Summary
From september 2000 to september 2001, 32 consecutive patients with ruptured intracranial aneurysms were examined with rotational and 3D reconstruction angiography using an Integris V5000 Philips Medical System: 39 aneurysms were detected.
After a selective cerebral artery was catheterized with a 5F or 4F-catheter, 35 ml of contrast medium was intra-arterially administered at a rate of 4 ml/s and a 180° rotational angiography was performed in eight seconds. This information was transferred to a computer (Silicon Graphics Octane) with software (Integris 3D-RA, Philips Integris Systems) and a three-dimensional reconstruction was made.
The information provided by Angio-3D was useful for evaluating the parent artery, aneurysmal sac, aneurysmal neck and arterial branches. It was also very useful in selecting the therapeutic method.
For open surgery, this technique provides preoperative images that are useful for planning microsurgical approaches, especially in cases of large aneurysm showing complex surrounding arteries.
For endovascular embolization, various anatomic characteristics of the aneurysm such as neck and sac size, shape, lobularity, parent artery and arterial branches adjacent to the aneurysmal neck must be demonstrated. This is very important to determine the best projection for embolization and to avoid multiple series. This is also essential in the choice of the first coil to create a good basket producing total occlusion.
Microaneurysms are demonstrated well with this technique whereas this is difficult to do with conventional arteriography.
The Angio-RM and Angio-CT literature show a lower sensitivity and specificity in comparasion with our experience with 3D IA-ROT-DSA. For this reason, we believe that 3D IA-ROT-DSA is now the gold standard for patients presenting intracranial aneurysms.
Key words: intracranial aneurysm, embolization, endovascular treatment, Guglielmi detachable coils (GDC), digital angiography, 3D angiography, rotational angiography, interventional neuroradiology
Introduction
Treatment for patients with intracranial aneurysms depends on many factors such as size of the aneurysm neck, morphology of the sac, parent and efferent arteries1-3.
Though intracranial digital subtraction angiography (IA-DSA) is the standard method to demonstrate and evaluate cerebral aneurysms, in many cases the information is incomplete and unable to answers questions regarding treatment of a complex aneurysm2.
With intra-arterial rotational angiography and three dimensional reconstruction (3D IA-ROT-DSA) we can obtain a correct evaluation of the key point of the aneurysms to decide and plan the best treatment (endovascular embolization, open surgery or conservative treatment).
Material and Methods
From september 2000 to may 2001, 23 consecutive patients with acute SAH due to ruptured cerebral aneurysms were admitted to our hospital. 3D IA-ROT-DSA -with a Rotational Angiographic System, Philips Integris V5000 (Philips, the Netherlands)-was performed on all these patients.
A selective catheterisation of the vessel involved was done using a 4-F or 5-F catheter via a femoral approach (Seldinger's technique) either under local or general anesthesia.
Thirty-five milliliters of nonionic contrast material (Iopamidol-“Iopamiro 3007®”) were injected into the internal carotid or vertebral artery with a power injector at 4 mL/s and 500 psi. Rotational angiography was performed with a 180° rotation of the C-arm for eight seconds (rotational DSA can fulfil to 30°, 90° or 120°). This information is transferred to a computer (Silicon Graphics Octane) with software (Integris 3D-RA, Philips Integris Systems) that allows a three-dimensional reconstruction in six minutes.
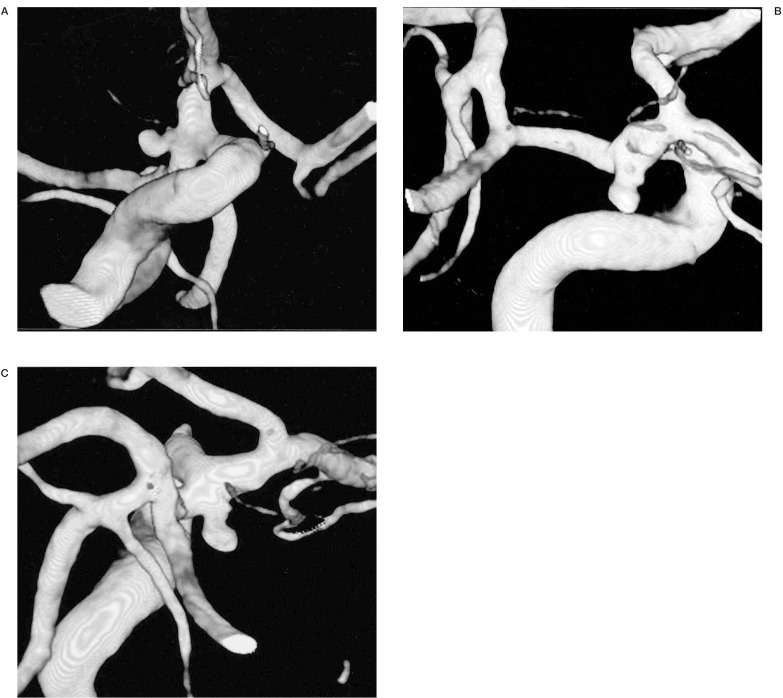
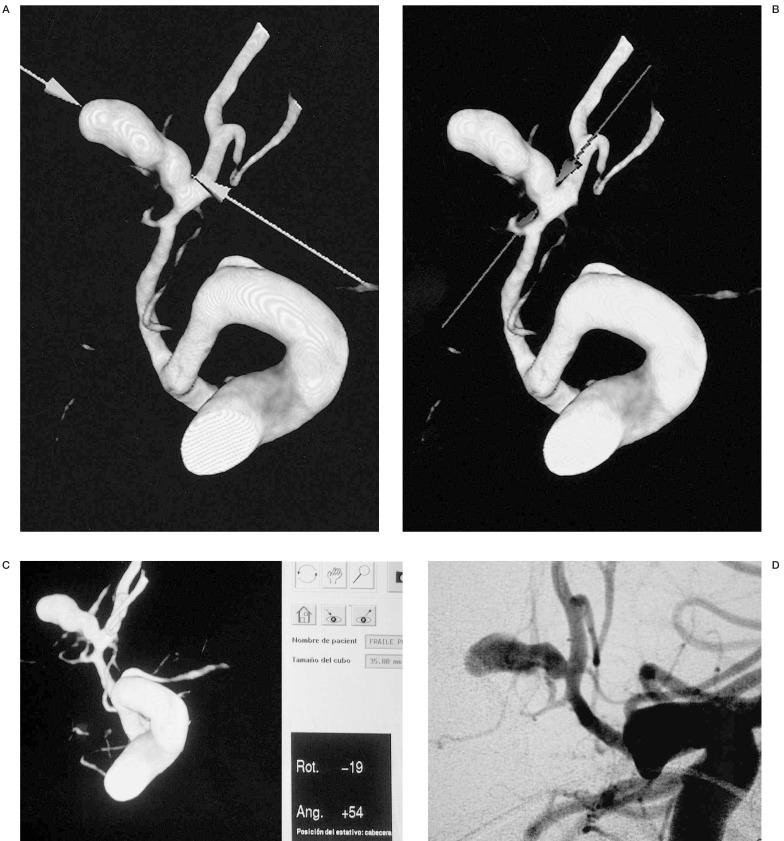
Following the reconstruction, we were able to evaluate the complex aneurysm from any angle (figure 1), demonstrate the shape, lobularity and measure key points such as diameter of aneurysm sac, neck, parent and efferent arteries (figure 4). The arterial branches adjacent to the aneurysmal neck were well demonstrated. This method has an additional advantage of providing the exact rotation and angulation to reproduce the best similar projection on angiography (figure 4).
Figure 1.
In this posterior communicating aneurysm, we can evaluate its characteristics (neck, sac, parent and efferent arteries) from any angle and provide anatomical information useful for endovascular or surgical planning.
After this evaluation we discuss the best modality of treatment whether endovascular or surgical based on the state of the patient, morphology of the aneurysm and its neck/sac ratio. A good neck/sac ratio will favour complete embolization whereas a wide neck or a poor neck/sac ratio shifts the management towards surgery.
For endovascular treatment, the C-arm is positioned to the angulation and rotation degrees indicated by the 3D-RA Philips Integris software (figure 4). The size of the first coil is chosen according to the diameter and morphology of the sac.The 3D information assisted in the surgical approach and the radiological anatomy was compared to the surgical anatomy.
Results
In 32 patients (16 men and 16 women, ages ranging from 26 to 74 years with an average age of 42 years) a total of 39 aneurysms were detected by 3D IA-ROT-DSA.
Endovascular embolization with GDCs was performed in 23 patients. In another 14 patients clipping was performed because radiological findings revealed that the aneurysms were not suitable for endovascular treatment. Management was conservative in two patients as the morphology of the aneurysm did not permit a good endovascular treatment or open surgery with a low risk.
In all patients, we evaluated the characteristics of the aneurysms (neck, sac, parent and efferent arteries) and provided useful anatomical information for endovascular or surgical planning.
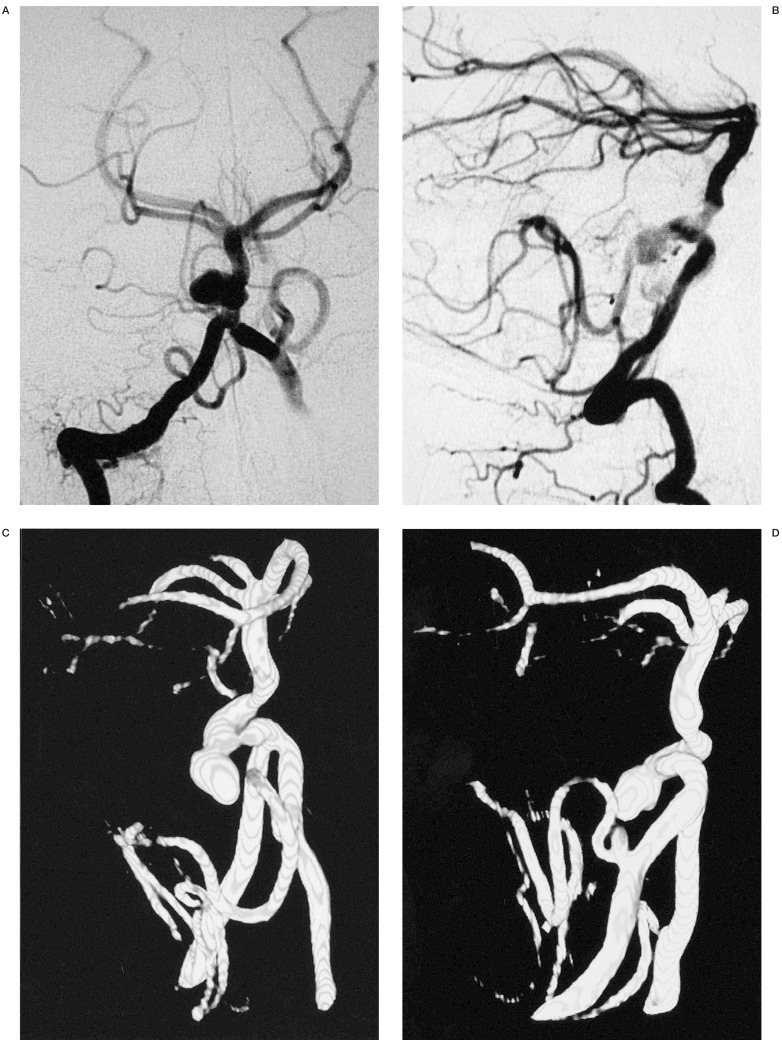
In our experience, 3D IA-ROT-DSA was very useful to assess suitability for treatment (endovascular or open surgery) (figures 1,2).
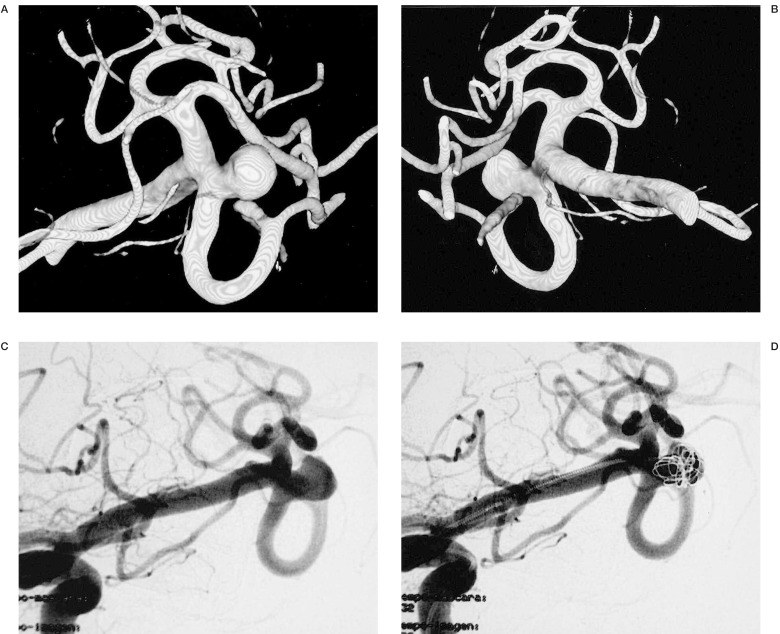
Figure 2.
We can see the neck, sac, parent and efferent arteries of this aneurysm of the Middle Cerebral Artery. This information advocates the correct choice of initial coil to build a basket permitting occlusion of the aneurysm.
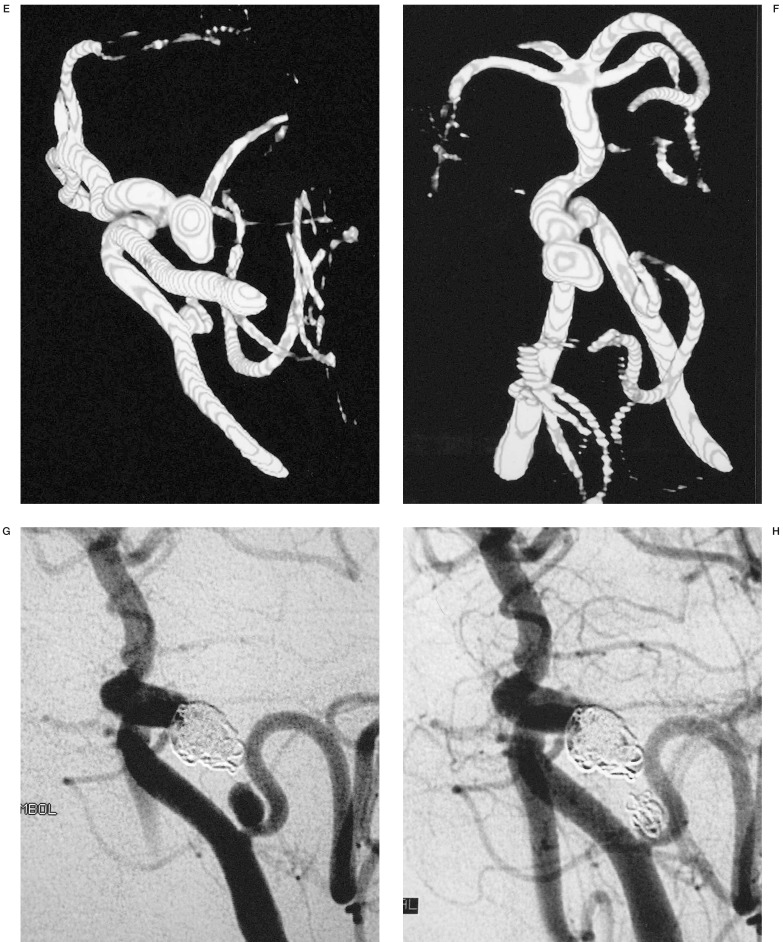
In cases where embolization was the modality of treatment, 3D IA-ROT-DSA showed the best projection to demonstrate the neck, parent and efferent arteries and to choose the initial coil to build an acceptable basket resulting in total occlusion (figures 4,5).
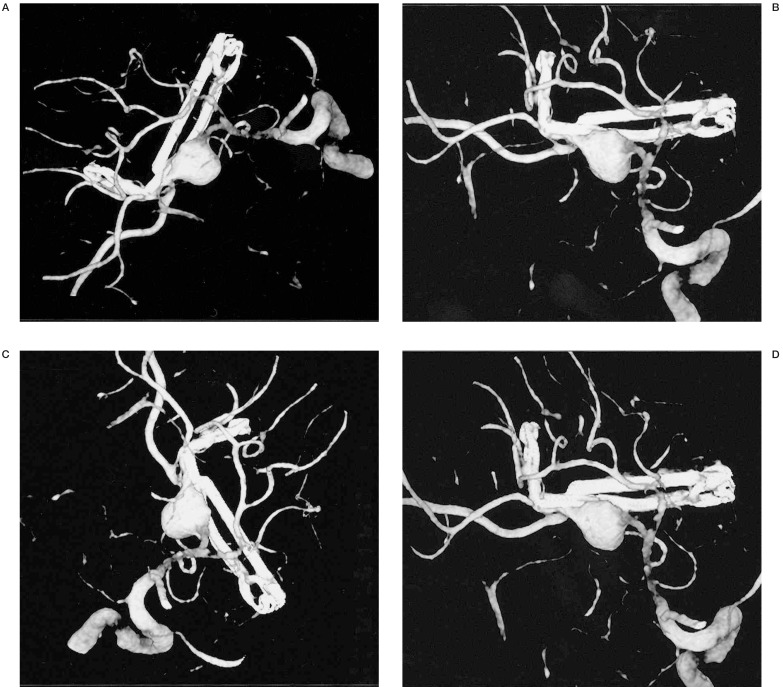
Figure 3.
Post-clipping control of a giant aneurysm of the one branch of the middle cerebral artery. We can evaluate its characteristics from any angle.
Figure 4.
3D IA-ROT-DSA clearly shows the best projection to disclose the anatomical relationship between neck, parent and efferent arteries and we can measure key points such as diameter of aneurysm sac and neck. A useful aspect of this technique is the capacity to provide accurate angulation and rotation degrees of the C-arm to obtain the best projection for embolization.
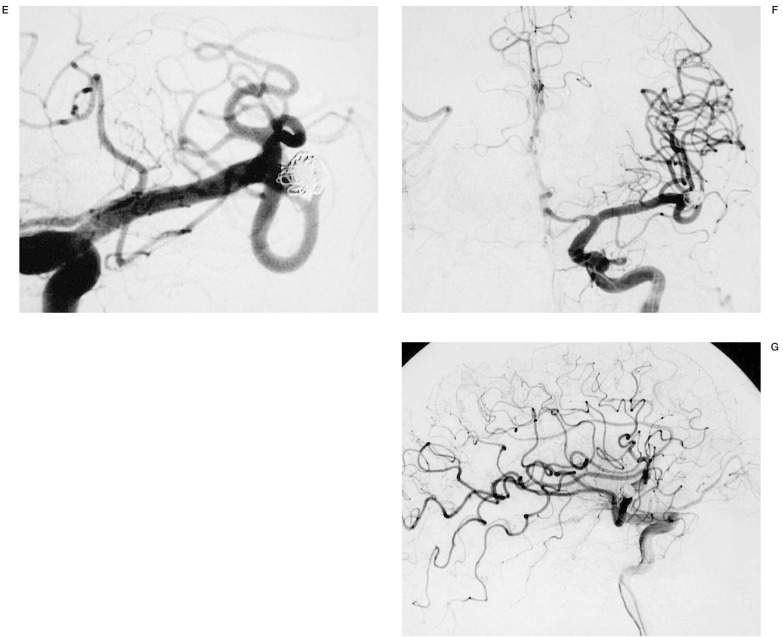
Figure 5.
The three-dimensional nature of this technique and the ability to view the three-dimensional imaging volume at multiple angles was helpful in identifying the relationship of this aneurysm of the basilar artery and evaluates the neck of the aneurysm beneath the overlapping tortuous basilar artery and demonstrates a second aneurysm of the posterior inferior cerebellar artery. This ability to depict the aneurysmal neck and the projection of the aneurysm relative to the parent artery is advantageous for evaluating the outcome of clipping or embolization of the aneurysm. In this particular case, this information advocates the best protection and the correct choice of initial coil to build a basket permitting total occlusion of the aneurysm.
In patients where the modality of treatment was a surgical approach, there was good surgical anatomy correlation with the 3D IA-ROT-DSA radiological anatomy.
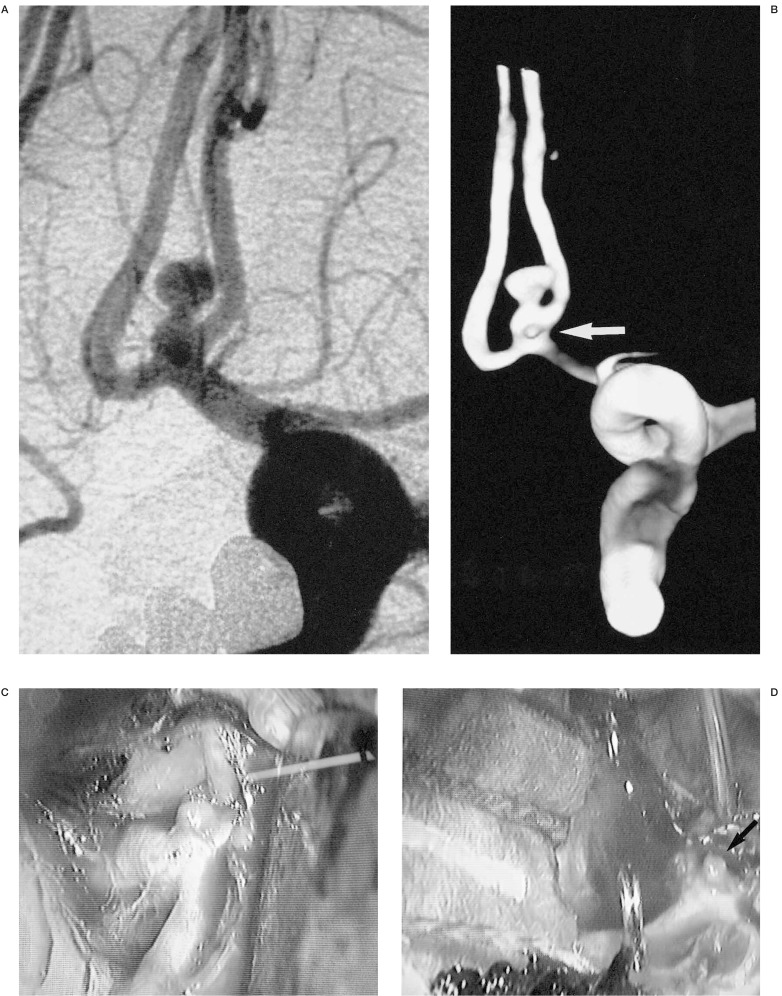
In one patient, the 3D IA-ROT-DSA revealed a microaneurysm which was not demonstrated by conventional angiography (figure 6).
Figure 6.
3D IA-ROT-DSA demonstrates this microaneurysm that conventional angiography failed to reveal.We can see the surgical verification.
In another patient referred from a different center with a presumed Middle Cerebral Artery (MCA) aneurysm diagnosed by IA-DSA, the 3D IA-ROT-DSA we performed demonstrated instead an infundubulum arising from the MCA that gave off early temporal branches.
Discussion
Endovascular embolization using platinum coils has been accepted as a treatment for patients with intracranial aneurysms, but safe and complete embolization depends of many factors such as size of the neck, morphology of the sac, parent and efferent arteries1-3.
However, open surgery continues to be a factor in the treatment of any aneurysms and their results depended on the same factors for embolization and others such as localisation, approach and complex arterial branches or cranial nerves adjacent to the aneurysmal neck 4.
Intraarterial digital substraction angiography (IA-DSA) is a safe imaging method with a low rate of systemic and neurologic complications (the prevalence of stroke -i.e., permanent neurologic deficit-was 0.3% and the overall prevalence of systemic complications was 1.8%)5.
Although IA-DSA is the standard method to demonstrate cerebral aneurysms and evaluate their characteristics, in many cases this information is incomplete and it is incapable of giving all the answers for the questions at the time of treatment of a complex aneurysm2.
New techniques such as computed tomographic angiography (CT-Angiography) and magnetic resonance angiography (MR-Angiography) were performed with the hope of finding a minimally invasive technique that offers equal or better information than IA-DSA 6-8. Unfortunately, MR-angiography failed to demonstrate the aneurysm to the standard of IA-DSA due to its lower resolution (the spatial resolution of 3D gradient-echo MRA is inferior to that of conventional angiography8,9) and other limitations7,8 (table 1).
Table 1.
Limitations of MR-Angiography
| 1 | Slow and/or turbulent flow. Turbulent flow induces chaotic phase shifts, which cannot be compensated by any gradient motion refocusing, and slow flow reduces inflow enhancement due to saturation effects, the result of either is substantial loss of signal within the vessels, that cannot be detected by MIP algorithm7,9,14. |
| 2 | The rapid switching of the gradients produced considerable noise, which can be intolerable to patients with acute subarachnoid haemorrhage and the patient might move7,8. |
| 3 | MR contrast media, may improve visualization of peripheral vessels, but introduce new problems such as imaging arteries and veins together and implies a small risk of contrast medium intolerance7 and raises the cost. |
| 4 | When vasospasm is present, the 3D TOF technique tends to overestimate it8. |
| 5 | The relatively narrow coverage of the transverse 3-D slab used does not always allow coverage of all po- tential sites of an aneurysm with one slab as in the case of the A2-A3 anterior cerebral artery aneurysm14. |
| 6 | In acute SAH, the blood significantly impaired the quality of the reconstructed MR angiographic image because of the hyperintensity of metahemoglobin in some blood clots, an artefact due to the short T1 of haemorrhage may obscure an aneurysm on 3D TOF images14,30. Improvements in phase-contrast MR an- giography may solve the problem14. |
A 0.5-tesla MR-Angiogram yielded a sensitivity of 91% to 93% and a specificity of 100%10, whereas a 1.5-tesla magnet yielded a sensitivity of 79 to 93% and a specificity of 92% to 100% for the detection of aneurysms larger than 3 mm in diameter 11,12. It had a 60% accuracy rate for aneurysms equal to or less than 3 mm 13. Even though giant (> 25 mm) and large (> 15 < 25 mm) aneurysms were demonstrated, the 3-DTOF MR angiographic images were of inferior optimal quality and the signal of the lumen of the aneurysm was often weak 14 due to the flow in giant aneurysms being sluggish 14.
In 40 patients with cerebrovascular disease, the MR angiogram was considered to be of diagnostic quality in 29 (72,5%), acceptable quality in 17 (42,5%), useful for diagnosis but potentially causing diagnostic errors in 12 (30%), not useful for diagnosis in seven (18%) and four angiograms (10%) were considered uninterpretable 9.
An advantage of MRA is that a partially or completely thrombosed aneurysm can appear as a vascular structure on MR-angiography. The detection of the free lumen is possible in the unreconstructed data sets with a combined re-/dephased sequence which differentiates stationary and moving protons or with additional MR images. In contrast, angiography shows only the perfused vascular lumina, sometimes leading to significant underestimation of the size of the aneurysm 7.
Three-dimensional time-of-flight (TOF) MR is more sensitive than three-dimensional phase-contrast (PC) or standard MR imaging for detection of aneurysms 13.
The quality of the representation of the aneurysm and its neck in most cases is better with Voxel View than MIP14. This improvement in the characterisation of cerebral aneurysms obtained with Voxel View can be related to the true 3-D volume processing of the data and the stereoscopic visualization provides a more realistic view of the detailed anatomy of the cerebral vasculature 14.
A statistically significant improvement of aneurysmal features, neck definition, and delineation of adjacent arterial anatomy was seen on both TOF (time of flight) and PC (phase contrast) MR angiograms with STANDOUT (a method for postprocessing data, soft thresholding and depth cueing of unrestricted techniques). Feature definitions were improved for smaller aneurysms. TOF and PC MR angiograms with STANDOUT showed improved elimination of artefactual intraluminal signal loss in normal large vessels, reduced artifactual narrowing of vessel caliber, and showed better small vessel delineation 15.
Some papers have compared MRA and DSA 7,8,14. In the study by Schuierer et Al 7, MRA displayed 19 out of the 22 aneurysms detected (86.4%); in the study by Gouliamos et Al 8, the presence and site of the aneurysms were correctly assessed by MRA in 12 of the 13 cases in which an aneurysm was confirmed (92%); while in the study byy Maeder et Al14, 94% of all intracranial aneurysms were recognized on MR angiography.
However, multislab three-dimensional time-of-flight MR angiography is an accurate and feasible method of noninvasive screening for intracranial aneurysms in individuals at high risk (families with a history of aneurysmal subarachnoid haemorrhage, cerebrovascular disease, minor headaches or families with a history of polycystic kidney disease). However, conventional angiography is still necessary before treatment16.
Imaging asymptomatic IA patients may differ from imaging symptomatic IA patients for several reasons. The aneurysms in asymptomatic patients tend to be smaller and require higher image resolution than those in symptomatic patients. On the other hand, these otherwise healthy individuals are better able to tolerate the relatively long imaging time of MR angiography than patients who have experienced SAH. Movement artifacts are less common in asymptomatic patients, and the potential arterial spasm or intra-aneurysmal fresh thrombus seen in patients with SAH are not present to interfere with the flow conditions and signal intensity16.
The advent of spiral computed tomography has led to the development of computed tomographic angiography (CT-Angiography), a technique that allows imaging of the intracranial arteries after an intravenous bolus administration of contrast material and rapid sequence CT scanning 6. The overall sensitivity and specificity of CT-Angiography for detection of aneurysm presence has been found to vary, with ranges of 77 to 97% and 50 to 100% respectively6,17-23.
Anderson et Al6 reported that anatomical information provided by CTA was excellent and helpful for surgical planning. For endovascular embolization using GDCs, various anatomic characteristics of the aneurysms such a neck size, shape, lobularity and arterial branches adjacent to the aneurysmal neck must be determined. In certain cases, CTA was superior to DSA in determining aneurysm shape and contour6.
Matsumoto et Al 24 detected one hundred ruptured aneurysms, including 41 associated unruptured lesions, using 3D-CT angiography in 100 consecutive patients with SAH. All of the ruptured aneurysms were confirmed at surgery and treated successfully. They believe that 3D-CT angiography can replace conventional angiography in the diagnosis of ruptured aneurysms and that surgery can be performed in almost all acutely ruptured aneurysms by using only 3D-CT angiography without conventional angiography. Furthermore, they performed surgery in basilar artery tip aneurysms on the basis of 3D-CT angiography alone because they were able to visualize the course of the vein of Labbé for the surgical approach.
The CT data can be processed to produce vascular images in three different ways: shaded surface display (SSD), maximum intensity projection (MIP) and multi or curved planar reformatting (CPR). CPR allows better demonstration of the body and neck of an aneurysm at the carotid siphon, which has a tortuous course and is surrounded by complex bony structures, but this technique has some limitations (spatial relations and the projection and direction of an aneurysm to all other structures cannot always be seen and it is highly operator-dependent) 25. Young et Al19 now use the surface display almost exclusively, because it provides a much better visual cast of aneurysms with depth and shading, useful particularly in the identification of very small aneurysms.
The adjustment of the time delay to obtain the maximum contrast concentration resulted in excellent arterial visualisation, and the 3D CT images could be generated at higher threshold levels (150-200 HU)26.
In published reports, 3D-CT angiography has been found to be superior to conventional angiography in depicting an aneurysm's shape, its relations to surrounding vessels and the adjacent skull, and in demonstrating the anatomical variation of vessels 17,18,27,28.
The facility for rotating the 3D-CT angiography device from any angle desired is of particular value in cases that are difficult to assess on conventional angiography 29, and produced additional 3D information for evaluating the neck of the aneurysm, which could not be assessed using conventional angiography 29, providing useful surgical information, such as the presence of calcification of the aneurysm neck and its anatomical relationship to the skull27.
3D-CT angiography sometimes can reveal an aneurysm that conventional angiography cannot 18 and is useful in the detection of cerebral aneurysms in patients with SAH in whom angiography revealed no diagnostic findings 18.
The sensitivity of helical CT in detecting calcium may be advantageous to surgeons, as calcium in the neck of an aneurysm may cause difficulty during clipping 30.
Intraluminal thrombus has the potential to embolize distally and may be better appreciated at helical CT than at conventional angiography or MR angiography30.
The minimum size of an aneurysm detected on 3D-CT angiography varies depending on the helical scanner used. According to various studies, the smallest aneurysm detected on 3D-CT angiography is approximately 2 mm in diameter17,19,26,31,32, and aneurysms as small as 1 mm were not usually detected by this method26, In the Matsumoto et Al series24, the smallest aneurysm visualized on 3D-CT angiography was 0.8 mm in diameter. These variations in detectability may be caused by technical differences. Some disadvantages of CTA can be seen in table 2.
Table 2.
Disadvantages of CT-Angiography
| 1 | It could not demonstrate collateral vessels, small perforating vessels (such as the recurrent artery of Heub- ner, the anterior choroidal artery, and thalamoperforating arteries), arteriosclerotic changes or vasospasm as clearly as IA-DSA6,24,26. |
| 2 | The inability to determine temporal (time-related) characteristics of artery and aneurysm filling 6, and 3D-CT angiography does not supply dynamic information on the cerebral circulation, such as collateral flow 24,26. |
| 3 | Its sensitivity is relatively low. With a sensitivity of 77 to 97%6,17-23,27, this method may be acceptable in an emergency situation in which time is limited. Otherwise, this level of sensitivity is too low for the routine workup of stable patients with aneurysmal SAH37,38. Therefore, in elective situations, all patients should un- dergo conventional femoral angiography as a preoperative procedure38 or 3D IA-ROT-DA that we now believe is the gold standard. However, the majority of “missed” aneurysms were smaller than 4 mm27,24. Though Matsumoto et Al38 have a 100% sensitivity, we believe that a multicentric study is necessary to clarify this point. |
| 4 | High loads of contrast are required. This is approximately 1.5 times the volume of similar iodinated con- trast that is used for routine four-vessel cerebral angiography27,37,39. |
| 5 | Cost-effectiveness of CTA. With a 77 to 97% sensitivity, it is not possible to predict the cost incurred by 23- 3% of the patients who would have had aneurysms not detected or would have undergone explorations showing negative results37. However, the average cost of a three-dimensional computed tomographic an- giogram was 31% of the average cost of a cerebral digital subtraction angiographic study and 72% of the average cost of a cerebral magnetic resonance angiographic study28. |
| 6 | The simultaneous demonstration of both arteries and veins on 3D-CT angiography makes their discrimi- nation difficult24. |
| 7 | The aneurysm located close to the skull base may not be visible against the bone structures27. |
| 8 | CTA does not provide a definitive diagnosis in dissecting aneurysms18,24. |
| 9 | The potential for a subarachnoid haemorrhage to obscure an aneurysm, due to the similarity in attenua- tion of blood and contrast material30. |
| 10 | CT angiography requires considerable dependence on operator skill, experience, and for the timing of the scan we need to find the optimum opacity of the vessels19. |
| 11 | Considerable time is needed for the three dimensional reconstruction and close examination of the im- ages19. For this reason, Young et Al19 have not yet used this technique in emergency situations such as in- tracerebral haematoma due to aneurysms. |
According to Matsumoto et Al24, conventional angiography is required in: a) patients in whom cerebral infarct was demonstrated on unenhanced CT scans to evaluate stenotic or occlusive lesions of the intracranial arteries, cervical carotid or vertebral arteries; b) giant or large aneurysms, in which the haemodynamic information provided by conventional angiography is necessary for treatment; c) aneurysms close to bone structures, such as ICA-ophthalmic artery aneurysms, to obtain a precise diagnosis; d) patients with dissecting aneurysms, in which conventional angiography findings of true and false lumen are needed for treatment; e) patients who have discrepancies between the distribution of SAH on CT scans and the location of the aneurysms on 3D-CT angiography to confirm whether there is another aneurysm in the area in which SAH has been shown.
Spiral CT angiography was useful in the detection of cerebral aneurysms in patients with SAH in whom angiography revealed no diagnostic findings 18. Anterior communicating artery aneurysms are generally well hidden in these types of SAH18.
Schwartz et Al 30 in an evaluation of cerebral aneurysms with helical CT compared with conventional angiography and MR angiography, reported that all aneurysms 3 mm or larger in maximum dimension were seen with helical CT and MR angiography. However aneurysms smaller than 3 mm were not apparent with either method. Small aneurysms (3-5 mm) close to the skull base were occasionally not seen well on the reconstructed images but were always visualized on the axial source images. Of 21 aneurysms demonstrated with both helical CT and MR angiography, 11 were seen equally well with both techniques; six were seen better with helical CT owing to flow-related or motion artefacts at MR angiography, and fourwere seen better with MR angiography because calcium partially obscured them at helical CT30.
Although angiographic verification before aneurysm surgery is preferable, in the moribund patient with intracerebral hematoma form a ruptured aneurysm, infusion CT scanning provides sufficient information concerning vascular anatomy to allow rational emergency craniotomy and aneurysm clipping33.
Rotational angiography provided better definition of the aneurysms neck and greater clarity of aneurysms than IA-DSA. It also improved the level of confidence in predicting the presence or absence of aneurysms, especially in the anterior communicating artery 34,35. The lack of subtraction artefacts from the surgical clips and multiple angles of view allowed better assessment of the presence or absence of a residual neck in postoperative cases 35.
With helical CT Angiography with intraarterial contrast medium administration (IAC), evaluation of collateral vessels is difficult. However, small arterial branches such as the posterior communicating artery were observed in some cases 2. Helical CT angiography with IAC is superior to rotational IA-DSA in the evaluation of the aneurysmal neck. Rotational DSA only clearly demonstrated the aneurysmal neck in 56.2% as the aneurysmal domes overlaps the neck. On the other hand, helical CTAngiography with IAC clearly showed the aneurysm neck in all cases 2. In addition, the aneurysmal shape was more clearly and spatially demonstrated by helical CT Angiography with IAC than rotational DSA, as this technique can provide multiple views of aneurysms. Concerning arterial branches adjacent to the aneurysmal neck, helical CTA was also superior to rotational DSA, especially in large aneurysms 2. The disadvantage of helical CT angiography with intraarterial contrast is the high capital cost of the equipment, as you need the CT scanner in the angiography room.
With intraarterial rotational DSA and three-dimensional reconstruction (3D IA-ROT-DSA) we can see the aneurysmatic complex from every angle. This allows a correct evaluation of key points such as: aneurysmal size, lobularity and shape, neck size, parent and efferent arteries and arterial branches adjacent to the aneurysmal neck 36, which in our experience is present in all patients.
The three-dimensional nature of this technique and the ability to view the three-dimensional imaging volume at multiple angles was helpful in identifying the relationship of the aneurysm to the parent vessel and evaluating the neck of an aneurysm beneath overlapping tortuous vessels. For large or giant aneurysms, the neck, parent and efferent arteries can be delineated. The ability to depict the aneurysmal neck and the projection of the aneurysm relative to the parent artery is advantageous for evaluating the outcome of clipping or embolization of the aneurysm (figure 5).
Published data of Tanoue et Al36 demonstrated that the 3-D reconstructed images correlated well with surgical findings. All the lesions were found during surgery in exactly the same locations depicted by 3-D DSA, and there were no false-positives or false-negatives. Therefore the sensitivity and specificity was 100% in the detection of the aneurysms. Nipple on the aneurysm was demonstrated, and each nipple was found to be exactly in the same position at surgery as on the 3D reconstruction, and were found at locations identical to the point of rupture 36.
On the other hand, neither an aneurysm's relationship to adjacent structures (bone, veins, and brain parenchyma) nor calcification of the aneurysm or the major arteries can be shown using 3-D DSA36.
It is important to note that in the Tanoue et Al series36, 3-D rotational angiography tended to underestimate the size of the neck. This should be kept in mind when aneurysms are being evaluated using this new modality and various treatment options are considered 40. Although this was not true in our study, we believe that a multicentric study with different angiographic systems is necessary to verify this point.
A useful aspect of this technique is its capacity to give information on angulation and rotation degrees of the C-arm to obtain the best projection for endovascular embolization (figure 4). This information saves contrast media and time as it avoids the search for various different projections to obtain this same information.
3D IA-ROT-DSA can disclose small cerebral aneurysms that conventional angiography fails to reveal (figure 6). However, it remains unclear to what extent this method can be used to distinguish patients with cerebral aneurysms in whom angiography did not reveal diagnostic findings and individuals who experience SAH of unknown cause.
Conclusions
3D IA-ROT-DSA is a new technique with the ability to view the 3D imaging volume at multiple angles. This permits complete visualisation of the aneurysm from any one angle and allows correct evaluation of the key features such as: aneurysmal size, lobularity and shape, neck size, parent and efferent arteries and arterial branches adjacent to the aneurysmal neck.
This technique demonstrated microaneurysms in patients with SAH undetected by conventional angiography.
For endovascular embolisation, this technique provides accurate angulation and rotation degrees of the C-arm, to obtain the best projection. This avoids multiples series, saves contrast media and time. The anatomic characteristics of the aneurysm suck as neck size, shape, lobularity and arterial branches adjacent to the aneurysmal neck can be determined. All this information advocates the correct choice of initial coil to build up an acceptable basket that permits total occlusion.
3D IA-ROT-DSA can provide useful anatomical information for surgical planning, especially in giant aneurysms with an arterial complex adjacent to the aneurysmal neck.
For these reasons, we believe that 3D IA-ROT-DSA is now the gold standard for patients with SAH of aneurysmal origin.
Acnowledgements
The authors are grateful to residents and nurses for assistance and maintenance of excellent standards, to Prof. Pierre Lasjaunias for his advice and constructive comments, to Dr. Jeyaledchumy Mahadevan and Rose Hickman for English editing and Pablo Ayesta for photographic assistance.
References
- 1.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 2.Nomura M, Kida S, et al. Pre-embolization study of ruptured cerebral aneurysms with helical TC. Interventional Neuroradilogy. 1999;5(suppl 1):219–223. doi: 10.1177/15910199990050S142. [DOI] [PubMed] [Google Scholar]
- 3.Murayama Y, Viñuela F, et al. Endovascular treatment of incidental cerebral aneurysms. Interventional Neuroradiology. 1999;5(suppl 1):79–81. doi: 10.1177/15910199990050S114. [DOI] [PubMed] [Google Scholar]
- 4.Rhoton AL. Microsurgical anatomy of saccular aneurysms. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill Book Company; 1990. pp. 1330–1340. [Google Scholar]
- 5.Waugh JR, Sacharias N. Arteriographic Complications in the DSA Era. Radiology. 1992;182:243–246. doi: 10.1148/radiology.182.1.1727290. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GB, Findlay JM, et al. Experience with computed tomographic angiography for the detection of intracranial aneurysms in the setting of acute subarachnoid haemorrhage. Neurosurgery. 1997;41:522–528. doi: 10.1097/00006123-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Schuierer G, Huk WJ, Laub G. Magnetic resonance angiography of intracranial aneurysms: comparison with intra-arterial digital subtraction angiography. Neuroradiology. 1992;35:50–54. doi: 10.1007/BF00588279. [DOI] [PubMed] [Google Scholar]
- 8.Gouliamos A, Gotsis E, et al. Magnetic resonance angiography compared to intra-arterial digital subtraction angiography in patients with subarachnoid haemorrhage. Neuroradiology. 1992;35:46–49. doi: 10.1007/BF00588278. [DOI] [PubMed] [Google Scholar]
- 9.Masaryk TJ, Modic MT, et al. Intracranial Circulation: Preliminary Clinical Results with Three-dimensional (volume) MR Angiography. Radiology. 1989;171:793–799. doi: 10.1148/radiology.171.3.2717754. [DOI] [PubMed] [Google Scholar]
- 10.Grandin CB, Mathurin P, et al. Diagnosis of Intracranial Aneurysms: Accuracy of MR Angiography at 0.5 T. Am J Neuroradiol. 1998;19:245–252. [PMC free article] [PubMed] [Google Scholar]
- 11.Horikoshi T, Fukamachi A, et al. Detection of intracranial aneurysms by three-dimensional time-of-flight magnetic resonance angiography. Neuroradiology. 1994;36:203–207. doi: 10.1007/BF00588131. [DOI] [PubMed] [Google Scholar]
- 12.Ida M, Kurisu T, et al. MR angiography of ruptured aneurysms in acute subarachnoid haemorrhage. Am J Neuroradiol. 1997;18:1025–1032. [PMC free article] [PubMed] [Google Scholar]
- 13.Huston J, III, Nichols DA, et al. Blinded prospective evaluation of sensitivity of MR angiography to know intracranial aneurysms: importance of aneurysm size. Am J Neuroradiol. 1994;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 14.Maeder PP, Meuli RA, de Tribolet N. Three-dimensional volume rendering for magnetic resonance angiography in the screening and preoperative workup of intracranial aneurysms. J. Neurosurg. 1996;85:1050–1055. doi: 10.3171/jns.1996.85.6.1050. [DOI] [PubMed] [Google Scholar]
- 15.Atlas SW, Listerud J, et al. Intracranial Aneurysms: Depiction on MR Angiograms with a Multifeature-Extraction, Ray-Tracing Postprocessing Algorithm. Radiology. 1994;192:129–139. doi: 10.1148/radiology.192.1.8208924. [DOI] [PubMed] [Google Scholar]
- 16.Ronkainen A, Puranen MI, et al. Intracranial aneurysms: MR Angiographic Screening in 400 Asymptomatic Individuals with Increased Familial Risk. Radiology. 1995;195:35–40. doi: 10.1148/radiology.195.1.7892491. [DOI] [PubMed] [Google Scholar]
- 17.Alberico RA, Patel M, et al. Evaluation of the circle of Willis with three-dimensional CT angiography in patients with suspected intracranial aneurysms. Am J Neuroradiol. 1995;16:1571–1580. [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto H, Iida J, et al. Use of spiral computerized tomography angiography in patients with subarachnoid haemorrhage in whom subsraction angiography did not reveal cerebral aneurysms. J Neurosurg. 2000;92:278–283. doi: 10.3171/jns.2000.92.2.0278. [DOI] [PubMed] [Google Scholar]
- 19.Young N, Dorsch NWC, et al. Spiral CT scanning in the detection and evaluation of aneurysms of the Circle of Willis. Surg Neurol. 1998;50:50–61. doi: 10.1016/s0090-3019(98)00015-9. [DOI] [PubMed] [Google Scholar]
- 20.Hope JKA, Wilson JL, Thomson FJ. Three-dimensional CT angiography in the detection and characterization of intracranial berry aneurysms. Am J Neuroradiol. 1996;17:439–445. [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa T, Okudera T, et al. Cerebral aneurysms: evaluation with three-dimensional CT angiography. Am J Neuroradiol. 1996;17:447–454. [PMC free article] [PubMed] [Google Scholar]
- 22.Vieco PT, Shuman WP, et al. Detection of circle of Willis aneurysms in patients with acute subarachnoid haemorrhage: a comparision of CT angiography and digital subtraction angiography. Am J Radiol. 1995;165:425–430. doi: 10.2214/ajr.165.2.7618571. [DOI] [PubMed] [Google Scholar]
- 23.Velthuis BK, van Leeuwen MS, et al. Computerized tomography angiography in patients with subarachnoid haemorrhage: form aneurysm detection to treatment without conventional angiography. J Neurosurg. 1999;91:761–767. doi: 10.3171/jns.1999.91.5.0761. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, Sato M, et al. Three-dimensional computerized tomography angiography-guided surgery of acutely ruptured cerebral aneurysms. J Neurosurg. 2001;94:718–727. doi: 10.3171/jns.2001.94.5.0718. [DOI] [PubMed] [Google Scholar]
- 25.Ochi T, Shimizu K, et al. Curved planar reformatted CT angiography: Usefulness for the evaluation of aneurysms at the carotid siphon. Am J Neuroradiol. 1999;20:1025–1030. [PMC free article] [PubMed] [Google Scholar]
- 26.Nakajima Y, Yoshimine T, et al. Computerized tomography angiography of ruptured cerebral aneurysms: factors affecting time to maximum contrast concentration. J Neurosurg. 1998;88:663–669. doi: 10.3171/jns.1998.88.4.0663. [DOI] [PubMed] [Google Scholar]
- 27.Anderson GB, Steinke DE, et al. Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;45:1315–1322. doi: 10.1097/00006123-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Harbaugh RE, Schlusselberg DS, et al. Three-dimensional computed tomographic angiography in the preoperative evaluation of cerebrovascular lesions. Neurosurgery. 1995;36:320–327. doi: 10.1227/00006123-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Strayle-Batra M, Skalej M, et al. Three-dimensional spiral CT angiography in the detection of cerebral aneurysm. Acta Radiol. 1998;39:233–238. doi: 10.1080/02841859809172186. [DOI] [PubMed] [Google Scholar]
- 30.Scwartz RB, Tice HM, et al. Evaluation of Cerebral Aneurysms with Helical CT: Correlation with Conventional Angiography and MR Angiography. Radiology. 1994;192:717–722. doi: 10.1148/radiology.192.3.8058939. [DOI] [PubMed] [Google Scholar]
- 31.Hsiang JNK, Liang EY, et al. The role of computed tomographic angiography in the diagnosis of intracranial aneurysms and emergent aneurysm clipping. Neurosurgery. 1996;38:481–487. doi: 10.1097/00006123-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Zouaoui A, Sahel M, et al. Three-dimensional computed tomographic angiography in detection of cerebral aneurysms in acute subarachnoid haemorrhage. Neurosurgery. 1997;41:125–130. doi: 10.1097/00006123-199707000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Le Roux PD, Dailey AT, et al. Emergent Aneurysm Clipping without Angiography in the Moribund Patient with Intracerebral Haemorrhage: The use of Infusion Computed Scans. Neurosurgery. 1993;33:189–197. doi: 10.1227/00006123-199308000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Tu RT, Cohen WA, et al. Digital Subtraction Rotational Angiography for Aneurysms of the Intracranial Anterior Circulation: Injection Method and Optimization. Am J Neuroradiol. 1996;17:1127–1136. [PMC free article] [PubMed] [Google Scholar]
- 35.Hoff DJ, Wallace Ch, et al. Rotational Angiography Assessment of Cerebral Aneurysms. Am J Neuroradiol. 1994;15:1945–1948. [PMC free article] [PubMed] [Google Scholar]
- 36.Tanoue Sh, Kiyosue H, et al. Three-dimensional Reconstructed Images after Rotational Angiography in the Evaluation of Intracranial Aneurysms: Surgical Correlation. Neurosurgery. 2000;47:866–871. doi: 10.1097/00006123-200010000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Vishteh AG, Spetzler RF, Comments of Anderson GB, Findlay JM, et al. Experience with computed tomographic angiography for the detection of intracranial aneurysms in the setting of acute subarachnoid haemorrhage. Neurosurgery. 1997;41:522–528. doi: 10.1097/00006123-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Solomon RA, Comments to Anderson GB, Steinke DE, et al. Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;45:1315–1322. doi: 10.1097/00006123-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Dorsch NWC, Young N, et al. Early Experience with Spiral CT in the Diagnosis of Intracranial Aneurysms. Neurosurgery. 1995;36:230–238. doi: 10.1227/00006123-199501000-00037. [DOI] [PubMed] [Google Scholar]
- 40.Barrow DL, Comments to Tanoue Sh, Kiyosue H, et al. Three-dimensional Reconstructed Images after Rotational Angiography in the Evaluation of Intracranial Aneurysms: Surgical Correlation. Neurosurgery. 2000;47:866–871. doi: 10.1097/00006123-200010000-00016. [DOI] [PubMed] [Google Scholar]