Abstract
BACKGROUND
Blood group A and B antigens are expressed only weakly on platelets (PLTs) of most individuals but are very strongly expressed on PLTs from approximately 1 percent of normal subjects (Type II high expressers). The implications of this trait for transfusion medicine are undefined.
STUDY DESIGN AND METHODS
A family was studied in which two Group B infants were born with neonatal thrombocytopenia, whereas a third infant whose blood group was A2 had a normal PLT count at birth.
RESULTS
Serologic studies demonstrated a maternal antibody that reacted strongly with PLTs from the father and the two group B children in flow cytometry and with GPIIb/IIIa from their PLTs in solid-phase assays. No PLT-specific antibodies were detected in maternal serum sample, but it contained a high-titer immunoglobulin G antibody specific for blood group B. All PLT-reactive antibody in the mother’s serum was removed by absorption with pooled, washed group A and B red cells (RBCs). Studies with monoclonal anti-B and measurement of serum B-glycosyltransferase activity showed that the father and both group B children were Type II high expressers of blood group B.
CONCLUSIONS
The findings indicate that high-titer blood group antibodies acquired from the mother can cause thrombocytopenia in infants possessing the Type II high-expresser phenotype despite competition for antibody binding by blood group antigens expressed on RBCs and other tissues.
Neonatal alloimmune thrombocytopenia (NAIT) is caused by transplacentally acquired maternal antibodies reactive with platelet (PLT)-specific alloantigens (HPA antigens) and occurs in about 1 of every 1000 births.1-4 Many cases are mild and remit spontaneously, but thrombocytopenia can be severe and numerous fatalities have been recorded.2-5 Unlike its red blood cell (RBC) counterpart, hemolytic disease of the newborn (HDN), almost two-thirds of NAIT cases occur in firstborn infants.5
Numerous human PLT alloantigens (HPAs) are capable of inducing maternal immunization during pregnancy and causing NAIT.5-7 Maternal-fetal incompatibility for the high-frequency alloantigen HPA-1a (PlA1, Zwa) accounts for 75 to 85 percent of the reported cases.3,4,7 A specific serologic diagnosis, however, is made in fewer than half of the suspected cases.7 Non-HPA antigens such as ABH and Class I HLA are shared by PLTs and other tissues. Although anecdotal reports have claimed that HLA-specific antibodies can cause NAIT,8-10 there is no consensus on this point because infants born to multiparous women immunized against Class I HLA antigens nearly always have a normal PLT count.
Fetal-maternal incompatibility for blood group A or B has not previously been implicated as a cause of NAIT and one report actually suggested that incompatibility for ABH may protect against NAIT as it does against HDN associated with fetal-maternal Rh incompatibility.11 It is not surprising that a role for ABH incompatibility in NAIT has not been considered because A and B antigens are expressed very weakly on PLTs of most normal individuals.12,13 Recent studies have shown, however, that a subset of A and B antigen positive normal subjects has PLTs that carry many times more than the usual number of A or B determinants12-14 and that these determinants are located on various PLT membrane glycoproteins (GPs), especially GPIIb and PECAM-1 (CD31).12 Curtis and colleagues12 showed that group A1 individuals can be divided into three subgroups; approximately two-thirds have PLTs with low A antigen expression (fewer than 2000 epitopes per PLT). A second group constituting approximately 30 percent has moderately increased A antigen expression (2-6,000 epitopes per PLT). Approximately 1 to 2 percent, however, have PLTs that carry 10 to 20,000 copies of A or B per PLT. The latter two groups were shown by statistical analysis to be distinct subpopulations and were designated “Type I” and “Type II” high expressers (H-Exp), respectively.12 The distribution of B antigen expression on PLTs appears to be similar to that of A antigen, but has been less well studied.12,14
Ogasawara and coworkers14 showed that PLTs from a “high-expresser” of blood group B were rapidly destroyed upon being transfused to a group O patient. Except for this single observation, however, the clinical significance of the H-Exp trait has not been critically examined. Here we describe a family in which two group B infants who inherited the Type II H-Exp phenotype from their father were born with moderately severe thrombocytopenia and present evidence that this complication was caused by transplacentally acquired, high-titer maternal immunoglobulin G (IgG) antibodies specific for blood group B.
CASE REPORT
The first child, born full term to a group O mother and A2B father both of Hispanic ancestry, required ventilatory and metabolic support and corticosteroids for treatment of meconium aspiration syndrome, from which it recovered after several weeks of hospitalization. Hematologic studies performed on the second day of life revealed a hemoglobin (Hb) level of 10.4 g per dL, a reticulocyte concentration of 4.4 percent, a PLT count of 33 × 109 per L, a positive antiglobulin test (IgG only), and RBCs positive for blood group B. Mild hyperbilirubinemia developed in the first week, but did not require phototherapy. PLT transfusions were given on Days 1, 3, and 5 to sustain PLT counts and a RBC transfusion was given on Day 5. PLT count increased to 220 × 109 per L on Day 11. PLTs and Hb remained in the normal range thereafter. The mild hemolytic anemia was attributed to maternal-fetal incompatibility for blood group B. The child is developing normally at 7 years of age.
A spontaneous abortion occurred 2 years later and a second full-term infant was born 1 year subsequently. Hematologic studies performed on the first day of life revealed a Hb level of 10.0 g per dL, a reticulocyte concentration of 14.5 percent, a PLT count of 61 × 109 per L, a positive antiglobulin test (IgG only), and RBCs positive for blood group B. Bilirubin was 17.4 mg per mL on the second day. Intravenous (IV) IgG, 0.5 g per kg, was infused on Day 2 for treatment of HDN and presumptive alloimmune thrombocytopenia. The PLT count increased to 123 × 109 per L on Day 5 and was normal at 2 weeks. No PLT or RBC transfusions were required. With phototherapy, bilirubin declined to 7.6 mg per mL after 1 week and was normal after 2 weeks.
A third full-term infant born the following year was blood group A2 and had a PLT count of 248 × 109 per L. The direct antiglobulin test was negative and there was no evidence of hemolysis or hyperbilirubinemia. The mother had a normal PLT count at the time of each delivery and no history of thrombocytopenia.
MATERIALS AND METHODS
Antibodies
Monoclonal antibodies (MoAbs) BRIC145 (anti-A), BGRL2 (anti-B), and BRIC198 (anti-H) were purchased from the International Blood Group Reference Laboratory (Bristol, UK). MoAbs specific for GPIIb/IIIa (AP2) and GPIa/IIIa (MBC202.2) used in the modified antigen capture enzyme-linked immunosorbent assay (MACE) were produced by the Hybridoma Core Laboratory, BloodCenter of Wisconsin, and have been described previously.12 The nonreactive murine monoclonal IgG1 antibody, MOPC-21 was from Sigma Chemical Co. (St Louis, MO). Fluorescein isothiocyanate (FITC)-labeled F(ab’)2 goat anti-human IgG-Fc, FITC-F(ab’)2 goat anti-mouse IgG-Fc, and alkaline phosphatase–labeled mouse anti-human IgG-Fc were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Serologic studies
PLT-reactive antibodies were characterized with a combination of serologic assays described previously.15-17 In brief, serum was screened for antibodies against a panel of group O target PLTs expressing HPA-1a, -1b, -2a, -2b, -3a, -3b, -4a, -5a, and -5b in a flow cytometric assay. PLT-bound immunoglobulins were detected with FITC–anti-human IgG-Fc with a flow cytometer (FACSCalibur, Becton Dickinson, San Jose, CA). Antibodies reactive with Class I HLA antigens and GPIb/IX and GPIV were detected with a commercial enzyme-linked immunosorbent assay (ELISA) kit PAK-2MP (GTI, Inc.,Waukesha,WI). Antibodies against HPA antigens carried on GPIIb/IIIa and GPIa/IIa were detected by MACE.15-17
HPA genotyping
Genotyping of parental DNA for PLT-specific antigens of the HPA-1, -2, -3, -4, -5, -6, -9, and -15 systems was performed with a multicode PLX assay developed with Eragen Biosciences (Madison, WI).18,19 Isolated genomic DNA was amplified by multiplex polymerase chain reaction (PCR), followed by HPA allele-specific primer extension with incorporation of synthetic oligonucleotides (EraCodes) and a biotin label. EraCode-labeled PCR products were incubated with beads (Luminex, Luminex Corp., Austin, TX) coated with complementary EraCode oligonucleotides (Eragen Biosciences). Beads were then incubated with streptavidin-phycoerythrin (PE) for 30 minutes at room temperature. PE fluorescence of PCR products bound to specific “addresses” on the beads was measured in an analyzer (Luminex-100, Luminex Corp.). Assignment of PLT antigen genotypes was made with a customized software program developed by Eragen. Typing for mutations encoding low-frequency antigens of the HPA-7, -8, -10 to -14, and -16 systems was done by PCR amplification of exons known to contain the relevant mutations.20-26 PCR primers corresponding to intronic sequence surrounding exons of interest were designed to yield PCR products ranging from 500 to 1900 nucleotides in length. Exons examined include GPIIIa exons 2 (HPA-10), 3 (HPA-16), 9 (HPA-7), 10 (HPA-14), and 11 (HPA-8, -11); GPIbb exon 1 (HPA-12); and GPIa exon 20 (HPA-13). PCR was carried out with 0.2 mg of genomic DNA with a PCR system (Fast Start High Fidelity PCR system, Roche Diagnostics, Indianapolis, IN). Amplification conditions varied among the alleles but all were based on computer software (Optimase Protocol Writer, Transgenomics, Inc., Omaha, NE). Primer sequences and amplification conditions are available upon request. Automated sequence analysis was performed in both directions on a genetic analyzer (ABI 3100, Applied Biosystems, Foster City, CA) as described.17
Isoagglutinin titers
One drop of a 5 percent suspension of washed normal group A1 or B RBCs was incubated with 0.1 mL of serially diluted serum for 15 minutes at 37°C. The cells were washed three times in phosphate-buffered saline (PBS), suspended in two drops of commercial anti-human IgG, Fc-specific (Immucor, Norcross, GA), centrifuged for 15 seconds, and graded for agglutination by shaking. The endpoint was taken as the highest dilution producing at least 1+ agglutination.
Measurement of ABH antigen expression
PLTs and RBCs were incubated with saturating quantities of A, B, or H-specific MoAbs for 30 minutes at room temperature, washed three times in 0.02 mmol/L PBS, 9 mmol/L Na2-ethylenediaminetetraacetate, 0.2 percent bovine serum albumin, pH 7.2, and incubated with FITC-labeled F(ab’)2 goat anti-mouse IgG. PLT-bound fluorescence was detected by flow cytometry as previously described.12
Serum B-transferase (d-galactosyltransferase) activity
As previously described,12 5 μL of papain-treated, washed, group O RBCs were incubated for 2 hours at 37°C with 50 μL of 1.6 mmol per L UDP-galactose (Sigma Chemical Co.), 25 μL of 0.1 mol per L cacodylic acid, pH 6.0, containing 0.1 mol per L MnCl2, and 100 μL of serum. The cells were then washed three times in PBS, and the newly synthesized B antigens were detected in flow cytometry by adding saturating amounts of the B-specific MoAb and BGRL2 and detecting bound MoAb with FITC-labeled F(ab’)2 goat anti-mouse IgG.
RESULTS
A maternal antibody reacted strongly with paternal PLTs and paternal GPIIb/IIIa but was not specific for recognized PLT-specific alloantigens
Maternal and paternal blood samples were studied for the first time during the second trimester of the third pregnancy because of a history suggestive of NAIT in the first two children. The mother’s serum sample contained an IgG antibody that reacted strongly with father’s PLTs in flow cytometry but only very weakly with PLTs from two group O donors carrying antigens of the HPA-1, -2, -3, and -5 systems and the high-frequency antigen HPA-4a (Fig. 1). Maternal serum reacted strongly in MACE with GPIIb/IIIa from the father’s PLTs but not with GPIIb/IIIa from the same two group O normal subjects that were positive in flow cytometry (Fig. 2). Weak antibodies specific for Class I HLA antigens, but no antibodies reactive with GPIb/IX, GPIa/IIa, or GPIV (CD36), were detected with the PAK-2MP ELISA, which utilizes target GPs isolated from group O PLTs. Genotyping of parental DNA for each allele of the HPA-1, -2, -3, -4, -5, -6, -9, and -15 systems identified only two antigens, HPA-2b and -3b, that were present in the father but not the mother. As noted, however, the mother’s serum failed to react in flow cytometry and MACE against PLTs from group O donors positive for these antigens. Because these findings could be explained by maternal immunization against a “rare” PLT-specific antigen carried on father’s PLTs,6,17 the father was genotyped for HPA-7bw, -8bw, -10 to -14bw, and -16bw with negative results.
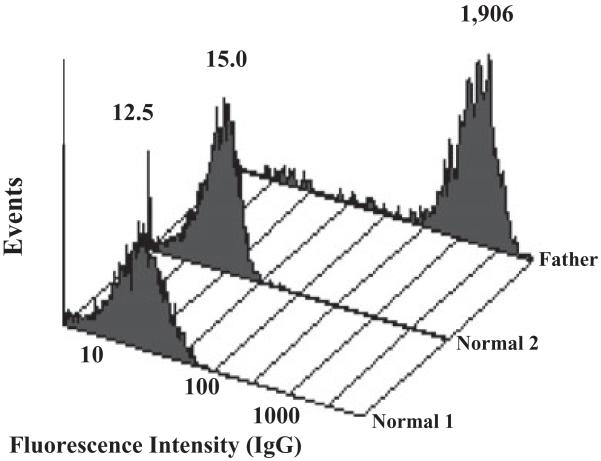
Fig. 1.
Maternal serum reacted strongly with paternal PLTs, but not with PLTs from two normal group O subjects. Values above each flow cytometry histogram indicate mean fluorescence intensity (MFI) of the PLT population. The MFI of PLTs incubated with normal serum was 5.2 (not shown)
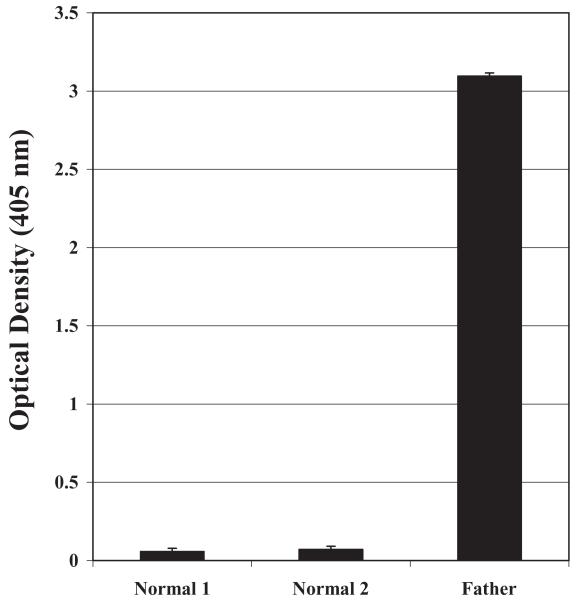
Fig. 2.
Maternal IgG reacted with GPIIb/IIIa captured from father’s PLTs, but not normal group O PLTs. Optical density values from MACE results are shown. Error bars denote + 2 SD
The maternal antibody was specific for blood group B
Failure to identify a specificity for the strong maternal antibody reactive with father’s PLTs by these approaches, together with the fact that blood groups of the father and mother were A2B and O, respectively, led us to investigate the possibility that maternal-fetal incompatibility for blood group B might have played a role in causing thrombocytopenia in the two B+ infants. As shown in Fig. 3, reactions of maternal serum with father’s PLTs, normal group B PLTs, and the father’s GPIIb/IIIa were abolished by absorption with pooled group A1 and B RBCs (to assure removal of antibodies with A,B specificity) washed repeatedly to remove essentially all contaminating PLTs and white blood cells.
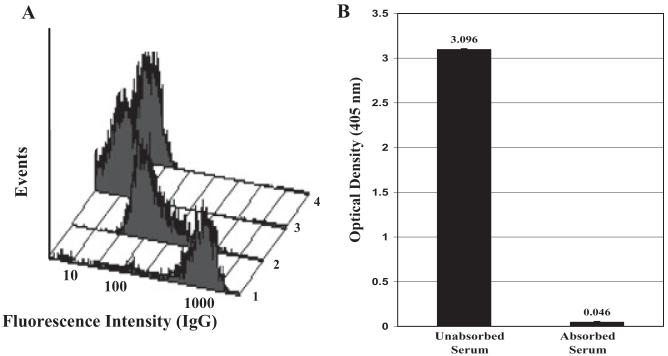
Fig. 3.
Absorption of maternal serum with normal A and B RBCs completely abolished reactivity with the father’s group B PLTs. (A) Unabsorbed maternal serum reacted strongly with the father’s PLTs (Lane 1) and normal group B PLTs (Lane 2). These reactions were completely abolished by absorption with washed, pooled group A and B RBCs (Lanes 3 and 4). (B) Similarly, absorption with RBCs abolished the reaction of maternal serum with fathers GPIIb/IIIa in MACE. Error bars denote + 2 SD
Titration studies showed that the mother’s IgG anti-B reacted with group B RBCs at an inverse dilution of 16,348. Inverse IgG isoagglutinin titers in serum from five randomly selected group O female blood donors and eight group O multiparous (3 or more pregnancies) women were 16, 32, 64, 128, 256, 32, 64, 128, 64, 512, 1,024, 1,024, and 8,192, respectively.
The father and both affected infants were Type II high expressers of B antigen
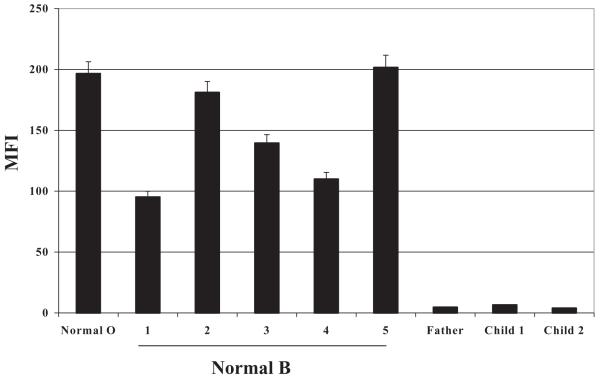
Reactions of monoclonal anti-B with PLTs from the father and the two affected infants are shown in Fig. 4. The sharp peak, shifted far to the right, is typical of “Type II high expression” of B and differs from the broad histogram obtained with PLTs from most group B subjects (second histogram).12 Values obtained for mean fluorescence intensity indicated that PLTs from the father and the two children carried 25, 23, and 23 times the amount of B antigen found on PLTs of a “normal” group B individual. Serum from the father and both children also contained extremely high levels of α-d-galactosyltransferase (B-glycosyltransferase) activity relative to serum from unselected group B normal donors (Table 1), a finding typical of the Type II high-expresser phenotype.12 Finally, H antigen was virtually undetectable on PLTs from all three group B high expressers, in contrast to significant amounts of H present on cells of most Group B individuals (Fig. 5). The likely explanation for this is that essentially all H substance, the substrate for synthesis of A and B antigens, is converted to B by the highly active B-glycosyltransferase.12 H substance was also barely detectable on RBCs of the father (not shown), in contrast to RBCs from normal group B persons.12
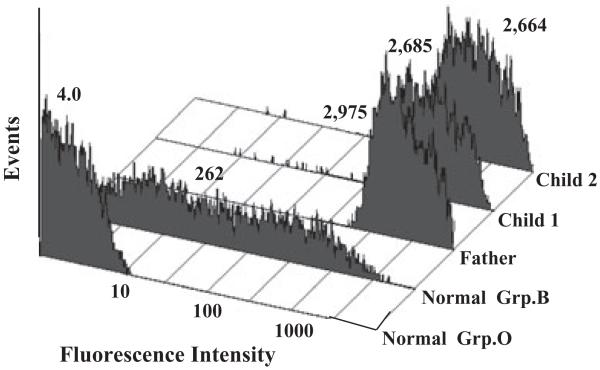
Fig. 4.
B antigen was strongly expressed on PLTs from the father and the two group B children affected with NAIT. The anti-B MoAb BGRL2 was used to measure B antigen expression. Reactions of BGRL2 with normal group O and B PLTs are shown in the first two flow cytometry histograms. Values above each histogram indicate MFI of the PLT population
TABLE 1. Serum B-glycosyltransferase activity.
| Serum | MFI* | p Value |
|---|---|---|
| Normal 1 | 59.5 | |
| Normal 2 | 50.2 | |
| Normal 3 | 38.0 | |
| Normal 4 | 30.2 | |
| Normal 5 | 93.7 | |
| Normal 6 | 109.4 | |
| Type I high B expresser | 714.4 | <0.05† |
| Father | 3502.2 | <0.001† |
| Child 1 | 1225.7 | <0.001† |
| Child 2 | 1956.5 | <0.001† |
MFI = mean fluorescence intensity obtained by flow cytometry with monoclonal anti-B.
p Values determined by comparison (dependent t test) to mean for the six normal serum values.
Fig. 5.
H antigen was virtually absent from PLTs of the father and both group B infants but was readily detected on PLTs from five normal group B persons and a group O donor. H antigen expression was measured by flow cytometry with the H-specific monoclonal BRIC198. Error bars denote + 2 SD
DISCUSSION
The first two children born into this family had mild HDN associated with maternal-fetal incompatibility for blood group B, whereas the third infant, who inherited the A2 antigen, was unaffected. Although fetuses and newborns with severe HDN may have moderate thrombocytopenia,27 PLT levels are normal in mildly affected infants who do not require exchange transfusion.28 Thrombocytopenia in a newborn with HDN can also be due to concomitant alloimmunization of the mother against a PLT-specific antigen such as HPA-5b.29 A careful study of maternal serum obtained postnatally, however, failed to demonstrate antibodies reactive with any of the common alloantigens and isoantigens carried on PLT GPIIb/IIIa, GPIb/IX, GPIa/IIa, or IV and genotyping for all 16 of the recognized HPA alloantigen systems identified only two antigens (HPA-2b and -3b) that were present in the father but not the mother. Essentially complete removal of maternal antibody by absorption with washed, pooled group A1 and B RBCs (Fig. 3) also argues strongly against the possibility that an antibody specific for an HPA antigen was present in maternal serum. Weak antibodies reactive with Class I HLA antigens were present in maternal serum, but this finding is common in multiparous women and has not been shown convincingly to be a cause of NAIT.
Despite these noninformative findings, the strong reaction of maternal serum with paternal PLTs in flow cytometry (Fig. 1) and with paternal GPIIb/IIIa in MACE (Fig. 2), together with the fact that the mother’s blood group was O and the father’s was A2B, led us to examine B antigen expression on PLTs of the father and the two affected children. Demonstration of extremely high levels of PLT B antigen, virtual absence of H substance on PLTs and RBCs, and high levels of serum B-glycosyltransferase showed that all three were Type II high expressers of B antigen.12 This finding, together with failure to demonstrate maternal antibodies reactive with PLT-specific antigens and complete removal of maternal antibody by absorption with pooled group A1 and B RBCs, argues strongly that neonatal thrombocytopenia in the two affected infants was caused by maternal IgG antibody specific for blood group B. This possibility is further supported by the fact that a third child, who inherited the A2 blood group antigen, had a normal PLT count at birth. The findings suggest that measurement of A or B antigen expression on paternal PLTs can be helpful in evaluating cases of NAIT that are unresolved by conventional serologic studies.
Findings made in this family indicate that transplacentally acquired maternal anti-B can cause destruction of fetal PLTs carrying high levels of B antigen, despite competition for antibody binding by B substance expressed on RBCs and in other tissues. In ABO HDN the group O mother nearly always has a high-titer IgG antibody specific for A or B.30 The very high anti-B titer in the mother studied here suggests that this may also be a requirement for destruction of fetal PLTs expressing high levels of A or B antigen.
Although only 1 to 2 percent of A and B antigen positive individuals are Type II high expressers,12-14 up to 30 percent (Type I high expressers) have PLTs that carry significantly higher levels of A and B antigen than other group A+ and B+ individuals.12 The known incidence of NAIT (about 1 in 1000 live births) indicates that this fetal phenotype, in itself, does not lead to NAIT in an infant incompatible with its mother for A or B. Studies to determine whether the relatively common Type I high-expresser phenotype may increase the severity of “conventional” NAIT caused by maternal alloimmunization against a PLT-specific antigen such as HPA-1a (PlA1), however, especially if the mother has a high-titer A- or B-specific antibody, appear indicated.
Acknowledgments
Supported by Grant HL-13629 from the National Heart, Lung, and Blood Institute.
ABBREVIATIONS
- GP(s)
glycoprotein(s)
- MACE
modified antigen capture enzyme-linked immunosorbent assay
- NAIT
neonatal alloimmune thrombocytopenia
REFERENCES
- 1.Dreyfus M, Kaplan C, Verdy E, Schlegel N, Durand-Zaleski I, Tchernia G. Frequency of immune thrombocytopenia in newborns: a prospective study. Immune Thrombocytopenia Working Group. Blood. 1997;89:4402–6. [PubMed] [Google Scholar]
- 2.Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, Hughes D, Jobson S, Ouwehand WH. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–7. [PubMed] [Google Scholar]
- 3.Bussel JB. Alloimmune thrombocytopenia in the fetus and newborn. Semin Thromb Hemost. 2001;27:245–52. doi: 10.1055/s-2001-15254. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan C. Platelet alloimmunity: the fetal/neonatal alloimmune thrombocytopenia. Vox Sang. 2002;83(Suppl 1):289–91. doi: 10.1111/j.1423-0410.2002.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 5.Newman PJ, McFarland J, Aster RH. The alloimmune thrombcytopenias. In: Loscalzo J, Schafer A, editors. Thrombosis and hemorrhage. Blackwell Scientific Publications; Oxford: 2003. pp. 441–56. [Google Scholar]
- 6.Metcalfe P, Watkins NA, Ouwehand WH, Kaplan C, Newman P, Kekomaki R, De Haas M, Aster R, Shibata Y, Smith J, Kiefel V, Santoso S. Nomenclature of human platelet antigens. Vox Sang. 2003;85:240–5. doi: 10.1046/j.1423-0410.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 7.Davoren A, Curtis BR, Aster RH, McFarland JG. Human platelet antigen-specific alloantibodies implicated in 1162 cases of neonatal alloimmune thrombocytopenia. Transfusion. 2004;44:1220–5. doi: 10.1111/j.1537-2995.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 8.King KE, Kao KJ, Bray PF, Casella JF, Blakemore K, Callen NA, Kennedy SD, Kickler TS. The role of HLA antibodies in neonatal thrombocytopenia: a prospective study. Tissue Antigens. 1996;47:206–11. doi: 10.1111/j.1399-0039.1996.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 9.Saito S, Ota M, Komatsu Y, Ota S, Aoki S, Koike K, Tokunaga I, Tsuno T, Tsuruta G, Kubo T, Fukushima H. Serologic analysis of three cases of neonatal alloimmune thrombocytopenia associated with HLA antibodies. Transfusion. 2003;43:908–17. doi: 10.1046/j.1537-2995.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- 10.Thude H, Schorner U, Helfricht C, Loth M, Maak B, Barz D. Neonatal alloimmune thrombocytopenia caused by human leucocyte antigen-B27 antibody. Transfus Med. 2006;16:143–9. doi: 10.1111/j.1365-3148.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- 11.Gratwohl AA, Shulman NR. [ABO compatibility and isoimmune neonatal thrombopenia] Schweiz Med Wochenschr. 1977;107:1464. [PubMed] [Google Scholar]
- 12.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–81. [PubMed] [Google Scholar]
- 13.Cooling LL, Kelly K, Barton J, Hwang D, Koerner TA, Olson JD. Determinants of ABH expression on human blood platelets. Blood. 2005;105:3356–64. doi: 10.1182/blood-2004-08-3080. [DOI] [PubMed] [Google Scholar]
- 14.Ogasawara K, Ueki J, Takenaka M, Furihata K. Study on the expression of ABH antigens on platelets. Blood. 1993;82:993–9. [PubMed] [Google Scholar]
- 15.Curtis BR, Ali S, Glazier AM, Ebert DD, Aitman TJ, Aster RH. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion. 2002;42:1173–9. doi: 10.1046/j.1537-2995.2002.00176.x. [DOI] [PubMed] [Google Scholar]
- 16.Harrison CR, Curtis BR, McFarland JG, Huff RW, Aster RH. Severe neonatal alloimmune thrombocytopenia caused by antibodies to human platelet antigen 3a (Baka) detectable only in whole platelet assays. Transfusion. 2003;43:1398–402. doi: 10.1046/j.1537-2995.2003.00533.x. [DOI] [PubMed] [Google Scholar]
- 17.Peterson JA, Balthazor SM, Curtis BR, McFarland JG, Aster RH. Maternal alloimmunization against the rare platelet-specific antigen HPA-9b (Max a) is an important cause of neonatal alloimmune thrombocytopenia. Transfusion. 2005;45:1487–95. doi: 10.1111/j.1537-2995.2005.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson SC, Marshall DJ, Harms G, Miller CM, Sherrill CB, Beaty EL, Lederer SA, Roesch EB, Madsen G, Hoffman GL, Laessig RH, Kopish GJ, Baker MW, Benner SA, Farrell PM, Prudent JR. Multiplexed genetic analysis using an expanded genetic alphabet. Clin Chem. 2004;50:2019–27. doi: 10.1373/clinchem.2004.034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietz BC, Warden MB, DuChateau BK, Ellis TM. Multiplex genotyping of human minor histocompatibility antigens. Hum Immunol. 2005;66:1174–82. doi: 10.1016/j.humimm.2005.08.243. [DOI] [PubMed] [Google Scholar]
- 20.Kuijpers RW, Simsek S, Faber NM, Goldschmeding R, van. Wermerkerken RK, dem Borne AE. Single point mutation in human glycoprotein IIIa is associated with a new platelet-specific alloantigen (Mo) involved in neonatal alloimmune thrombocytopenia. Blood. 1993;81:70–6. [PubMed] [Google Scholar]
- 21.Peyruchaud O, Bourre F, Morel-Kopp MC, Reviron D, Mercier P, Nurden A, Kaplan C. HPA-10w(b) (La(a)): genetic determination of a new platelet-specific alloantigen on glycoprotein IIIa and its expression in COS-7 cells. Blood. 1997;89:2422–8. [PubMed] [Google Scholar]
- 22.Simsek S, Folman C, van der Schoot CE, dem Borne AE. The Arg633His substitution responsible for the private platelet antigen Gro(a) unravelled by SSCP analysis and direct sequencing. Br J Haematol. 1997;97:330–5. doi: 10.1046/j.1365-2141.1997.502696.x. [DOI] [PubMed] [Google Scholar]
- 23.Sachs UJ, Kiefel V, Bohringer M, Afshar-Kharghan V, Kroll H, Santoso S. Single amino acid substitution in human platelet glycoprotein Ibbeta is responsible for the formation of the platelet-specific alloantigen Iy(a) Blood. 2000;95:1849–55. [PubMed] [Google Scholar]
- 24.Santoso S, Amrhein J, Hofmann HA, Sachs UJ, Walka MM, Kroll H, Kiefel V. A point mutation Thr(799)Met on the alpha(2) integrin leads to the formation of new human platelet alloantigen Sit(a) and affects collagen-induced aggregation. Blood. 1999;94:4103–11. [PubMed] [Google Scholar]
- 25.Santoso S, Kiefel V, Richter IG, Sachs UJ, Rahman A, Carl B, Kroll H. A functional platelet fibrinogen receptor with a deletion in the cysteine-rich repeat region of the beta(3) integrin: the Oe(a) alloantigen in neonatal alloimmune thrombocytopenia. Blood. 2002;99:1205–14. doi: 10.1182/blood.v99.4.1205. [DOI] [PubMed] [Google Scholar]
- 26.Jallu V, Meunier M, Brement M, Kaplan C. A new platelet polymorphism Duv(a+), localized within the RGD binding domain of glycoprotein IIIa, is associated with neonatal thrombocytopenia. Blood. 2002;99:4449–56. doi: 10.1182/blood.v99.12.4449. [DOI] [PubMed] [Google Scholar]
- 27.Saade GR, Moise KJ, Jr, Copel JA, Belfort MA, Carpenter RJ. Fetal platelet counts correlate with the severity of the anemia in red-cell alloimmunization. Obstet Gynecol. 1993;82:987–91. [PubMed] [Google Scholar]
- 28.Koenig JM, Christensen RD. Neutropenia and thrombocytopenia in infants with Rh hemolytic disease. J Pediatr. 1989;114(4 Pt 1):625–31. doi: 10.1016/s0022-3476(89)80709-7. [DOI] [PubMed] [Google Scholar]
- 29.Schild RL, Hoch J, Plath H, Geissen C, Fahnenstich H, Dame C, Hansmann M. Perinatal management of fetal hemolytic disease due to Rh incompatibility combined with fetal alloimmune thrombocytopenia due to HPA-5b incompatibility. Ultrasound Obstet Gynecol. 1999;14:64–7. doi: 10.1046/j.1469-0705.1999.14010064.x. [DOI] [PubMed] [Google Scholar]
- 30.Voak D, Bowley CC. A detailed serological study on the prediction and diagnosis of ABO haemolytic disease of the newborn (ABO HD) Vox Sang. 1969;17:321–48. doi: 10.1111/j.1423-0410.1969.tb00406.x. [DOI] [PubMed] [Google Scholar]