KOR agonist U69 593 increases immobility (Mague et al, 2003). U69 593 increases ICSS thresholds in the lateral hypothalamus (Todtenkopf et al, 2004), which is indicative of anhedonic behavior, and the KOR agonist U50,488 produces a dose-dependent conditioned place aversions in rats (Bals-Kubik et al, 1989; Mucha and Herz, 1985) and increases distress-induced USVs in rat pups (Barr et al, 1994) in a nor-BNI reversible manner. Collectively, these data suggest that the DYN/KOR system contributes to negative affective states. These same negative affective states induced by DYN are seen in alcohol withdrawal. It was previously shown that alcohol-dependent rats in withdrawal demonstrate increased prodepressive-like behaviors (Williams et al, 2012), such as increased immobility in the FST. In addition, other studies have shown that rats spend increased time in the closed arm of the EPM during alcohol withdrawal (Valdez et al, 2004). Therefore, both alcohol withdrawal and KOR agonists increase negative affective states in rodents.
A primary question is whether the DYN/KOR system regulates alcohol withdrawal-induced negative affect. Studies have supported this link as alcohol withdrawal has been shown to increase DYN levels in both the nucleus accumbens (Acb) and amygdala (Lindholm et al, 2000). The same study also found increased DYN levels in the Acb of rats 30 min and 21 days after chronic alcohol exposure, indicating that chronic alcohol consumption leads to both short- and long-term changes in the DYN/KOR system. Interestingly, alcohol-preferring mice (C57BL/6j strain) have lower basal levels of prodynorphin mRNA and KORs in the Acb than alcohol-avoiding mice (Jamensky and Gianoulakis, 1997). Therefore, C57BL/6j mice may prefer alcohol because they experience less of the dysphoric effects produced by the DYN/KOR system following alcohol consumption. In addition, the selective KOR antagonist nor-BNI decreases escalated alcohol self-administration in alcohol-dependent rats (Walker and Koob, 2008; Walker et al, 2011; Nealey et al, 2011). This evidence suggests that alterations in the DYN/KOR system are important in the physiological and motivational alterations that occur in dependence and withdrawal, including negative affective states that occur in withdrawal (Chartoff et al, 2012).
Some evidence indicates that KOR agonists can attenuate place preferences for alcohol (Logrip et al, 2009), while other evidence indicates that KOR agonists can potentiate place preferences for alcohol (Sperling et al, 2010). This apparent contradiction can be explained based on methodological differences related to the timing of the KOR agonist administration (Walker et al, 2012). This study developed its timing parameters for KOR agonist infusion based on those shown to promote increased place preferences (Sperling et al, 2010), rather than decreased place preferences.
We hypothesized that cues associated with a DYN-induced negative affective state would increase alcohol consumption in the presence of that cue. Cues associated with negative affective states may pose as risk factors for the initial dysregulation of alcohol consumption and promote relapse. Aside from alcohol dependence and withdrawal, there is emerging evidence for the role of the DYN in fear conditioning, and relapse to drugs of abuse (Land et al, 2009). One study showed that nor-BNI could block fear reinstatement, while U50,488 could potentiate fear renewal (Cole et al, 2011). Another study found that the KOR antagonists nor-BNI and JDTic could attenuate conditioned fear in the fear-potentiated startle paradigm (Knoll et al, 2007). Other research has shown that the KOR agonist CI-977 increased alcohol preference and intake during alcohol deprivation in long-term alcohol-experienced rats (Holter et al, 2000), indicating that the DYN/KOR system may be important in relapse to drinking. Furthermore, our data implicate the DYN/KOR system in the initial dysregulation of alcohol self-administration via conditioned affective processes, pointing to activity in the DYN/KOR system serving as a risk factor for developing alcohol dependence.
While the cue associated with KOR activation did result in a significant increase in alcohol self-administration, it should be noted that the cued self-administration levels, when measured in g/kg, resulted in a ∼50% increase in alcohol consumption approximating 0.7 g/kg. Based on a previous systematic evaluation of orally administered alcohol and the resulting BALs (Walker and Ehlers, 2009), that level of alcohol consumption would be predicted to produce BALs in the range 70 mg/dl, which is slightly less than the 80 mg/dl threshold previously posited as the ideal goal for binge and dependence models (Walker et al, 2008). However, the current affective cue experiments were not designed to develop a binge-drinking model, but instead to investigate whether affective-linked cues could alter the consumption of alcohol in non-dependent animals, which they did. One explanation for this consumption level is that only the k1 receptor was activated, whereas the pharmacologically validated, but not cloned, k2 subtype of the KOR (Mansour et al, 1994) was not activated by U50,488. Thus, the consumption levels of<80 mg/dl could be due to U50,488 being a selective k1 ligand, whereas the endogenous DYNs could activate both k1 and k2 subtypes of the KOR to induce an increase in negative affect.
DYN's relationship with alcohol and other drugs stems from its ability to modulate other neurotransmitters such as dopamine (DA), GABA, glutamate, and serotonin. Activation of the DYN/KOR system has been found to modify DA in the Acb following cocaine (Thompson et al, 2000) and ethanol use (Doyon et al, 2006). The DYN/KOR system also modulates dopaminergic neurons projecting to the prefrontal cortex (Margolis et al, 2006). The KOR agonist, U69593, leads to a decrease in GABA and glutamate in the Acb (Hjelmstad and Fields, 2003); both neurotransmitters that are important in addiction (Walker and Koob, 2007; Walker and Ettenberg, 2001; Ezequiel and Nobre, 2012). Activation of the p38 mitogen-activated protein kinase system via stress-induced negative affect is KOR dependent (Bruchas et al, 2009), and p38 activation also regulates the serotonergic system (Samuvel et al, 2005), which has recently been shown to innervate the Acb and is susceptible to KOR regulation (Land et al, 2009). Thus, in a single area such as the Acb, KORs have the ability to modulate at least four different neurotransmitters, which identifies a need for research to identify the precise mechanism through which the DYN/KOR system can contribute to dysregulated alcohol consumption.
The results presented here are important because of the high comorbidity of alcoholism with affective disorders such as depression (Boden and Fergusson, 2011), as well as the interaction of negative affect and relapse to alcohol use (Higley et al, 2011). Furthermore, understanding how the DYN/KOR system is involved in alcohol withdrawal-induced negative affect and escalated alcohol drinking provides additional targets for pharmacotherapeutic development. In addition to the establishment that withdrawal-induced increases in 22-kHz USVs are sensitive to KOR antagonism and that KOR agonists induce 22-kHz USVs, we are the first to demonstrate that cues associated with the activation of KORs (and the resulting negative affective state) can dysregulate alcohol consumption in non-dependent animals. Importantly, the negative affective cue-induced increases in self-administration were attenuated by KOR antagonism. Collectively, these data indicate that the DYN/KOR system is important in the pathogenesis of alcohol withdrawal-induced negative affect and implicates the DYN/KOR system as a mediator of excessive alcohol consumption related to affective cues in non-dependent rats.
Acknowledgments
This work was supported, in part, by R01AA020394-01 from the National Institute on Alcohol Abuse and Alcoholism, RGA 11-014 from the Hope for Depression Research Foundation, and research grants from the WSU Alcohol and Drug Abuse Research Program awarded to BMW and ALB according to the State of Washington Initiative Measure No. 171. We thank Jessica Kissler, Mitchell Kissler, Georgia Schaffer, and Chelsea Smith for their technical assistance. The authors are particularly appreciative of the assistance provided by Dr Jaak Panksepp and Paolo Iacobucci with the technicalities related to USV measurement. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health, or the State of Washington.
The authors declare no conflict of interest.
References
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Barr GA, Wang S, Carden S. Aversive properties of the kappa opioid agonist U50,488 in the week-old rat pup. Psychopharmacology (Berl) 1994;113:422–428. doi: 10.1007/BF02245218. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106:906–914. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Richardson R, McNally GP. Kappa opioid receptors mediate where fear is expressed following extinction training. Learn Mem. 2011;18:88–95. doi: 10.1101/lm.2049511. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH. Models of depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- Ezequiel LL, Nobre MJ. The negative effects of alcohol hangover on high-anxiety phenotype rats are influenced by the glutamate receptors of the dorsal midbrain. Neuroscience. 2012;213:93–105. doi: 10.1016/j.neuroscience.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Higley AE, Crane NA, Spadoni AD, Quello SB, Goodell V, Mason BJ. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacology (Berl) 2011;218:121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Holter SM, Henniger MS, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology (Berl) 2000;153:93–102. doi: 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- Jamensky NT, Gianoulakis C. Content of dynorphins and kappa-opioid receptors in distinct brain regions of C57BL/6 and DBA/2 mice. Alcohol Clin Exp Res. 1997;21:1455–1464. [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T, Barry AE. Alcohol as a gateway drug: a study of US 12th graders. J Sch Health. 2012;82:371–379. doi: 10.1111/j.1746-1561.2012.00712.x. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Pohorecky LA. An air-puff stimulus method for elicitation of ultrasonic vocalizations in rats. J Neurosci Methods. 1995;62:1–5. doi: 10.1016/0165-0270(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA. Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol. 2009;43:359–365. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Meng F, Akil H, Watson SJ. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. Kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier, Academic Press: San Diego, CA; 2005. [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. PNAS. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Schindler AG, Martinelli E, Gustin RM, Bruchas MR, Chavkin C. Stress-induced activation of the dynorphin/kappa-opioid receptor system in the amygdala potentiates nicotine conditioned place preference. J Neurosci. 2012;32:1488–1495. doi: 10.1523/JNEUROSCI.2980-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. 2010.
- Thompson AC, Zapata A, Justice JB, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Harshberger E. Kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol Biochem Behav. 2012;102:44–47. doi: 10.1016/j.pbb.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol, Biochem Behav. 2009;91:560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Benzodiazepine modulation of opiate reward. Exp Clin Psychopharmacol. 2001;9:191–197. doi: 10.1037//1064-1297.9.2.191. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol. 2012;46:359–370. doi: 10.1016/j.alcohol.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and ‘relapse' in morphine-addicted rats. Psychopharmacologia. 1967;10:255–284. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]
- Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, et al. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in wistar rats. Psychopharmacology (Berl) 2012;223:75–88. doi: 10.1007/s00213-012-2691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]