Summary
We report a case of fatal cardiovascular collapse that occurred during Ethanol sclerotherapy of a venous malformation in a 21-year-old woman. The malformation was located on the anterior part of the thigh. Fifty ml of a mixture of Ethanol, Ethibloc and Lipiodol containing 35 ml of Ethanol (0.52 ml / kg) were injected under fluoroscopy. A major drop in arterial pressure was recorded after release of the tourniquet placed at the thigh root. The patient died after four hours of intensive cardiac reanimation. Her blood alcohol level was 0.4 g/l one hour after the end of the intervention. The cardiac toxicity of ethanol depends more on the potential acute venous contamination than on the blood alcohol concentration. The currently admitted “safety limit” of 1 ml/kg of bodyweight for ethanol sclerotherapy of venous malformations is certainly unsafe and must be redefined.
Key words: complication, alcohol, sclerotherapy, venous malformation
Introduction
Ethanol has demonstrated its efficacy as an agent to sclerose venous malformations. However, due to its general toxicity, a maximum volume of ethanol has been defined and most authors accept the limit of 1 ml/kg of body-weight 1-4. We report a lethal complication due to the cardiac toxicity of Ethanol that occurred during sclerotherapy with an injected ethanol volume of 0.52 ml/kg, i.e. twice below the “safety limit”.
Case Report
A 21-year-old woman (67-kg bodyweight, 180 cm tall) had been diagnosed two years before with a venous malformation (VM) of the anterior part of the left thigh (figure 1). Because of several painful episodes, sclerotherapy was decided.
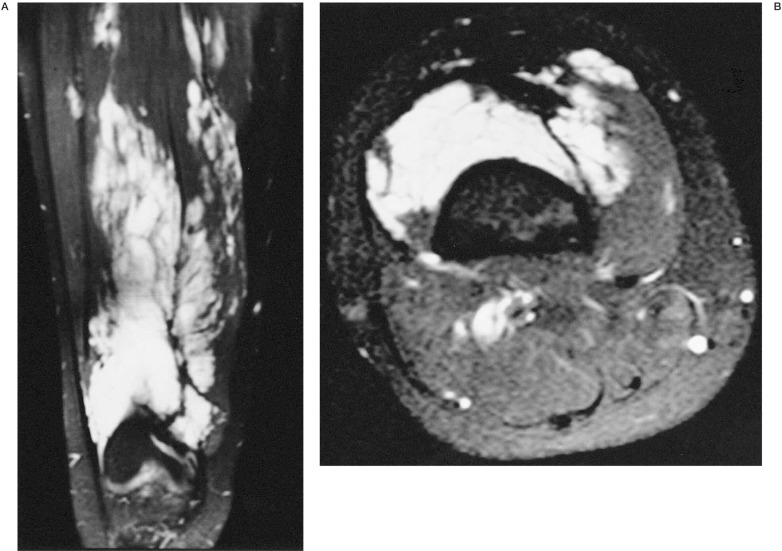
Figure 1.
A) Sagittal view and B) axial view of MR of the left thigh showing the venous malformation.
The intervention was conducted under general anesthesia using Propofol, Sufentanyl and N2O2 (50%). Blood pressure (non invasive), electrocardiogram and oxygen saturation (Pulse Oxymeter) were monitored during the intervention. A tourniquet was placed at the proximal part of the thigh and the VM was punctured using 20 Gauge needles. Contrast material was injected to control the correct placement of the needle in the VM and the absence of contrast egress into normal veins (figure 2). Contrast material was then re-aspirated and the sclerosing mixture was injected. Three punctures were performed using the same protocol. Sclerosis was performed using a mixture associating Ethanol, Lipiodol ultra-fluide (Guerbet Inc, Aulnay sous Bois, France) and Ethibloc (Ethicon Inc, Issy les Moulineaux, France) in a respective proportion of 70%- 15%- 15%. A total volume of 50 ml of this mixture (containing 35 ml of Ethanol) was injected over 30 minutes. The tourniquet was maintained for 20 minutes after the last injection and was released under fluoroscopy. No migration of the radio-opaque cast was seen. However, a few seconds after release, the mean arterial blood pressure dropped from 100 to 20 mm Hg. Ephedrine was injected with 500 ml of Plasmion®. The arterial blood pressure rose to normal for ten minutes but dropped again with a decrease of cardiac frequency to 50 beats/min associated with an enlargement of the cardiac complexes on the electrocardiogram. Arterial blood pressure rose after Epinephrine injection and the patient was transferred from the radiology floor to the ICU where the whole anesthesiologist team performed multiple attempts at resuscitation. Blood samples were drawn from a brachial vein and several dosages were performed about one hour after the last ethanol injection. Blood alcohol level was measured at 0.4 g/l. Several episodes of collapse were recorded despite all attempts at resuscitation. A transthoracic echocardiogram performed during one collapse episode showed a profound and diffuse alteration of cardiac contraction. The patient died after four hours of resuscitation.
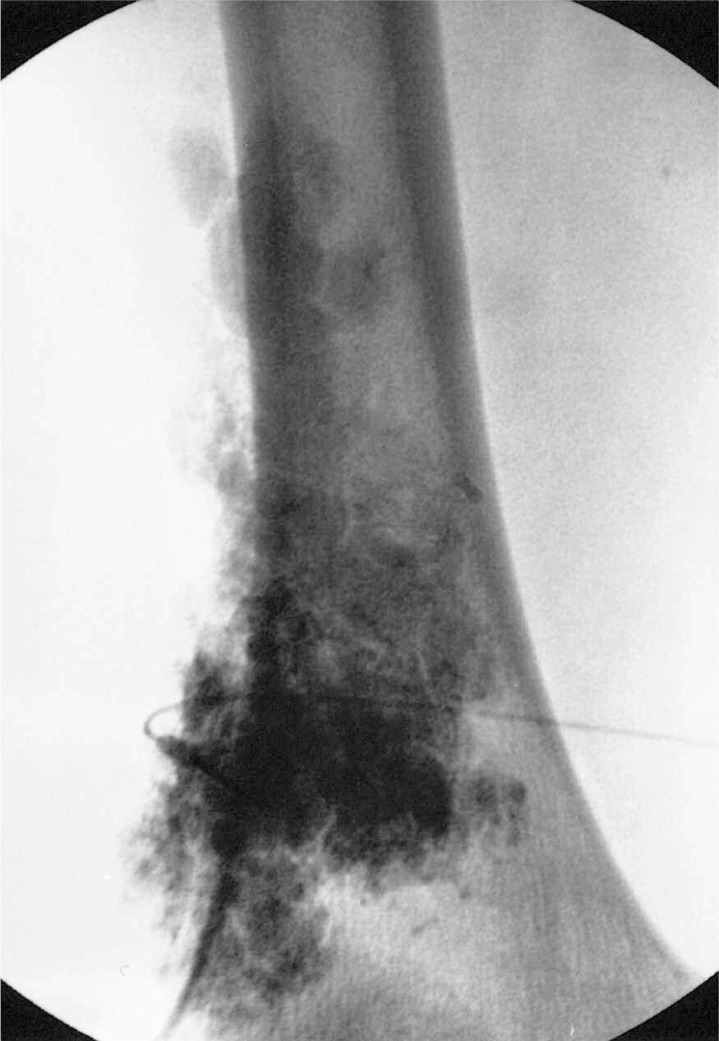
Figure 2.
Plain film of the left thigh at the end of the intervention showing the filling of the venous malformation by the sclerosing mixture.
Autopsy did not disclose any macroscopic lesion of the heart and lungs, especially with no pulmonary embolism.
Discussion
After data analysis, our conclusion was that death was due to Ethanol cardiac toxicity induced by the Ethanol bolus effect occurring after the tourniquet release.
The sclerosis technique used in this case is comparable to that reported in many articles: use of Lipiodol to opacify the Ethanol5 and use of a tourniquet to avoid outflow of the sclerosing agent in normal veins4. The Ethanol dose was below the maximum accepted in most articles2,4 and textbooks1,3. Resuscitation was performed by staff anesthesiologists using all “state of the art” techniques.
This observation raises three major problems.
The first concerns the “safety dose” of Ethanol. The limit of 1 ml/kg of bodyweight has been deducted from the alcohol blood level at equilibrium, i.e. after dilution in the total body free water (2/3 the bodyweight). Such concentration is thought acceptable because it is within the range of legally defined tolerable toxic levels. However, the present complication occurred with an alcohol blood level of 0.4 g/l, which was the maximum limit for driving in France in the recent past. It corresponds to the expected concentration after injection of 0.5 ml/kg 4. However, before reaching the equilibrated concentration, Ethanol can migrate to the vein as a bolus, which may have toxic effects. The question then could be: “Which volume of Ethanol can be released to avoid bolus effect?” In the light of the above discussion, one can also question the recommended use of a tourniquet in such a procedure. In itself, it can promote the retention of all ethanol injected, even at a “safe” quantity, because of a bolus recirculation when the tourniquet is removed. In the absence of such a tourniquet, injected ethanol is then released in time in small quantities into the circulation, warning us to stop the procedure when heart toxicity occurred.
The second problem concerns the medical attitude facing such complications in this specific field. Although rare, some observations of cardiac toxicity due to Ethanol have already been reported6,7. In these observations, toxic effects were observed with Ethanol doses sometimes inferior to 1 ml/kg. Surprisingly, those publications have not modified the concept of the “safety dose”. This may relate to acceptance of an unpredictable “individual” reaction but one must remember that individual variation is the rule in biology. Otherwise, how can we explain the concept of lethal dose 50 that defines the dose of drug able to kill 50% animals, meaning implicitly that 50% of the animals that were exposed to the same dose survive ?
The third question challenges the regulatory process governing the use of such toxic agents (Ethanol, Dimetylsulfoxide) in medicine, especially when intoxication cases have been reported. They cannot be considered inert devices (coils, stents), for which accidents have to be reported to a “material safety use” committee. Since they are also not drugs, for which adverse effects have to be reported to a “pharmacovigilance” committee, there is no official committee to report to and to fix the rules for use.
Because our case led to patient death, we considered such a report as an ethical duty for the community. In the absence of any mistake during the procedure, the occurrence of such a fatal complication should stimulate specialists to organize a survey structure providing a database necessary to fix future guidelines.
References
- 1.Lasjaunias P, Berenstein A. Surgical Neuroangiography. Vol. 2. Springer Verlag; 1987. Endovascular treatment of craniofacial lesions. Ethyl Alcohol; pp. 40–43. [Google Scholar]
- 2.Yakes WF, Haas DK, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. 1989;170:1059–1066. doi: 10.1148/radiology.170.3.2916057. [DOI] [PubMed] [Google Scholar]
- 3.Yakes WF. Management of extracranial head and neck and paraspinal vascular malformations. In: Connors JJ, Wojak JC, editors. Interventional Neuroradiology. Strategies and practical techniques. WB Saunders; 1999. pp. 327–337. [Google Scholar]
- 4.Mason KP, Michna E, et al. Serum ethanol levels in children and adults after ethanol embolization or sclerotherapy for vascular anomalies. Radiology. 2000;217:127–132. doi: 10.1148/radiology.217.1.r00se30127. [DOI] [PubMed] [Google Scholar]
- 5.Suh JC, Shin KH, et al. Venous malformations: sclerotherapy with a mixture of ethanol and lipiodol. Cardiovasc Intervent Radiol. 1997;20:268–273. doi: 10.1007/s002709900150. [DOI] [PubMed] [Google Scholar]
- 6.Gelczer RK, Charboneau JW, et al. Complications of percutaneous ethanol ablation. J Ultrasound Med. 1998;17:531–533. doi: 10.7863/jum.1998.17.8.531. [DOI] [PubMed] [Google Scholar]
- 7.Garel L, Mareschal JL, et al. Fatal outcome after ethanol renal ablation in child with end-stage kidneys. Am J Roentgenol. 1986;146:593–594. doi: 10.2214/ajr.146.3.593. [DOI] [PubMed] [Google Scholar]