Summary
A new acrylic glue, Glubran 2, is now available on the European market. It bears the CE mark with several surgical indications and the specific indication for neuroradiological endovascular use. Despite this approval, to our knowledge its use is still limited to surgery and no injections have been made in human patients.
This study was designed to evaluate the behavior of Glubran 2 in endovascular injection in a simulation of brain AVM.
Six sheep were operated on opening a fistula between the right common carotid artery and the jugular vein. This fistula modifies blood flow in the skull base rete mirabilis, which then functions as an AVM. In two sheep, the rete mirabilis was occluded by injection of 1.5 ml of Histoacryl diluted 1:1 and 1:3 with Lipiodol. In two sheep, the rete was embolized by injection of 1.5 ml of Glubran 2 diluted 1:1 with Lipiodol. The last two sheep were embolized by injection of Glubran 2 diluted 1:3 with Lipiodol. The procedures were documented by DSA angiographic acquisitions and by fluoroscopic VHS. The sheep were killed immediately after the procedures and the rete mirabilis isolated for histologic examination.
Embolization was obtained with both kinds of glue. Glubran 2 diffuses in a very similar way to Histoacryl with an apparently more complete diffusion. Reflux in the ascending pharyngeal artery showed that Glubran 2 tended not to produce bubbles but diffused more homogeneously.
The subjective conclusion of this work is that Glubran 2 can be used in endovascular embolizations. Before approaching brain AVMs, a further study will investigate embolization of the external carotid territory.
Key words: AVM, acrylic glue, embolization, experimental neuroradiology
Introduction
Selective arterial embolization was first undertaken in the Sixties to treat brain arteriovenous malformations.
The technique was subsequently applied in the surgical and neurosurgical treatment of hypervascular tumours, haemorrhage following head injury, renal and gastrointestinal tumours and vascular head and neck lesions. In addition, embolization can be used as an adjunct procedure in orthopaedic oncology prior to surgery or as a palliative treatment for pain from metastases.
For more than twenty years, an acrylic glue, Histoacryl (Braun, Aesculap AG, Tuttingen, Germany), has been used for embolization of cerebral and extracerebral vascular diseases, especially brain arteriovenous malformations which are delicate conditions difficult to treat1,7.
In recent years, attention has focused on the fact that Histoacryl has no official recognition and its endovascular use is prohibited. This situation raises major legal problems deriving from its improper use.
A new acrylic glue, “Glubran 2” (GEM Srl, Viareggio, Italy), was first marketed a couple of years ago. This glue is authorised for surgical use and for “endovascular use in neuroradiology” with a CE mark for “internal use”. Glubran 2 is not the same as other cyanoacylate glues currently available (cf. Histoacryl) as it has a different chemical composition making it a comonomer rather than a simple monomer. The comonomer of Glubran 2 surgical glue is the result of mixing two monomers: N butyl 2 cyanoacrylate (NBCA) and MS (a monomer owned by GEM Srl). NBCA is a cyanoacylate monomer common to many other glues like Histoacryl which are CE approved solely for topical use. The addition of MS to the basic monomer allows the NBCA to polymerize with an exothermic reaction at around 45° C, and a slightly higher polymerization time than Histoacryl thereby reducing the toxicity of the basic monomer. In fact, the longer the carboxylic side chain of the minor cyanoacrylate is, the less cytotoxic the resulting adhesive8. MS also gives surgical Glubran 2 a significant anti-inflammatory effect. Based on this premise, the addition of MS to NBCA yielded a glue complying with European regulations and hence receiving CE certification for internal and endovascular use.
Although Glubran 2 can be legally used for cerebral embolization procedures, practical clinical experience with the substance is lacking. For this reason, we performed an experimental animal study to test the endovascular behaviour of the glue. The study protocol conformed to Italian legislation (Decree Law n° 116 dated 27/01/1992) and was approved by the central vetinary service for the protection of animals used for experimental purposes.
Material and Methods
We set out to simulate the conditions of brain AVM according to Qian et Al5 by creating a fistula between the common carotid artery and the jugular vein thereby inverting arterial blood flow in the skull base rete mirabilis, which then simulates the conditions of an AVM: afferent arterial vessels from the internal maxillary artery, anastomotic arteries on the left side transformed into eferents on the right side.
Six sheep underwent surgery according to Italian legislation governing the protection of animals used for experimental purposes (Legislative Decree 116/92) and the “Guide for the Care and Use of Laboratory Animals” and Animal Welfare Assurance No. A5424-01 of the National Institute of Health (NIH-Rockville Maryland USA); the research protocol was authorized by the Bologna University Ethical Committee.
Animals
Six crossbred adult sheep (aged three years), weighing 75 ±5 kg, were housed in individual cages with a grid floor at room temperature and controlled humidity: 20 ± 0.5° C and 50 ± 5%, with ventilation of ten air changes an hour. The animals were fed a standard maintenance diet (Mucedola, Settimo Milanese, Italy), clover and water ad libitum.
General anaesthesia
After five days'quarantine, the animals underwent surgery in general anaesthesia. As preoperative preparation, the sheep were fasted for 48 hours without water for the 24 hours prior to the operation. The animals were premedicated by subcutaneous administration of atropine sulphate 0.125 mg/kg and intramuscular injection of ketamine 10mg/kg (Ketavet Farmaceutici Gellini Spa, Italy) and xylazine 0.3 mg/kg (Rompun, Bayer, Italy). General anaesthesia was induced by bolus infusion of thiopentone sodium (Pentothal, Hoechst AG, Germany) in 2.5% solution at a dose of 6 mg/kg. On loss of the swallowing reflex, an endotracheal tube and a ruminant probe were inserted to avoid meteorism. Anaesthesia was maintained with assisted ventilation (Servo Ventilator 900D Siemens, Germany) with administration of an O2/N2O (50/50%) mixture with fluothane at 2.3% (Halothan, Hoechst AG, Germany).
Intra-operative monitoring
The animals were monitored throughout surgery using a modular system for three-way ECG (M1001 A ECG Module Hewlett Packard, Germany), end-tidal CO2 (M1016A CO2 Module Hewlett Packard, Germany), average arterial pressure (AAP) using a 20 G catheter inserted into the cephalic artery (Component Monitoring System Hewlett Packard, Germany) and body temperature measured by a rectal probe (M1029º Temperature Module, Hewlett Packard, Germany). Assisted ventilation was administered at a frequency of 12 breaths/min maintaining an end-tidal CO2 of 30-35 mmHg: these parameters were maintained throught the operation. The temperature of the operating theatre was kept stable at 23 ± 2° C throughout the procedure and the animals' vital parameters kept constant.
Surgical procedure
All surgical procedures were carried out under sterile conditions with the animals in supine decubitus.
The surgical approach involved incision in the right laterocervical neck region along the anterior margin of the sternocleidomastoideus muscle, revealing and isolating a 5 cm long segment of the right external jugular vein and common carotid artery. After careful removal of perivascular tissue, each part of the isolated vessels was clamped with vascular miniclamps to block blood flow for the duration of shunt preparation. A 2 cm incision was made in both vessels, washing ay residual blood within the vessels with saline solution. A laterolateral shunt was created using prolene suture thread 7-0. When completed, the clamps were removed and the common carotid artery isolated 1 cm below and the jugular vein 1 cm above the shunt. After the procedure the incision was sutured with separate stitches along the anatomical planes.
Postoperative course
After surgery the animals underwent postoperative treatment for five days with intramuscular administration of cephalosporin at a dose of 1 gr/die (Totacef, Bristol Myers Squibb SPA, Italy) and 500 mg/die ketoprofen (Orudis, Rhone-Poulenc-Rorer SPA, Italy); the surgical wound was medicated daily with betadine. Clinical vetinerary examination was performed daily and the animals presented no lateral signs after surgery: they were able to stand two hours after the procedure and organic functions resumed after twelve hours.
Angiography and embolization
Angiography and embolization were subsequently performed under the folllowing anaesthesia protocol: pre-operative fasting from solid food for 36 h, fluids for 12 h; Premedication: t.- 30 min atropine sulphate (0.04 mg/Kg i.m.); t.-15 min acetylpromazine maleate (0.25 mg/Kg i.m.) + xylazine ( 0.5 mg/Kg i.m.) in a single syringe; Induction: t. 0 min sodium thiopental dilution 1g/ 20 ml to action; Orotracheale intubation + ruminant probe; Deep anaesthesia: isoflurane 2.5% in O2 (0.10 l / Kg/ min); Maintenance: isoflurane 1.25% average in O2 (0.10 l/ Kg/ min) administered in an open circular system under assisted spontaneous breathing.
Arteriography was subsequently performed under general anaesthesia (G.E. OEC 9800 System, Courtesy of General Electric Medical Systems) and microcatheterization with access through the left femoral artery (Microcateteri Elite, courtesy of Boston Scientific). The rete mirabilis was embolized by injection of: 1.5 ml of Histoacryl diluted 1:1 and 1:3 with Lipiodol: one sheep each.
1.5 ml of Glubran 2 diluted 1:1 with Lipiodol: two sheep. 1.5 ml of Glubran 2 diluted 1:3 with Lipiodol: two sheep.
Histology
The sheep were killed immediately by i.v. injection of a solution of Embutramide (200 mg/ ml) mebenzoniim iodide (50 mg/ ml) and tetracaine chloride (50 mg/ ml) (Tanax®) at a dosage of 0.15 ml/ Kg at the end of the procedures and the rete mirabilis isolated for histological examination. Specimens were fixed in 10% buffered formalin with paraffin embedding and staining with haematoxylin-eosin. Histological features were evaluated blindly. The pathologist (FR) did evaluate the distribution of the material within the rete mirabilis, endothelial damage or necrosis. Acute and chronic inflammation, giant cell reactions and fibrosis were beyond the scope of the study because of too short a time between the injections animal sacrifice.
Results
The main angiographic findings showed that:
– embolization was obtained with both Histoacryl and Glubran 2;
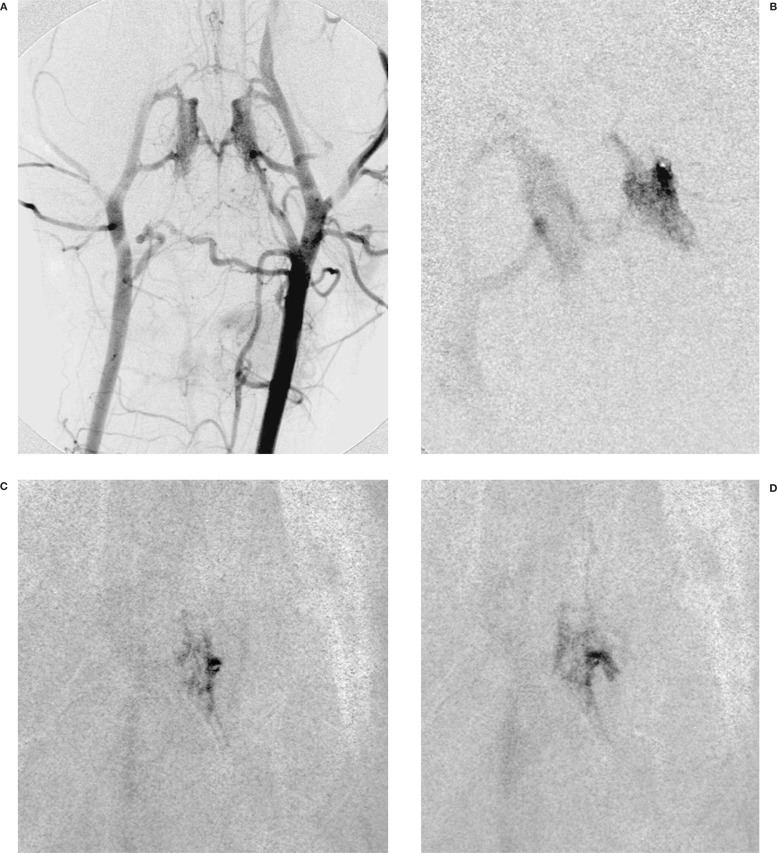
– Glubran 2 diffuses in a way very similar to Histoacryl closely corresponding to the angiographic appearance of superselective injection of contrast media (figure 1);
Figure 1.
A) Left Carotid angiography with clear visualisation of the rete mirabilis. Flow inversion in the right carotid artery, due to the carotid-giugular fistula. B) Superselective injection of the rete mirabilis through a left anastomotic artery. C,D) Injection of the glue, Glubran 2: Lipiodol 1:1.
– reflux in the ascending pharyngeal artery showed that Glubran 2 tended not to produce bubbles but diffused homogeneously;
– the microcatheter did not stick to Glubran 2;
– we did not observe changes in sheep vital parameters during injection of Glubran, which could be linked to the absence of an exothermic reaction (and pain) due to glue polymerization.
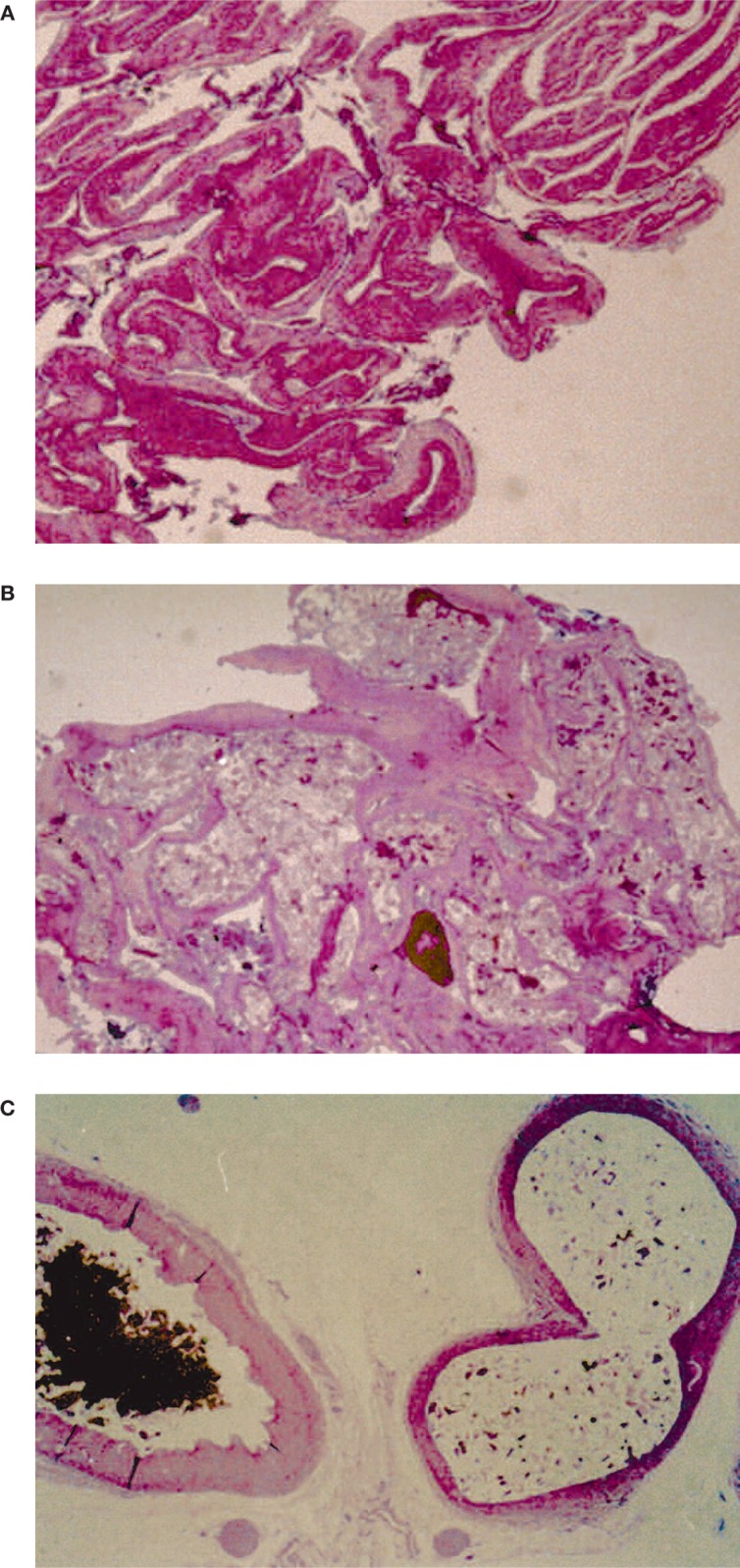
The pathological findings demonstrated that distribution of both glues in the rete mirabilis was complete with vascular stretching and dilation (figure 2).
Figure 2.
Histological findings: A) rete mirabilis. B) rete mirabilis filled with glue: Glubran 2: Lipiodol 1:1. C) at higher magnification, on the left side a normal rete mirabilis capillary vessel; on the right side a capillary filled with glue.
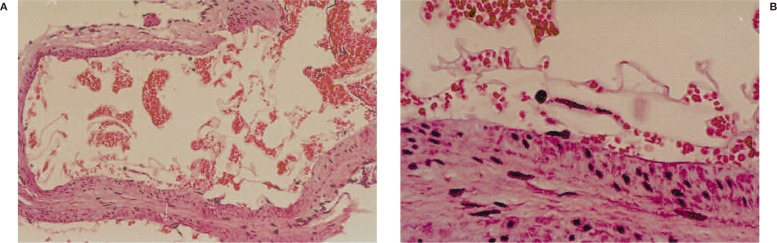
Focal necrosis was detected with both glues 4, as described for Histoacryl 2,3,6,9. Focal endothelial damage was disclosed in one sheep (figure 3), subsequently identified as Histoacryl injection: Histoacryl/Lipiodol 1/1, and interpreted as probably due to the high thermic reaction of Histoacryl polymerization 8-9. The pathologist failed to find significant differences between the two glues
Figure 3.
Histological findings: endothelial damage after injection of Histoacryl: Lipiodol 1:1. A) Panoramic view, B) magnification: modest detachment of the endothelium probably due to the thermic reaction to polymerization.
Conclusions
Glubran 2 behaves in a very similar way to Histoacryl and is probably less harmful because of the lower thermic level of polymerization. It is suitable for use in endovascular embolisation procedures.
Before tackling human brain AVMs a further study will investigate embolization of the external carotid territory and spinal orthopaedic tumors and metastases.
References
- 1.Alethic VA, Debrungh GM. Intracranial AVMs: the approach and technique of cyanoacrylate embolization. In: Connors JJ, Wojak JC, editors. Interventional Neuroradiology. Philadelphia: WB Saunders Co; 1999. [Google Scholar]
- 2.Berenstein A, Hieshima G. Clinical vs experimental use of isobutyl-2-cyanoacrylate. J Neurosurg. 1987;63:318–319. doi: 10.3171/jns.1987.67.2.0318. [DOI] [PubMed] [Google Scholar]
- 3.Brothers MF, Kauffmann JCE, et al. NBCA: a substitute for IBCA in interventional neuroradiology. Hystopathologic and polymerization time studies. Am J Neuroradiol. 1989;10:777–786. [PMC free article] [PubMed] [Google Scholar]
- 4.Montanaro L, Arciola CR, et al. Cytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical use. Biomaterials. 2001;22(1):59–66. doi: 10.1016/s0142-9612(00)00163-0. [DOI] [PubMed] [Google Scholar]
- 5.Qian Z, Climent S, et al. A simplified AVM model in sheep: feasibility study. Neuroradiology. 1999;20:765–770. [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel SM, Vinuela F, et al. Adjusting polymerization time for isobutyl-2-cyanoacylate. Am J Neuroradiol. 1986;7:109–112. [PMC free article] [PubMed] [Google Scholar]
- 7.Valavanis A, Yasargyl MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Standard Neurosurg. 1998;24:131–214. doi: 10.1007/978-3-7091-6504-1_4. [DOI] [PubMed] [Google Scholar]
- 8.Vinters HV, Galil KA, et al. The histotoxicity of cyanoacrylates. Neuroradiology. 1985;27:279–291. doi: 10.1007/BF00339559. [DOI] [PubMed] [Google Scholar]
- 9.Woodward SC, Herrmann JB, et al. Histotoxicity of cyanoacrylate tissue adhesive in the rat. Ann Surg. 1965;54:113–122. doi: 10.1097/00000658-196507000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]