Abstract
A number of addictions have been linked with decreased striatal dopamine (DA) receptor availability and DA release. Stress has a key role in cannabis craving, as well as in modulation of dopaminergic signaling. The present study aimed to assess DA release in response to a laboratory stress task with [11C]-(+)-PHNO positron emission tomography in cannabis users (CU). Thirteen healthy CU and 12 healthy volunteers (HV) were scanned during a sensorimotor control task (SMCT) and under a stress condition using the validated Montreal imaging stress task (MIST). The simplified reference tissue model (SRTM) was used to obtain binding potential (BPND) in striatal subdivisions: limbic striatum (LST), associative striatum (AST), and sensorimotor striatum (SMST). Stress-induced DA release (indexed as a percentage of reduction in [11C]-(+)-PHNO BP ND) between CU and HV was tested with analysis of variance. SMCT BPND was significantly higher in CU compared with HV in the AST (F=10.38, p=0.003), LST (F=4.95, p=0.036), SMST (F=4.33, p=0.048), and whole striatum (F=9.02, p=0.006). Percentage of displacement (change in BPND between SMCT and MIST PET scans) was not significantly different across groups in any brain region, except in the GP (−5.03±14.6 in CU, compared with 6.15±12.1 in HV; F=4.39, p=0.049). Duration of cannabis use was significantly associated with stress-induced [11C]-(+)-PHNO displacement by endogenous DA in the LST (r=0.566, p=0.044), with no effect in any other brain region. In conclusion, despite an increase in striatal BPND observed during the control task, chronic cannabis use is not associated with alterations in stress-induced DA release.
Keywords: psychosocial stress, dopamine, cannabis use disorder, positron emission tomography, [11C]-(+)-PHNO
INTRODUCTION
Cannabis is the most widely used illicit substance around the world (Bauman and Phongsavan, 1999; Kleber and Dupont, 2012), and the one most commonly used by people with psychosis (Menezes et al, 1996; van Os et al, 2011). Although many individuals report recreational use of cannabis, a third of individuals experimenting with this drug progress to develop abuse (Gruber and Pope, 2002), and of those who try to quit, an estimated 71% fail within 6 months (Moore and Budney, 2003). Despite the widespread use (World Drug Report 2012), cannabis produces dependence less readily than most other illicit drugs. For example, while 15% and 24% of those who try cocaine and heroin, respectively, develop dependence, only 9% of those who try cannabis develop dependence. Low striatal dopamine (DA) receptor (D2/3) availability and low amphetamine-induced DA release in the ventral striatum have been observed with several substance-use disorders, including alcoholism (Martinez et al, 2005; Volkow et al, 1996), heroin (Martinez et al, 2012), cocaine (Volkow et al, 1993), and methamphetamine (Volkow et al, 2001) use. Less is known about the changes in DA transmission in chronic cannabis use.
The active component of cannabis, Δ9-THC, is known to cause DA release in both the nucleus accumbens and medial prefrontal cortex in animals (Chen et al, 1990; Tanda et al, 1997), and there is robust evidence that cannabis can exacerbate pre-existing psychotic symptoms or trigger their re-emergence in those with psychosis (D'Souza et al, 2004; Mathers and Ghodse, 1992). Interestingly, an early neuroimaging observation that cannabis increased DA release in a drug-free patient with schizophrenia (Voruganti et al, 2001) has been followed by more recent controlled studies showing that Δ9-THC is not associated with DA release in humans (Stokes et al, 2008), despite causing the expected behavioral changes. Consistent with these publications, two recent studies reported no baseline difference in D2 availability between cannabis users (CU) and healthy volunteers (HV) (Sevy et al, 2008; Urban et al, 2012), with no effect of cannabis use on amphetamine-induced DA release (Urban et al, 2012).
Stress is a potent trigger of relapse in addiction (Sinha, 2011; Sinha et al, 2011) and an important risk factor for chronic use of cannabis (see Sinha (2011) for review). Furthermore, cross-sensitization between Δ9-THC and stress have been recently reported in animals (Suplita et al, 2008), suggesting that the putative physiological and psychological effects of cannabis could be potentiated in individuals experiencing adverse environmental stress. Epidemiologically, cannabis use at a young age (Andreasson et al, 1987; Moore et al, 2007) and early experience of adverse (stressful) life events have been associated with increased risk of developing schizophrenia (Read et al, 2005), implicating cannabis use and sensitivity to stress as risk factors for psychiatric disorders, particularly schizophrenia. Despite the wealth of research suggesting a strong relationship between stress response and substance use disorders (Robinson and Berridge, 2000), no studies have directly investigated the relationship between the neurochemical response to stress and chronic cannabis use.
The unique binding profile of the DA agonist radiotracer [11C]-(+)-PHNO used in this study includes preferential binding to the D3 DA receptor subtype, which increases its sensitivity and allows quantification of changes that a D2/3 antagonist radiotracer such as [11C]raclopride may not detect. The DA D3 receptor subtype has lately been the subject of intense interest, due to its postulated involvement in the biochemical mechanism of drug dependence and relapse. Its preferential localization in the mesolimbic DA system in rats and humans, as well as animal studies showing sensitivity of cocaine self-administration to D3 antagonists and partial agonists, suggest that the D3 subtype is a key factor in the regulation of motivation, reward, and emotion (Murray et al, 1994; for review see Ikemoto and Panksepp, 1999) and thus likely involved in the neurochemical processes underlying substance abuse and dependence.
Based on the potential cross-sensitization between stress and cannabis, and our ability to quantify D2 and D3 receptor availability in-vivo, here we propose to test the hypothesis that CU during early abstinence have altered dopaminergic responses (increased [11C]-(+)-PHNO displacement) to a validated psychosocial stress challenge (Pruessner et al, 2004).
MATERIALS AND METHODS
Subjects
This study was approved by the local Research Ethics Board at the Centre for Addiction and Mental Health (CAMH) and University of Toronto. Thirteen CU with no current or past psychotic disorder and 12 matched HV (assessed by a psychiatrist using the SCID) were recruited from the community through online postings. All subjects signed written informed consent after the study procedures were fully explained.
CU
Inclusion criteria. (1) Male or female between 18 and 40 years old; (2) capacity to provide informed consent in English; (3) no family history (in first-degree relatives) of schizophrenia, schizoaffective disorder, schizotypal personality disorder, or any other disorder involving psychotic symptoms; and (4) regular cannabis use at least three times weekly or meeting DSM-IV criteria for cannabis dependence and positive drug screen both at screening and the days of the positron emission tomography (PET) scans.
Exclusion criteria. (1) Current or lifetime Axis I disorder; 2) current or lifetime treatment with psychotropic medication; (3) substance abuse, other than cannabis, in the past 6 months; and (4) metal implants that would preclude magnetic resonance imaging (MRI).
HV
For HV, inclusion criteria were items (1), (2), and (3), and exclusion criteria were (1)–(4) with item (3) not allowing cannabis use more than five times in the lifetime. Additionally, HV must not have had any personal or family history (first degree relative) of any axis 1 disorder.
Psychopathology Measures
All subjects completed the SCID questionnaire as administered by a trained psychiatrist (RM). In addition, they completed (1) the 12-item Marijuana Craving Questionnaire (MCQ) consisting of four subscales: Compulsivity (evaluating the inability to control cannabis use), Emotionality (evaluating the anticipation of relief from negative mood or withdrawal), Expectancy (evaluation of anticipation of positive outcomes) and Purposefulness (intention and planning to use cannabis for positive outcomes) (Heishman and Singleton, 2006); 2) the 24-item Parental Bonding Instrument (Parker et al, 1979) scale, which provides information on parental bonding for subject's mother and father, given its effects on stress-induced DA changes in HV (Pruessner et al, 2004); 3) the State-Trait Anxiety Inventory, state version (SAQ; (Spielberger et al, 1983) and visual analog scales administered immediately before and after each PET scan.
Montreal Imaging Stress Task
All subjects underwent two PET scans at the same time of the day on two different days, at least 5 days apart: first while undergoing a sensorimotor control task (SMCT) and second while undergoing the Montreal imaging stress task (MIST). To reduce the novelty of the task for the first scan, all subjects performed the non-stressful (SMCT) version of the task before the PET imaging sessions. SMCT scan was always performed first, in order to avoid any residual effects of the stress task. Psychosocial stress was induced using the MIST, which has been validated in previous fMRI and PET studies (Lederbogen et al, 2011; Mizrahi et al, 2012; Pruessner et al, 2004). Briefly, subjects performed mental arithmetic on a computer screen that also displays information about the total number of errors, expected average number of errors, time spent on the current problem, and performance feedback for each problem (correct, incorrect, timeout). During the stress condition, subjects completed six 6-min block segments of arithmetic while lying in the scanner. The time constraint is adjusted to be slightly beyond each individual's abilities. Because of the manipulation of the difficulty level, the average performance was set at 20–30% correct answers. In addition, subjects were given negative verbal feedback by the investigator for ∼2 min between each block, telling them that they need to improve their performance to reach minimum performance requirements. Before the stress task, subjects performed the sensory motor control PET session (non-stress), a similar arithmetic task but without time constraints or negative verbal feedback. In all the experiments, the control or stress task was started ∼6–8 min before tracer injection, with 6 min of mathematical questions and ∼1–2 min for either neutral or negative feedback and salivary cortisol measurement.
Physiological Measures
Saliva samples were collected every 12 min throughout the experiment. Saliva-derived cortisol was analyzed using a time-resolved fluorescence immunoassay (Dressendorfer et al, 1992) and the area under the curve (g/dl/min) was calculated for each subject and each scanning session as described in Pruessner et al (2003).
Image and Data Analyses
MRI acquisition. Subjects undertook a standard fast spin echo T1 (FSPGR, TE=5.3–15, TR=8.9–12, FOV=20 cm, matrix=256 × 256, slice thickness=1.5, NEX=1) and a proton density (TE=17, TR=6000, FOV=22 cm, matrix=256 × 256, slice thickness=2 mm, NEX=2) brain MRI acquired on a 1.5T Signa-GE scanner. These images were used for the analysis of the PET scans and to rule out structural lesions.
PET acquisition. Radiosynthesis of [11C]-(+)-PHNO was performed as previously described (Wilson et al, 2005). Each subject was administered ∼9–10 mCi of tracer (Table 1) and scanned for 90 min. Data were acquired using a high-resolution PET CT scanner, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN, USA) which measures radioactivity in 81 brain sections with a thickness of 2.0 mm each. A custom-fitted thermoplastic mask was made for each subject and used with a head fixation system during PET acquisition to minimize head movement. The images were reconstructed with a 2D filtered back projection algorithm with a ramp filter at Nyquist cutoff frequency.
Table 1. Demographics of the Study Groups.
| Demographics (SD) | Healthy volunteers (n=12) | Cannabis users (n=13) | |
|---|---|---|---|
| Age (years) | 26.08 (3.8) | 24.23 (4.9) | |
| Gender | Male | 7 | 6 |
| Female | 5 | 7 | |
| Mother PBI | 33.75 (6.9) | 42.62 (7.8) | |
| Smoking status | Non-smoker | 11 | 10 |
| Smoker | 1 | 3 | |
| Cannabis use | Lifetime use (joints) | n/a | 7859.85 (10566.6) |
| Years of cannabis use | n/a | 9.23 (4.9) | |
| Age at first use (years) | n/a | 14.92 (1.3) | |
| Number of joints per week | SMCT | n/a | 15.50 (15.5) |
| MIST | n/a | 14.55 (13.1) | |
| Hours since last joint smoked | SMCT | n/a | 9.18 (5.6; range 2–18.5) |
| MIST | n/a | 11.5 (7.5; range 1–21.5) | |
| Cannabinoids value (μg/l) | SMCT | n/a | 1558.7 (2934.2) |
| MIST | n/a | 2130.2 (3550.3) |
Presented as means and standard deviation (SD) showing no significant differences in age (t=1.042, df=1,23, p=0.308), gender (chi-square 0.371, p=0.543) and smoking status (chi-square 1.009, p=0.315). Cannabis users had a significantly higher mother pbi values (t=−2.998, df=1,23, p=0.006). In cannabis users, there was no significant difference in any cannabis use parameters between the control (smct) and stress (mist) tasks.
PET data analysis. Regions of interest (ROIs) were delineated using an automated method implemented in an in-house software (ROMI), abolishing subjectivity in manual ROI drawing (Rusjan et al, 2006). We delineated the globus pallidus (GP) and substantia nigra (SN) as per Tziortzi et al (2011). Time activity curves from the ROIs were obtained from the dynamic [11C]-(+)-PHNO PET images. PET data were evaluated in the striatal subdivisions based on their functional connections to the limbic, frontal executive, and motor brain regions: limbic striatum (LST, including the ventral striatum), associative striatum (AST, including the pre-dorsal putamen, pre-dorsal caudate, and the post-caudate striatum), and sensorimotor striatum (SMST, post-dorsal putamen) (Martinez et al, 2003). Activity from the right and left regions were averaged together, and a weighted average (weighted by subregion volume) was used to derive binding potential (BPND) with respect to the non-displaceable compartment in the brain for [11C]-(+)-PHNO (cerebellar cortex) applying the simplified reference tissue model (SRTM; Ginovart et al, 2007). SRTM provides an estimate of the BPND of the radiotracer, which is proportional to the more fundamental parameters of receptor number (Bmax) and affinity (1/Kd). Finally, [11C]-(+)-PHNO displacement was calculated as 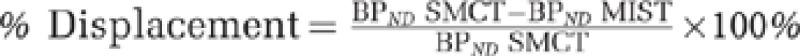 .
.
Voxel-wise images were generated using a data-driven method with reference region implemented in DEPICT (Gunn et al, 2002). Each parametric map was spatially normalized to an anatomical template (Montreal Neurological Institute) using Statistical Parametric Mapping (SPM) normalization and co-registration tools. Once in the same space, BPND maps were used to assess significant contrast between conditions (SMCT vs MIST) in each group (HV, CU) at the voxel level using an implicit mask of BPND>0.3. Difference between HV and CU BPND was evaluated by an independent t-test with FWE correction, as implemented in SPM5 (www.fil.ion.ucl.ac.uk/spm).
Statistical Analysis
Independent t-test and Chi-square tests were used to detect difference in demographics and injection parameters between groups. Once the SRTM BPND was obtained for each striatal region, changes between the SMCT and MIST BPND values were evaluated using paired t-tests. Main hypotheses were tested using analysis of variance to investigate differences between HV and CU in SMCT BPND and stress-induced DA release indexed as [11C]-(+)-PHNO % displacement between groups for each ROI. Subjective perceived stress, PBI measures, MCQ, length of cannabis use, and age of onset, as well as stress-induced cortisol release (quantified as the difference in area under the curve between SMCT and MIST scan) were related to PET data with linear regression analyses. All analyses were two tailed with the conventional α=0.05. ANCOVA with SMCT BPND as covariate was used to compare percentage of displacement between the CU and HV and obtain estimated marginal means for each group.
RESULTS
Both groups were comparable for demographics (Table 1) and scan parameters (Table 2). All CU and none of the HV met criteria for cannabis dependence and tested positive for cannabis both at screening and on the day of the scans. SMCT and MIST scans were performed on average 12.8±10.1 (HV) and 14.4±7.8 days apart (CU). Out of 13 CU, 7 had no exposure to other drugs, while the remaining 6 reported past occasional use, with no dependence, of MDMA (n=4), cocaine (n=1), hallucinogenic mushrooms (n=3), LSD (n=1), heroin (n=1), and ketamine (n=1). As expected, all subjects performed significantly worse on the MIST (number of errors 42.63±12.2 and 34.92±12.9 for HV and CU, respectively) than in the SMCT (errors 5.23±3.4 and 7.83±5.6 for HV and CU, respectively; F=104.67, p<0.001 and F=48.25, p<0.001 for HV and CU, respectively), showing that the MIST was able to adapt to the level of performance of each person and produce a tailored programmed failure within each group. We found no significant difference in the number of errors committed by CU or HV during either the MIST (t=1.531, p=0.139) or SMCT (t=−1.388, p=0.178). Following the MIST scan, comparison of post-scan SAQ outcomes revealed that all subjects were less calm (F=29.99, df=4,42, p<0.001) and less satisfied (F=34.35, df=1,42, p<0.001) but more tense (F=21.14, df=1,42, p<0.001), more strained (F=24.99, df=1,42, p<0.001), upset (F=47.38, df=1,42, p<0.001), and confused (F=23.62, df=1,42, p<0.001) compared with the SMCT scan, suggesting that the stress paradigm was effective in eliciting an emotional response. These differences between SMCT and MIST SAQ held true for both the HV and CU subjects independently. Total SAQ scores assessed immediately following the scans were significantly elevated following the MIST as compared with the SMCT (F=72.80, df=1,42, p<0.001) and displayed a trend level difference between HV and CU (F=4.07, df=1,20, p=0.057).
Table 2. PET Scan Parameters Showing No Significant Difference Between Groups or Scan Sessions.
| Parameter |
Healthy volunteers (n=12) |
Cannabis users (n=13) |
||
|---|---|---|---|---|
| Control task | Stress task | Control task | Stress task | |
| Activity injected (mCi) | 9.23±1.65 | 9.73±0.94 | 9.98±0.82 | 10.12±0.43 |
| Specific activity (Ci/mmol) | 1019.8±454 | 1135.7±445 | 1293.2±500 | 1338.3±444 |
| Mass injected (μg) | 2.50±0.69 | 2.32±0.62 | 2.09±0.57 | 2.06±0.67 |
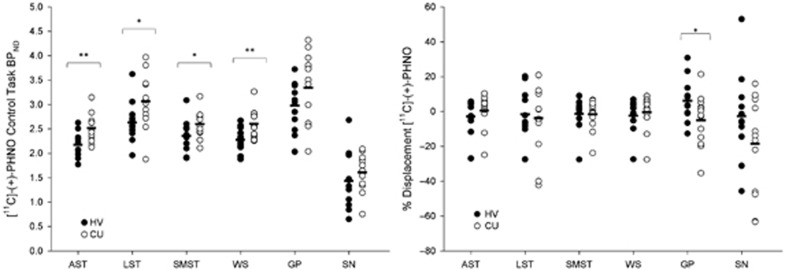
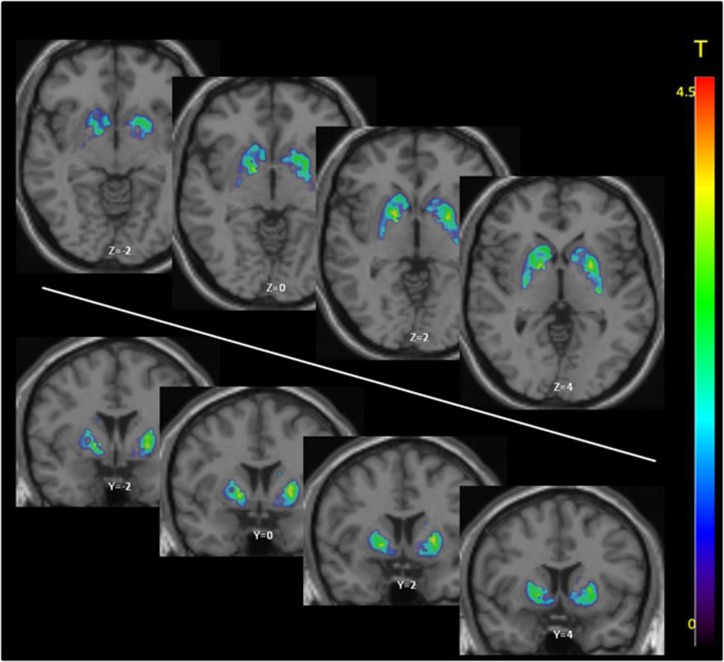
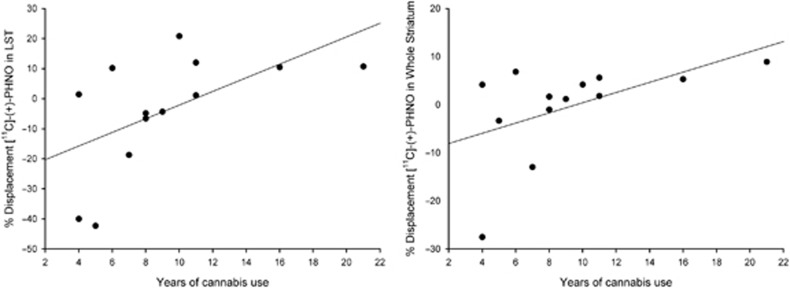
SMCT BPND was significantly different between groups in the AST (F=10.38, p=0.004), LST (F=4.95, df=1,23, p=0.036), SMST (F=4.33, df=1,23, p=0.049), and whole striatum (F=9.02, df=1,23, p=0.006), with HV having lower BPND as compared with CU in these regions but no difference in D3-rich regions (GP (F=2.12, df=1,23, p=0.159) and SN (F=0.89, df=1,23, p=0.354)) (Figure 1, Table 3). Similarly, voxelwise data comparing SMCT BPND parametric maps between HV and CU showed two clusters of significantly higher BPND in CU compared with HV at the level of the right caudate/GP and the left putamen (Figure 2). Percentage of displacement was not significantly different across groups in any brain region (Figure 1), except in the GP (F=4.39, df=1.23, p=0.049), with CU having less stress-induced changes (CU −5.03%) relative to controls (HV 6.15%). Voxelwise data confirmed the lack of difference between conditions. Interestingly, displacement in the LST was significantly different between CU (3.99%) and HV (−9.11%), when LST SMCT BPND was taken as a covariate. No other striatal region showed this effect. Years of cannabis use showed significant correlation with stress-induced [11C]-(+)-PHNO displacement in the LST (r=0.566, p=0.04; Figure 3) and a trend level correlation with [11C]-(+)-PHNO displacement in the entire striatum (r=0.522, p=0.067); however, these correlations are lost when age was added as a covariate. When SMCT BPND of the respective regions are taken as a covariate, the correlation with years of use in the LST remained significant (r=0.789, p=0.002). No correlation was observed between the PET-derived SMCT BPND, MIST BPND or percentage of displacement and PBI scores (maternal, paternal, or total subsets) or total lifetime cannabis use.
Figure 1.
Left: Control task (SMCT) BPND in HV (filled circles) and CU (empty circles). Right: [11C]-(+)-PHNO displacement in response to the MIST between HV and CU. *p<0.05.
Table 3. Binding Potential (BPND) of [11C]-(+)-PHNO Obtained During the Control (SMCT) and Stress (MIST) Task, and Tracer Displacement in Striatal Subregions, Whole Striatum, Globus Pallidus and Substantia Nigra of HV, CU and CU Who Had No Cannabis Use in the 8 h Preceding the PET Scans.
| ROI | SMCT BPND | MIST BPND | % Displacement | t | p | |
|---|---|---|---|---|---|---|
| HV | AST | 2.17±0.25 | 2.23±0.31 | −2.87±9.2 | −1.156 | 0.272 |
| LST | 2.63±0.41 | 2.64±0.23 | −1.69±13.4 | −0.052 | 0.959 | |
| SMST | 2.35±0.32 | 2.43±0.39 | −1.35±9.5 | −1.063 | 0.311 | |
| Striatum | 2.28±0.24 | 2.33±0.30 | −2.41±9.1 | −0.935 | 0.370 | |
| GP | 2.98±0.51 | 2.80±0.62 | 6.15 ±12.1 | 1.932 | 0.080 | |
| SN | 1.43±0.57 | 1.46±0.67 | −2.73±24.4 | −0.343 | 0.738 | |
| CU | AST | 2.52±0.28 | 2.50±0.32 | 0.48±9.4 | 0.266 | 0.795 |
| LST | 3.06±0.54 | 3.11±0.44 | −3.85±19.5 | −0.346 | 0.735 | |
| SMST | 2.60±0.27 | 2.64±0.31 | −1.69±8.5 | −0.674 | 0.513 | |
| Striatum | 2.60±0.30 | 2.61±0.34 | −0.40±9.9 | −0.066 | 0.948 | |
| GP | 3.34±0.72 | 3.45±0.60 | −5.03±14.6 | −0.864 | 0.404 | |
| SN | 1.61±0.38 | 1.87±0.58 | −18.51±28.1 | −2.188 | 0.049* | |
| CU >8 h | AST | 2.41±0.24 | 2.48±0.34 | −2.66±10.9 | −0.695 | 0.510 |
| LST | 2.92±0.54 | 3.12±0.37 | −10.04±22.5 | −0.935 | 0.381 | |
| SMST | 2.49±0.22 | 2.60±0.34 | −4.56±9.5 | −1.359 | 0.216 | |
| Striatum | 2.49±0.23 | 2.58±0.35 | −3.80±11.3 | −0.924 | 0.386 | |
| GP | 3.13±0.76 | 3.29±0.64 | −7.58±18.0 | −0.819 | 0.440 | |
| SN | 1.48±0.41 | 1.81±0.60 | −26.27±28.3 | −2.334 | 0.052 |
*p<0.05, comparison between the MIST and SMCT BPND value, paired-samples t-test.
Figure 2.
Voxelwise comparison between SMCT difference between HV and CU. Images were generated using DEPICT, with voxels showing significantly higher BPND in CU compared with HV (FWE-corrected, p<0.05) overlaid on standard MRI template. Two clusters of significant voxels were detected: a cluster encompassing 455 voxels in the right hemisphere with significant voxels in the right globus pallidus (MNI coordinates [16, 0, −2] corrected p=0.032, uncorrected p=0.005) and right caudate (455 voxels, MNI coordinates [14, 10, 14] corrected p=0.032, uncorrected p=0.005); and a 489 voxel cluster in the left putamen (MNI coordinates [−26,−12, 8] corrected p=0.024 uncorrected p=0.003).
Figure 3.
Relationship between years of use and percentage of displacement of [11C]-(+)-PHNO, showing significant correlation in LST (left panel; r=0.566, p=0.04) and a trend level relationship in the entire striatum (right panel; r=0.522, p=0.067). The correlation with years of use in the LST remains significant (r=0.789, p=0.002) when the percentage of displacement is corrected for the LST SMCT BPND.
PET scans took place on average 9.18±5.6 and 11.5±7.5 h (SMCT and MIST, respectively) since last cannabis use, with 5 of 13 CU subjects reported using cannabis <8 h before the SMCT or MIST scan (Table 1). The PET imaging outcomes from the sample excluding these subjects were analyzed separately to exclude any acute potential effects of cannabis on [11C]-(+)-PHNO binding (Table 3). No differences in BPND (SMCT or MIST) or tracer displacement were found between HV and this CU group. Additionally, no correlation was observed between hours since last cannabis use and any of the PET outcomes (BPND or displacement).
We also explored the effects of cannabis use as assessed with the MCQ on both BPND and stress-induced BPND change. We only found a significant correlation between change in compulsivity to use cannabis following the control scan and GP BPND (r=0.644, p=0.018) during SMCT. On the day of the stress scan, greater pre- and post-scan MCQ emotionality were significantly associated with high LST BPND values (r=0.581, p=0.037 and r=0.563, p=0.045, respectively). Lower stress-induced [11C]-(+)-PHNO displacement in the GP (r=−0.656, p=0.015) and trend level in the SN (r=−0.513, p=0.073) were significantly associated with greater MCQ compulsivity, as assessed before the MIST scan. A greater magnitude of cortisol response to stress was related to the greater displacement of [11C]-(+)-PHNO in the AST (r=0.426, p=0.034), but not in any other regions, when all subjects are considered. Stress-induced cortisol release was not significantly different between groups (HV −14.76±19.3, CU 2.80±23.4; F=4.15, p=0.053). Additionally, in CU, increased stress-induced cortisol release correlated with higher change in MCQ expectancy and purposefulness (r=0.809, p=0.001 and r=0.736, p=0.004, respectively).
DISCUSSION
Using [11C]-(+)-PHNO PET imaging and a validated psychosocial stress paradigm to measure tracer displacement, we observed no difference in stress-induced DA release between HV and chronic CU. Our findings support previous observations by Urban et al (2012), who found no difference between CU and HV in [11C]-raclopride binding following an acute amphetamine challenge. We did observe increased BPND in D2-rich areas in CU compared with HV during the performance of the control (SMCT) task. Taken together, these findings suggest that chronic cannabis use does not show a different DA response to stress.
DA agonist radiotracer [11C]-(+)-PHNO demonstrates an estimated ∼20-fold higher affinity for D3 vs D2 in vivo (Narendran et al, 2006b; Rabiner et al, 2009). Detected regional signal following [11C]-(+)-PHNO administration is therefore a function of the differential affinity as well as concentration of D3 vs D2 receptors in a given region. In D3-rich regions like the GP, D3 binding is thought to account for ∼67% of the [11C]-(+)-PHNO signal, while the SN represents 100% D3 binding; thus, its signal can be a sole marker of D3 effects (Searle et al, 2010; Tziortzi et al, 2011). In other regions like the dorsal striatum (caudate, AST and putamen, SMST), the relative concentration of D2 receptors is much higher and, therefore, only a small component of the signal, 10–40%, is attributable to D3 (Searle et al, 2010; Tziortzi et al, 2011). Consequently, observed elevated BPND suggest that chronic cannabis use may result in increased receptor availability in D2-rich regions (AST, LST, SMST and the whole striatum), while the D3-rich regions are relatively unaffected. However, any conclusions derived from the comparison of SMCT BPND measurements between groups are confounded by the fact that the SMCT scan was obtained while the subjects were performing a cognitive task, which cannot be considered a ‘true' baseline state. In contrast to our findings, increased [11C]-(+)-PHNO BPND was reported in D3-rich areas of chronic methamphetamine users (Boileau et al, 2012), which suggests that chronic use of dopaminergic drugs affects the brain in a distinct fashion from cannabis. Nevertheless, it is worth considering that although the presence of D3 autoreceptors in all SN DA neurons is well established (Diaz et al, 2000), there is still no clear evidence of a physiological role of these receptors in SN (Davila et al, 2003). The higher [11C]-(+)-PHNO BPND in CU during the control SMCT task in D2-rich regions could be interpreted as an increase in D2/3 receptor availability, reflecting either lower levels of endogenous DA (while performing a cognitive task, SMCT) or upregulation of D2 receptors. Recent studies evaluating the effect of chronic exposure to THC in animals using 3H-labeled version of (+)-PHNO have observed increased tracer binding, accompanied by increased D2 and D3 densities in the midbrain (Ginovart et al, 2012). Previous reports using [11C]-raclopride imaging in humans have demonstrated no change in (true) baseline striatal D2/3 receptor availability in chronic CU (Sevy et al, 2008; Stokes et al, 2012; Urban et al, 2012).
We found that the MIST was able to elicit a significant emotional stress response as indicated by the significantly elevated total SAQ scores following the MIST as compared with the SMCT. However, despite the increase in subjective report of stress following the MIST, we did not find a significant change of [11C]-(+)-PHNO BPND between SMCT and MIST scans in the HV (Table 3). Our finding is consistent with previous observations using the same psychosocial stress paradigm (Pruessner et al, 2004), in which the MIST elicited a significant change in D2/3 receptor binding but only in certain type of individuals, such as those with a history of low maternal care or with certain personality types (Suridjan et al, 2012).
The lack of effect on stress-induced DA release distinguishes chronic cannabis use disorder from alcoholism, stimulant, and heroin dependence, which have all been shown to reduce amphetamine-induced DA release (Martinez et al, 2007; Martinez et al, 2012; Volkow et al, 1993; Volkow et al, 1996). However, our findings of lack of difference in tracer displacement in response to stress are remarkably consistent with a recent study in chronic CU (Urban et al, 2012). Previous studies combining [11C]raclopride PET and in vivo microdialysis in rhesus monkeys have shown that very large increases in synaptic DA concentrations were reflected in comparatively smaller changes in [11C]raclopride binding (Breier et al, 1997; Endres et al, 1997). [11C]-(+)-PHNO is more sensitive to displacement by synaptic DA (Ginovart et al, 2006; Narendran et al, 2006a; Shotbolt et al, 2012). However, the relationship between synaptic DA outflow and [11C]-(+)-PHNO binding is unknown. It is therefore still possible that there was a significant difference in stress-induced DA release between the CU and healthy controls that did not translate into a significant difference in [11C]-(+)-PHNO percentage of displacement.
We observed no correlation between the age of onset of cannabis use and displacement of [11C]-(+)-PHNO in any of the regions studied. We did, however, find that the length of cannabis use (in years) correlated with stress-induced tracer displacement in the LST and whole striatum. This observation topographically corresponds to the dopaminergic alterations commonly reported in substance abuse, but the relationship, if replicated in a larger cohort, would suggest that chronic cannabis use sensitizes, rather than blunts, striatal dopaminergic signaling. Correlation between DA receptor availability (indexed as SMCT BPND) in LST with MCQ emotionality (cannabis use to alleviate negative mood) provides additional support for the involvement of ventral striatum in regulating aspects of substance use. Interestingly, significant elevations in the emotionality component of the MCQ has also been observed following the Trier Social Stress Task (McRae-Clark et al, 2011), while recent experience monitoring studies have linked social anxiety with cannabis cravings outside the laboratory environment (Buckner et al, 2012). The correlation between cortisol release and expectancy and purposefulness measures of the MCQ validates the linkage between stress and cannabis craving. Although a recent large prospective population study reported reduced cortisol stress response to a stress task in CU (van Leeuwen et al, 2011), we observed no difference in cortisol release between groups, likely due to the sample size.
Some limitations are typical in neurochemical brain imaging studies in humans. First, while abstinence from cannabis was not an inclusion criteria, out of 13 scanned subjects, 5 subjects have used cannabis in the 8 h before the SMCT or MIST scan. Although this acute use could conceivably affect the stress-response reported here, previous studies have shown, at best, a modest effect of acute cannabinoid administration on DA release (Bossong et al, 2009; Stokes et al, 2012). When those five subjects are excluded from the analysis, the main finding, that is the lack of a difference between CU and HV on the stress-induced DA release, remains unchanged (except for the loss of significance in the GP displacement; Table 3). Thus, either 2–3 weeks withdrawal (Urban et al, 2012) or current chronic use seem to produce similar effects on the DA system. The inclusion of an additional group composed of former CU with 2–3 months of abstinence would possibly rule out intoxication or short-term withdrawal effects and thus help differentiate between the ‘trait' and ‘state' aspects of cannabis use and their effect on the DA system. Second, as commonly observed with CU, 6 out of 13 CU reported past use of other drugs. Excluding these subjects from the study did not affect the main finding (except again for the GP displacement which is lost, suggesting a possible weak effect). Third, given that our HV group did not show a significant DA response to stress, an assertion of a ‘blunted' or ‘sensitized' DA response to stress cannot be entertained. Fourth, baseline estimates of D2 and D3 binding were not ‘true' baseline measures, as subjects were performing the cognitive (non-stressful) version of the MIST. Fifth, it has recently been suggested that [11C]-(+)-PHNO may not be at tracer dose in the D3-rich regions, which would hinder its accurate quantification (Gallezot et al, 2012; Rabiner and Laruelle, 2010). However, our significant findings were relatively specific to D2 regions, and scan sessions (SMCT and MIST) were carried out on separate days, at least 5 days apart (except for one subject who could not come on two occasions). Additionally, the nature of the stress task did not allow for the order of the scans to be randomized. With all the subjects in the study, SMCT control task was done during the first PET scan, followed by the stress task (MIST) during the second scan. The use of the social stress paradigm to induce DA release is appropriate for use in populations who have not been exposed to dopaminergic drugs and is therefore a safe alternative compared with studies performing imaging following amphetamine or methylphenidate administration. Finally, the lack of effects in the ventral striatum of the acute stress challenge may be related to the joint [11C]-(+)-PHNO binding to D2 and D3 receptors, which may increase sampling variability in this brain region (Narendran et al, 2006b).
In conclusion, the present work supports previously published observations that chronic cannabis use, unlike other addictions, does not affect DA release. Differences in BPND between the CU and HV during the control task provide support for future in vivo studies quantifying changes in striatal D2 receptor expression in chronic cannabis use. Additionally, our findings in the SN and GP suggest the need for further examination using larger samples of changes in D3 receptor expression and availability in cannabis use and in other substance-use disorders.
Acknowledgments
This work is supported by an operating grant from the Ontario Mental Health Foundation (OMHF). RM is supported by the New Investigator Award from Canadian Institute of Health Research (CIHR) and the OMHF New Investigator Fellowship. The initial pilot data was also supported by the Connaught New Staff matching grant and the Dean's fund from the University of Toronto. TPG was supported by the Chair in Addiction Psychiatry at the University of Toronto. We also wish to thank Armando Garcia, Winston Stableford, Peter Bloomfield and Matthew Mitchell (Siemens Molecular Imaging, Knoxville, TN, USA) for their technical assistance, Dr. Isabelle Boileau for valuable discussion, and Dr. Jens Pruessner for the development of the MIST.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Bauman A, Phongsavan P. Epidemiology of substance use in adolescence: prevalence, trends and policy implications. Drug Alcohol Depend. 1999;55:187–207. doi: 10.1016/s0376-8716(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Crosby RD, Wonderlich SA, Schmidt NB. Social anxiety and cannabis use: an analysis from ecological momentary assessment. J Anxiety Disord. 2012;26:297–304. doi: 10.1016/j.janxdis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. D3 dopamine autoreceptors do not activate G-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci. 2003;23:5693–5697. doi: 10.1523/JNEUROSCI.23-13-05693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Swaminathan S, DeJesus OT, Sievert M, Ruoho AE, Murali D, et al. Affinities of dopamine analogs for monoamine granular and plasma membrane transporters: implications for PET dopamine studies. Life Sci. 1997;60:2399–2406. doi: 10.1016/s0024-3205(97)00300-7. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, et al. Affinity and selectivity of [11C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, et al. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Tournier BB, Moulin-Sallanon M, Steimer T, Ibanez V, Millet P. Chronic delta(9)-tetrahydrocannabinol exposure induces a sensitization of dopamine D(2/3) receptors in the mesoaccumbens and nigrostriatal systems. Neuropsychopharmacology. 2012;37:2355–2367. doi: 10.1038/npp.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, et al. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab. 2007;27:857–871. doi: 10.1038/sj.jcbfm.9600411. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG. Marijuana use among adolescents. Pediatr Clin North Am. 2002;49:389–413. doi: 10.1016/s0031-3955(01)00011-6. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Gunn SR, Turkheimer FE, Aston JA, Cunningham VJ. Positron emission tomography compartmental models: a basis pursuit strategy for kinetic modeling. J Cereb Blood Flow Metab. 2002;22:1425–1439. doi: 10.1097/01.wcb.0000045042.03034.42. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG. Assessment of cannabis craving using the Marijuana Craving Questionnaire. Methods Mol Med. 2006;123:209–216. doi: 10.1385/1-59259-999-0:209. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Kleber HD, Dupont RL. Physicians and medical marijuana. Am J Psychiatry. 2012;169:564–568. doi: 10.1176/appi.ajp.2012.12030373. [DOI] [PubMed] [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474:498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, et al. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71:192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mathers DC, Ghodse AH. Cannabis and psychotic illness. Br J Psychiatry. 1992;161:648–653. doi: 10.1192/bjp.161.5.648. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, et al. Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology (Berl) 2011;218:49–58. doi: 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes PR, Johnson S, Thornicroft G, Marshall J, Prosser D, Bebbington P, et al. Drug and alcohol problems among individuals with severe mental illness in south London. Br J Psychiatry. 1996;168:612–619. doi: 10.1192/bjp.168.5.612. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71:561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. J Subst Abuse Treat. 2003;25:85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Murray AM, Ryoo HL, Gurevich E, Joyce JN. Localization of dopamine D3 receptors to mesolimbic and D2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci USA. 1994;91:11271–11275. doi: 10.1073/pnas.91.23.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006a;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, et al. Dopamine (D(2/3)) receptor agonist positron emission tomography radiotracer [(11)C]-(+)-PHNO is a D(3) receptor preferring agonist in vivo. Synapse. 2006b;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A parental bonding instrument. Br J Med Psychol. 1979;52:1–10. [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Laruelle M. Imaging the D3 receptor in humans in vivo using [11C](+)-PHNO positron emission tomography (PET) Int J Neuropsychopharmacol. 2010. pp. 1–2. [DOI] [PubMed]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, et al. in vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112:330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2:S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197:549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, et al. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA; 1983. [Google Scholar]
- Stokes P, Curran V, Grasby P. Effects of THC on dopamine release in the human striatum: An 11C-raclopride PET study. Biol Psychiatry. 2008;63 (7, Suppl. S:127S–127S. [Google Scholar]
- Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, et al. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol. 2012;26:144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- Suplita RL, Eisenstein SA, Neely MH, Moise AM, Hohmann AG. Cross-sensitization and cross-tolerance between exogenous cannabinoid antinociception and endocannabinoid-mediated stress-induced analgesia. Neuropharmacology. 2008;54:161–171. doi: 10.1016/j.neuropharm.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suridjan I, Boileau I, Bagby M, Rusjan PM, Wilson AA, Houle S, et al. Dopamine response to psychosocial stress in humans and its relationship to individual differences in personality traits. J Psychiatr Res. 2012;46:890–897. doi: 10.1016/j.jpsychires.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, et al. Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry. 2012;71:677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen AP, Creemers HE, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC. Hypothalamic-pituitary-adrenal axis reactivity to social stress and adolescent cannabis use: the TRAILS study. Addiction. 2011;106:1484–1492. doi: 10.1111/j.1360-0443.2011.03448.x. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2011;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Voruganti LN, Slomka P, Zabel P, Mattar A, Awad AG. Cannabis induced dopamine release: an in-vivo SPECT study. Psychiatry Res. 2001;107:173–177. doi: 10.1016/s0925-4927(01)00104-4. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.