Abstract
BACKGROUND
Constraint-induced (CI) movement therapy (also called forced use by some investigators and clinicians) has gained increasing popularity as a treatment mode for restoring function in the upper extremities of patients with stroke. The purpose of this article is to review the concept of constraint-induced movement therapy and provide a critical analysis of the existing data.
REVIEW SUMMARY
The evidence to date offers encouragement for the application of this procedure for patients who have some movement recovery out of synergy. Success may be contingent on patient cooperation and intense repetitive use with applications of retraining through practice and shaping. The extent to which each of the latter elements influences the magnitude of recovery is still unclear. However, task novelty and challenge seem important to recovery of function. There are several methods used to map cortical changes after stroke. At this time, transcranial magnetic stimulation is the primary vehicle used to assess motor cortical reorganization after CI therapy in humans.
CONCLUSIONS
Accumulating data indicate that the size of a cortical area representative of a muscle does expand and its center of gravity does change with CI therapy.
Keywords: stroke, rehabilitation, upper extremity, forced use, constraint-induced movement therapy, transcranial magnetic stimulation, neuroimaging
Present trends in health care have intensified the need for evidence-based practice in neurologic rehabilitation. Government agencies and third-party payer systems increasingly are petitioning clinicians to justify the need for and effectiveness of applied treatments (1). This increased accountability has forced neurorehabilitationists to evaluate the role of scientific theory, acknowledge how theory shapes and structures clinical practice (2), define those practices most amenable to successful physical improvements to overcome impairments, and use contemporary techniques to explore mechanisms to explain such improvements. One treatment approach that has attracted considerable attention is repetitive task practice. One form of this approach is constraint- induced (CI) therapy, also known as forced use, when formal intense training is not part of the therapy. Accordingly, the purposes of this review are to update neurorehabilitation practitioners on the present status of neuromuscular approaches to overcoming upper extremity (UE) impairments in patients after stroke, describe the fundamental aspects of CI therapy, and review data gathered through transcranial magnetic stimulation (TMS) that will help yield information about mechanisms for this treatment approach.
CURRENT STATUS OF NEUROMUSCULAR TREATMENT APPROACHES
More than 50 years ago, rehabilitation of the neurologic patient was considered an orthopedic problem, managed through bracing, surgery, and muscle reeducation (1). The goals in reeducating were to strengthen muscle and prevent compensatory movement patterns through the use of repetitive, active exercise, similar to some therapeutic and progressive resistive exercise regimens used in present clinical practice (2,3). Although this approach proved effective in the treatment of focal or individual muscle impairment, clinicians could not effectively manage the abnormal patterns of movement seen in many neurologic patients. Consequently, attention was directed to neurophysiological bases for multiple theories as foundations to explain restoration of movement control. In fact, many of the dominant rehabilitation models and techniques used in treating the neurologically impaired UE emerged from these theories.
In general, treatment of the UEs of patients with stroke followed principles of neurofacilitation techniques or of functional retraining. Collectively called the neurofacilitation approaches, Rood, Brunnstrom, Bobath, proprioceptive neuromuscular facilitation, and sensory integration therapy were all developed in response to reflex and hierarchic models of motor control (1,2,4). These models theorized that sensory input was a prerequisite for motor output with normal movement resulting from cerebral and cerebellar cortical control over spinal-level reflexes. Each neurofacilitation technique, although unique in its application, sought to facilitate normal movement through the provision of specific afferent input. Even today, many clinicians use modality treatments (i.e., biofeedback, electrical stimulation, transcutaneous neuromuscular stimulation, and ultrasound) to treat abnormal muscle activity before training functional movement, which adheres to reflex and hierarchic principles.
Conversely, rehabilitation specialists who treat impairments primarily through repetitive practice of functional, goal-directed activities rely more on present theories in motor control, collectively referred to as the system’s theory (5), in which the integration of many systems, inclusive of the central nervous system (CNS), results in organized, normal movement (2,3). In contemporary terms, the therapeutic approaches emerging from the system’s theory, referred to as task-oriented, traditional functional retraining or functional therapy, focus on the repetitive and meaningful performance of activities.
With such differences in treatment rationale, the argument has been made that one neuromuscular treatment approach should appear more efficacious than another in rehabilitating the UE of patients with stroke (6). Yet the evidence to support many techniques used by rehabilitation therapists lacks unanimity (5,7–20). Nonetheless, many clinical decisions are now driven by limited time and funds allocated for treatment of the UE of patients after stroke. In light of this fact, approaches that highlight repetitive task practice within the context of functional activities have gained considerable attention, and among these, CI therapy seems to be one of the more prominent approaches.
CONSTRAINT-INDUCED THERAPY
CI therapy is one of a few evidence-based neurorehabilitation treatments developed directly from basic science research, the fundamental theoretical constructs of which were subsequently applied to humans. Results from numerous studies (21–26) validate the effectiveness of this approach for improving UE motor function in patients after stroke. With increasing evidence, CI therapy has thus helped elucidate new appreciation for neurologic recovery, both in extent of recovery and in the ability to improve beyond the subacute stages after stroke.
Historic Basis of Constraint-Induced Therapy
The historic basis behind CI therapy emerged from more than seven decades of research. Tower (27) noted in 1940 that after unilateral lesions of the pyramidal tracts, monkeys would fail to use the affected limb; however, this use could be improved again with restraint of the unaffected limb. Taub (28) summarized several studies that explored the effects of the surgical abolition of somatic sensation in the forelimb of monkeys. In these studies, monkeys often quit using the insensate limb after multiple unsuccessful attempts. Consequently, to accomplish basic functional tasks, the monkeys learned compensatory techniques with the nonaffected arm, a phenomenon Taub and colleagues (28,29) referred to as learned nonuse. Taub and colleagues observed that successfully developing these compensatory strategies reinforced the nonuse of the affected limb. Thus, animals never realized the possible functional potential of that limb. However, they later discovered that learned nonuse could actually be reversed when the uninvolved UE of deafferented, adolescent monkeys was restrained for 3 days. Furthermore, when the restraint was maintained for a period of 1 to 2 weeks, this return in function could be long lasting.
These fundamental observations have led to numerous studies and reviews that either describe or evaluate the application of CI therapy in humans. These studies are summarized in Table 1 and include the number and characteristics of participants, intervention description, follow-up time period, outcome measures, and commentary. Ostendorf and Wolf (30) demonstrated the first application of this approach to humans in a single-subject pilot study. Their approach of exclusively implementing forced use of the hemiplegic UE was then applied in a larger study (21) involving patients with chronic strokes and traumatic brain injury. The unaffected UE was restrained during waking hours over a 2-week period. Patients performed the protocol at home, with supervision from caregivers. UE improvements in timed or force-generating activities were noted in 19 of 21 functional tasks, and improvements persisted up to 1 year after the intervention.
Table 1.
Summary of Constraint-Induced Therapy Articles
| Year | Author | Title | Patients | Sample Size | Intervention | Follow-Up | Outcome Measures |
|---|---|---|---|---|---|---|---|
| 1981 | Ostendorf and Wolf (30) | Effect of Forced Use of the Upper Extremity of a Hemiplegic Patient on Changes in Function: A Single-Case Design | CVA, chronic | 1 | 7 days of forced use (restraint only) | Post, 2 weeks, 4 weeks | Preliminary version of WMFT |
| 1989 | Wolf et al (21) | Forced Use of Hemiplegic Upper Extremities to Reverse the Effect of Learned Nonuse Among Chronic Stroke and Head Injured Patients | CVA/TBI, chronic | 21 | 14 days of forced use (restraint only) | 1, 2, 4 weeks, 12 months | WMFT |
| 1993 | Taub et al (24) | Technique to Improve Chronic Motor Deficit After Stroke | CVA, chronic | 9 | 14 days restraint plus 10 training days, 6 hours/day | 4 weeks, 2 years | WMFT, MAL, AMAT |
| 1994 | Taub et al (98) | An Operant Approach to Rehabilitation Medicine: Overcoming Learned Nonuse by Shaping | CVA, chronic | Report on previous 9 patients with details regarding shaping tasks | No new data reported | ||
| 1996 | Taub et al (31) | Effects of Motor Restriction of an Unimpaired Upper Extremity and Training on Improving Functional Tasks and Altering Brain Behaviors | CVA, chronic | Original 9 patients plus 13 more | Compares first 2 groups (Taub et al, 1993) to 3 additional groups with variations of same treatment | Post | No specific data reported |
| 1997 | Taub and Wolf (32) | Constraint Induction Techniques to Facilitate Upper Extremity Use in Stroke Patients | CVA, chronic | Original 9 patients plus 18 | Adds sixth group (intensive therapy only) to compare to 5 groups (Taub et al, 1996) | Post | No specific data reported |
| 1997 | Morris et al (99) | Constraint-Induced Movement Therapy for Motor Recovery After Stroke | CVA, chronic | Description of information of 6 groups from 1996 article and more detailed information about CIT protocol | No new data reported | ||
| 1998 | Taub et al (23) | Constraint-Induced Movement Therapy: A New Approach to Treatment in Physical Rehabilitation | CVA, chronic | Previous study patients plus 8 lower-level patients | 14 days of restraint plus 10 training days, 6 hours/day | Post | WMFT, MAL; no specific data reported |
| 1998 | Liepert et al (88) | Motor Cortex Plasticity During Constraint-Induced Movement Therapy in Stroke Patients | CVA, chronic | 6 | 2 weeks of CIT (no other description provided) | Post | TMS, MAL |
| 1999 | Miltner et al (25) | Effects of Constraint-Induced Movement Therapy for Motor Recovery in Chronic Stroke Patients | CVA, chronic | 15 | 12 days of restraint plus 8 training days, 7 hours/day | Post, 6 months | MAL, WMFT |
| 1999 | Kunkel et al (26) | Constraint-Induced Movement Therapy for Motor Recovery in Chronic Stroke Patients | CVA, chronic | 5 | 14 days of restraint plus 10 training days, 6 hours/day | Post, 3 months | AAUT, WMFT, MAL, AMAT |
| 1999 | Taub et al (36) | Constraint-Induced Movement Therapy: A New Family of Techniques With Broad Application to Physical Rehabilitation. A Clinical Review | CVA, chronic | Previous study patients plus 18 lower-level patients | 14 days of restraint plus 10 training days, 6 hours/day | Post | WMFT, MAL |
| 1999 | Vander Lee et al (22) | Forced Use of the Upper Extremity in Chronic Stroke Patients: Results From a Single-Blind Randomized Clinical Trial | CVA, chronic | 66 (33 control, 33 CIT) | CIT (2-week restraint with 10 training days, 6 hours/day) vs. bimanual NDT (similar training period) | Post, 1 year | RAP, ARA, Fugl-Meyer, MAL |
| 1999 | Blanton and Wolf (34) | An Application of Upper-Extremity Constraint-Induced Movement Therapy in a Patient With Subacute Stroke | CVA, subacute | 1 | 14 days of restraint plus 10 training days, 6 hours/day | Post, 3 months | AAUT, WMFT, MAL |
| 1999 | Kopp et al (100) | Plasticity in the Motor System Related to Therapy-Induced Improvement After Stroke | CVA, chronic | 4 patients previously reported (1999) | Additional analysis of previous study subjects | Post, 3 months | Dipole modeling of steady-state movement-related cortical potentials |
| 2000 | Liepert et al (77) | Treatment-Induced Cortical Reorganization After Stroke in Humans | CVA, chronic | 13 | 12 days of restraint plus 8 training days, 7 hours/day | Post, 4 weeks, 6 months | TMS, MAL |
| 2000 | Dromerick et al (33) | Does the Application of Constraint-Induced Movement Therapy During Acute Rehabilitation Reduce Arm Impairment After Ischemic Stroke | CVA, acute | 20 (CIT 11, control 9) | 14 days of restraint for 6 hours per day plus training 2 hours/day, 5 days/week for 2 weeks | Post | ARA, Barthel, FIM |
| 2001 | Sabari et al (101) | Constraint-Induced Motor Relearning After Stroke: A Naturalistic Case Report | CVA, acute | 1 | 12 days of restraint plus 8 training days, 7 hours/day | Post, 1 year | FIM, AMAT, MAL |
| 2001 | Liepert et al (102) | Motor Cortex Plasticity During Forced- Use Therapy in Stroke Patients: A Preliminary Study | CVA, 4 to 8 weeks | 9 | 1 week of conventional PT, followed by 1 week of CIT | Post | NHPT, fMRI Frenchay arm test, vigorometry |
| 2001 | Page et al (35) | Modified Constraint Induced Therapy: A Randomized Feasibility and Efficacy Study | CVA, subacute | 6 (2 CIT, 2 traditional therapy, 2 control) | CIT: 5 days/week, 5 hours of restraint plus 30 minutes of therapy 3 times a week for 10 weeks vs. regular therapy vs. no therapy | Post | Fugl-Meyer, WMFT, MAL, ARA |
| 2001 | Levy et al (66) | Functional MRI Evidence of Cortical Reorganization in Upper-Limb Stroke Hemiplegia Treated With CI Therapy | CVA, subacute | 2 | 14 days of restraint plus 10 training days, 6 hours/day | Post, 3 months | WMFT, MAL, fMRI |
| 2001 | Morris and Taub (40) | Constraint-Induced Therapy Approach to Restoring Function After Neurological Injury | CVA | Descriptive review of CIT protocol |
Taub et al (24) continued this work, adding 6-hour supervised practice sessions for 10 of the 14 days while patients wore a restraint. Four patients underwent unaffected UE restraint, and five subjects were assigned to the comparison group. The restraint was worn 90% of waking hours and only removed during water-based activities (i.e., showering and toileting), naps, and activities where balance might be compromised. The comparison group was given two sessions of passive movement of the affected extremity and instructions for self-range of motion exercises and told to focus attention on using the affected extremity. An earlier version of the Wolf Motor Function Test (WMFT) called the Emory Test and the Arm Motor Activity Test (AMAT) were administered to objectively evaluate UE motor function. Mean performance times decreased significantly on the two motor ability tests for the restraint group compared with the control group. Improvements in strength measures within the Emory Test were noted within the experimental group as well as quality of movement. The Motor Activity Log (MAL) was administered to assess the patients’ subjective impression of how well and how often movement is observed in the affected arm during basic activities of daily living (ADL) before, during, and after the intervention. On this 6-point scale, patients rate their own performance from 0 (cannot use the limb for that activity) to 5 (using the limb as well/as much as before the stroke). A score of 3 is the minimum value that indicates the patient is functionally able to use the limb to complete the task without use of the stronger limb. Statistically significant improvements were made only in the experimental group and were maintained 2 years after intervention. These subjects did exhibit a pronounced improvement in actual attempts to use the limb in all of the activities, a mean increase of 97% compared with pretreatment scores. In unstructured post-interviews, the restraint patients indicated a greatly expanded range of activities in their real-life situation, with two patients gaining the ability to write with their affected UE, an activity that was not previously attempted. Minor adverse events from training, described as typical overuse symptoms of stiffness and discomfort in the affected extremity, occurred in three of the four patients.
Expanding on previous work, Taub and coworkers published a series of case studies (23,31,32) involving a total of 27 patients. Six different modified treatment protocols were described, which included the following: (1) use of a resting splint/sling to restrain the less-affected arm and supervised task practice with the more-affected arm; (2) no restraint (control)—attention group; (3 sling restraint of the less-affected arm and shaping (operant conditioning) of movements of the more-affected arm; (4) half-glove on the less-affected hand as device to remind the subject to avoid using this hand while performing shaping of movements of more-affected arm; (5) no restraint, only shaping of the more-affected arm; and (6) intensive training 6 hours per day for 10 days (no glove or shaping but intensive physical therapy, including aquatic therapy, neurophysiological facilitation, and task practice).
In this context, the term shaping refers to a “specific behavioral training technique in which a desired motor or behavioral objective is approached in small steps, by successive approximations” (32, p. 40). Specific values for outcome measures on these additional patients were not provided. However, descriptively, the results interpreted from the MAL and WMFT changes suggested that the sling and shaping group and the half-glove and shaping group improved as well as the original group of restraint and supervised task practice. The shaping-only group improved as well as these groups, but the “transfer of improvement to the life situation, while substantial, was not as great as in the constraint groups (32, p. 51).” The intensive therapy group, receiving conventional therapy for 6 hours per day over a period of 10 days, improved just as much as the patients in the sling plus shaping group. From these results, the relative importance of restraint (in combination with intensive training) as a basis for improved function is unclear, but these data suggest that constraint of the less-affected limb assists in improving subsequent use of that limb during daily activities. Only the nine original participants from the first article in the series (24) were randomized. Although in the original study (24) and again in a later article (23), Taub and colleagues noted that patients underwent 14 days of restraint and 10 days of training, the other two studies in the series (31,32) suggest that patients received only 12 days of restraint and 8 days of training. From these conflicting reports, it is unclear which treatment interval was used for these groups.
Miltner et al (25) evaluated the effects of restraint with a resting hand splint for 12 days while using shaping techniques 7 hours on each of 8 days in 15 patients. Improvements were expressed as effect sizes rather than as percentages. The effect sizes on the WMFT and MAL were described as “extremely large by standards of the field (p. 25).” However, as in previous studies (24), the quality of movement portion of a primary outcome measure, the MAL, was administered each day of the treatment phase as well as before and after intervention. Familiarizing patients with the MAL questions on a daily basis may permit recollection of previous responses, thereby narrowing the choice points on the MAL, thus effectively reducing the variability and enhancing the effect size. Additionally, the possible confounding influences of the Hawthorne effect (i.e., effects from repeated testing) cannot be dismissed using this method of outcome measurement administration. The WMFT analysis showed significant improvements in each measure from pretreatment to posttreatment but no significant differences from baseline to pretreatment or posttreatment to follow-up. Follow-up data represented only 12 of the 15 patients for the MAL and 9 patients for the WMFT.
Kunkel et al (26) studied five patients who wore a restraint for 14 days while performing shaping procedures for 10 of those days. Improvements were seen in the MAL, the Actual Amount of Use Test (AAUT), the Arm Motor Ability Test (AMAT), and WMFT outcome measures. The MAL score improvements indicate that patients progressed from using the affected limb as merely an assist to using the limb to complete a task independently. Although the AAUT represents an objective and unobtrusive measure of motor function of the UE, actual reliability and validity levels of this test were not provided. For the WMFT and AMAT test results, the average improvement scores were 42% and 19%, respectively. Large effect sizes were noted for each of these four tests as well. Small sample size and absence of a comparison group limit the conclusiveness of these results. No data are offered regarding baseline measurement comparisons so that patient homogeneity could be determined; consequently, potential effects of intersubject variability are not addressed.
Vander Lee et al (22) have performed the largest controlled CI therapy trial to date, comparing CI therapy to treatment emphasizing bimanual activities. Their results showed that 1 week after the last treatment session, the change within the CI therapy group was significantly different compared with the group undergoing bimanual training for Action Research Arm test (ARA) and the MAL amount of use score. Other outcome measures (MAL quality of movement score, Rehabilitation Activities Profile, and the UE section of the Fugl-Myer Assessment scale) did not improve significantly. Only the ARA scores showed persistent change at 1-year follow-up. The greater treatment effect measured by the ARA was clinically relevant for CI therapy patients with sensory disorders, and the greater treatment effect measured by the MAL amount of use scores was relevant for CI therapy patients with hemi-neglect. Differences in kind and intensity of CI therapy intervention in this study compared with previous studies (23–26,31,32) may be responsible for these results. The experimental patients were treated in groups of four by one or two therapists, and no intensive shaping techniques were used. Except for the MAL, different outcome measures were used, also limiting comparisons to previous studies.
Preliminary studies using CI therapy have also addressed patients with acute and subacute stroke. Dromerick et al (33) evaluated the effects of the CI therapy protocol in a randomized controlled trial of 20 patients with stroke less than 3 months from the ictus. Their results showed the CI treatment group (CI therapy complementing regular treatment) attained significantly higher scores on total ARA and pinch subscale scores compared with the control group who received regular treatment only, but with control over total treatment duration for both groups. The differences in the mean ARA grip, grasp, and gross movement subscale scores did not reach statistical significance. No difference was noted between groups for UE ADL performance measures (Barthel and Functional Independence Measure). Most disability measures do not distinguish unilateral from bilateral ADL tasks; consequently, a lack of significance for the ADL measures may be attributable to the insensitivity of these measures to detect change in impaired UE function. This study illustrates the feasibility of applying a modified CI therapy protocol to the acute stroke population. Different outcome measures evaluating change and function compromise comparisons to other studies in the chronic stroke population.
Blanton and Wolf (34) reported one of the first applications of CI therapy for a patient with subacute stroke. This patient improved both MAL and WMFT scores, and these improvements were still present 3 months later. The CI therapy protocol used with this patient is similar to the intervention used in studies conducted by Taub and colleagues (23,31,32) with half-glove restraint and intensive task practice for 6 hours per day for 10 days. Although limited in relevance because of a case study format, the results from this case study support additional investigation of CI therapy for this patient population.
Most recently, Page et al (35) evaluated a modified CI therapy protocol in a randomized controlled trial of six subacute patients (between 3 and 9 months). Two patients were placed in each of the three groups: CI therapy (restraint of unaffected arm 5 days per week during 5 hours identified as times of frequent use in addition to 30-minute physical and occupational therapy sessions three times a week for 10 weeks), regular therapy (30-minute physical and occupational therapy sessions three times a week for 10 weeks), and no therapy. Substantial improvements were found for the CI therapy group in the Fugl-Myer Assessment of Motor Recovery, ARA, WMFT, and MAL. No specific statistical analysis was done on the small sample size, and comparisons with other studies are limited because of the use of a different, modified CI therapy protocol. These results are encouraging for the application of CI therapy in the subacute stroke population, but an evaluation of a much larger number of patients is needed.
In summary, CI therapy holds substantial potential to assist patients with stroke to improve UE function. Although multiple articles describe the benefits of CI therapy, conclusive evidence is limited because of the lack of any large randomized clinical trial (RCT). Presently, most of the evidence is presented in uncontrolled patient series and four small RCTs. Larger, controlled studies with uniform CI therapy intervention and measurement protocols will help to define more clearly the benefits of this intervention.
Is There an Appropriate Patient Population for Constraint-Induced Therapy?
Despite evidence that CI therapy may provide an avenue for significant functional improvements in the hemiparetic limb, not every patient recovering from a neurologic injury has benefited from this treatment approach. Although Taub et al (36) have suggested that CI therapy may be applicable to greater than 75% of the stroke population with chronic unilateral motor deficits, the validity of this possibility has yet to be demonstrated. The primary criteria for patient eligibility for a CI therapy intervention include adequate balance and safety while wearing the restraint and the ability to initiate at least 20 degrees of wrist extension and at least 10 degrees of extension at two digits in addition to the first digit of the affected hand. These criteria are derived from electromyographic biofeedback studies performed by Wolf and Binder-Macleod (37) that indicated voluntary movements of finger and wrist extension were a better predictor of future acquisition of independent limb use than was the ability to reduce the hyperactive responses from stretching UE flexor muscle groups. The number of patients that actually achieve this level of functional recovery in the upper limb after a stroke is estimated to be approximately 20% to 25% of the population (37). These criteria would seem to constitute important prerequisites to consider for reacquisition of important function using the CI therapy paradigm or any other treatment approach. Because most UE use involves grasping and manipulating objects, the preparatory movements of wrist and finger extension of the involved limb are essential to achieving at least some success within the patient’s ADLs without requiring assistance from the other limb or another person.
With such specific criteria to consider, neurorehabilitationists must realize that the generalizability of the CI therapy literature is limited to patients with the minimal movement criteria. Consideration must also be given to the need for solid family support, good cognitive skills, and emerging movement in the hand as well as other UE joints.
Elements of Intensive Training Associated With the Constraint-Induced Therapy Training
The two approaches used in CI therapy training include general task practice and “shaping” (adapted task practice). General task practice refers simply to the practice of a full functional task that may have multiple steps for completion (i.e., eating a meal, making coffee, or finding and dialing phone numbers from a city directory).
Shaping or adapted task practice is defined as a method in which a motor or behavioral objective is approached in small steps by successive approximations or by making the task more difficult in accordance with the patient’s motoric capabilities. In using a telephone, for example, one would practice grasping the telephone and holding the receiver before practicing pushing the telephone buttons. The task selection is based on the patient’s specific movement deficits and preference. Specific knowledge of results about a patient’s performance is given after each trial or practice session. Generally, this feedback is in terms of number of repetitions per unit of time or time required to perform a set task.
Winstein (38) and Schmidt (39) note that part-task training can be an effective way to retrain some activities if they can be naturally divided into units that reflect their inherent goals. Morris and Taub (40) note that shaping is unique because patients are given explicit feedback concerning even small improvements in performance. Activities are chosen that can be easily broken down into subtasks that are objectively measured, and then these subtasks are repeated for a specific number of times (i.e., at least 10 trials). Thus, with CI therapy, the amount and frequency of feedback and knowledge of results is increased, as is the typical number of repetitions for a given task. Consequently, the intensity of training for any given task is greater, based on the assumption that repetitively engaging relevant neural substrates will achieve greater plastic changes within the CNS.
Possible Mechanisms of Constraint-Induced Therapy
The two primary mechanisms proposed for the effects observed after CI therapy are believed to be overcoming learned nonuse of the affected limb or use-dependent cortical reorganization.
Learned nonuse
Neurorehabilitationists may propagate the development of learned nonuse (Figure 1) in patients with neurologic injury if patients are trained to attempt functional tasks using compensatory strategies rather than promoting appropriate movement control during functional use of the impaired limb. Unfortunately, attempting to maximize functional outcomes within the context of limited numbers of treatments may, at times, come at the expense of facilitating motor and sensory recovery of the hemiplegic limb. Carr and Shepherd (41) have noted that poor rehabilitation outcomes may result from not appreciating the long-term ineffectiveness of a false sense of independence after teaching compensatory behavior with the less-affected UE. In fact, Lettinga et al (42) suggest that the ultimate goal of maximum functional recovery should be expressed as lack of dependence or use of these compensatory strategies. Within the context of CI therapy, the treatment of learned nonuse (Figure 2) is achieved through restraint of the less-affected limb, referred to by Wolf and colleagues (21,30) as forced use and reinforced through the application of shaping or repetitive task practice approaches. Based on the early work of Taub et al (24) and Wolf and colleagues (21,30), 2 weeks is typically the interval for restraining the limb through use of a padded safety mitt. This simple device prevents prehension of the fingers while allowing use of the less-affected limb for balance if needed. Patients are encouraged to wear the mitt for 90% of their waking hours, except for activities that are water-based or might compromise the patient’s safety or balance.
Figure 1.
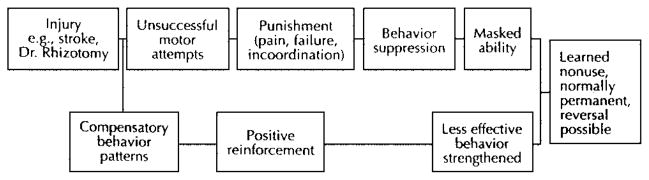
Schematic model for the development of learned nonuse. Reprinted with permission from Carlson JG, Seifert AR, Birbaumer N. Clinical Applied Psychophysiology. New York: Plenum Press; 1994:192.
Figure 2.
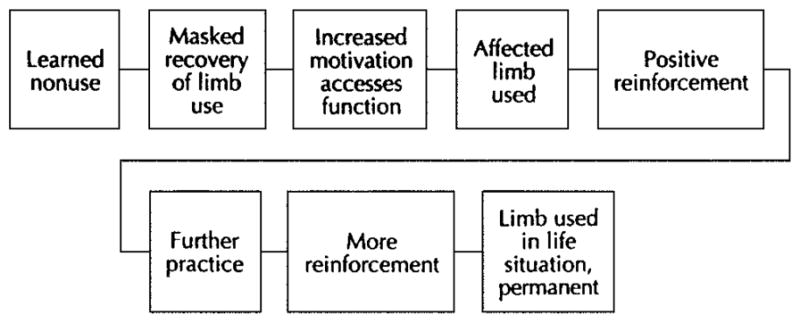
Schematic model of mechanism for overcoming learned nonuse. Reprinted with permission from Carlson JG, Seifert AR, Birbaumer N. Clinical Applied Psychophysiology. New York: Plenum Press; 1994:192.
Repetitive practice cortical reorganization
The potential for reorganization in the adult brain has been largely underestimated (43). Studies involving CI therapy represent one area in which issues regarding the interaction between behavioral and neural plasticity have been examined. A growing number of studies examining the scientific basis of recovery of function in rehabilitation provide evidence for the potential of the CNS to adapt and change. Although some plastic responses to injury seem to arise spontaneously, this term may reflect our lack of understanding of the underlying processes (44). The notion that practice induces plastic, dynamic changes in the CNS is a common belief (45). Behind this premise is the concept that in the undamaged or healthy brain, neuronal connections are continuously remodeled by experience and by the performance of specific, intensive, and complex movements used to solve motor problems and attain goals. Much like neurodevelopment in children, movements that are practiced throughout life may become more represented in the brain. However, even though simple repetition of movement can induce some cortical change, recent evidence suggests “actual motor skill acquisition, or motor learning is a prerequisite factor in driving representational plasticity in the primary motor cortex.” (46, p. 27)
Specific to rehabilitation of patients with stroke, Kwakkel et al (47) have found a small but significant intensity-effect relationship. Meta-analysis demonstrated a statistically significant summary effect size for activities of daily living (0.28±0.12). In a more recent single-blind randomized controlled trial (48), a greater intensity of arm rehabilitation resulted in more improvements in dexterity than seen in a control group that underwent only basic rehabilitation. Similar improvements occurred with focused lower extremity rehabilitation, thus providing additional evidence that exercise therapy primarily induces treatment effects on the abilities at which training is specifically aimed. This present evidence regarding the effect of intense targeted treatment and the efficacy of task-specific training supports the importance of the repetitive task practice aspect of the CI therapy paradigm. During CI therapy, patients wearing the constraint on the less-affected UE for 2 weeks also participate in an intensive 6-hour-per-day, functionally based rehabilitation program for 10 days. The intensity of training is manifest as decreased time to perform a task, increasing the number of repetitions per unit of time, changing the spatial domain in which the task is undertaken, or combinations of these temporal and spatial considerations.
Mapping Clinically Relevant Cortical Responses to Constraint-Induced Therapy
For any therapeutic intervention, there is a need to understand the mechanisms contributing to its effectiveness. Several methods have been used to map cortical changes in animals and humans after lesions. The technique most often used in nonhuman primates has been neurophysiological mapping; in humans, positron emission tomography (PET), functional magnetic resonance imaging (fMRI), and trans-cranial magnetic stimulation (TMS) have been used most often.
Changes in primary somatosensory or primary motor cortex have been observed with specific interventions or manipulations (49–51). The results in monkeys demonstrating the effects of rehabilitative training after stroke using restraint of the unimpaired arm are very similar to those found after CI therapy (52–54). Yet repetitive motor activity alone does not seem to produce functional reorganization of cortical maps (53), and it seems that motor skill acquisition, or motor learning, is a prerequisite factor in driving representational plasticity in M1 of the adult squirrel monkey (46).
Over the last 10 years, fMRI has been used to enhance the understanding of the underlying mechanisms of motor control during motor learning (55,56) and motor functional recovery after stroke using fMRI (57–61) and PET (62–64). However, few functional imaging experiments have investigated the effects of rehabilitative interventions (65), specifically CI therapy, on cortical reorganization after stroke.
At this time, there is only a single functional imaging study that attempts to evaluate the effect of upper limb CI therapy on cortical motor reorganization (66). Qualitative assessment of the images showed larger areas of activations bilaterally and in the peri-infarct area after 2 weeks of CI therapy. The study was hampered by design and methodological problems, leaving definitive conclusions of cortical reorganization after CI therapy problematic.
Continued use of these neuroimaging techniques will advance our understanding of the mechanisms of CI therapy. To date, the one approach in humans that has received the most attention in investigating the mechanisms of cortical change after CI therapy is transcranial magnetic stimulation.
Basic Overview of Principles Underlying Transcranial Magnetic Stimulation
Since its introduction by Barker in 1985, TMS has gained recognition as a safe, relatively painless and noninvasive method for mapping cortical motor representation areas (67–69). Recently, TMS has been used to investigate the possible mechanisms underlying both spontaneous and therapy-induced poststroke motor recovery.
TMS is based on the principle of electromagnetic induction. Electrical current is directed through a hand-held copper-stimulating coil, with the consequent production of a transient magnetic field. When held over the scalp, the rapidly changing magnetic field is able to induce a small electrical current in underlying brain tissue, without significant attenuation. When performed over the primary motor cortex at low stimulus intensities, TMS is thought to stimulate the corticospinal tract indirectly (trans-synaptically) via horizontal fiber depolarization (67,68,70–72). The resultant efferent volleys can then be recorded as motor evoked potentials (MEPs) via surface or indwelling electrodes at peripheral target muscles.
Measured TMS variables often include the location of the “hot spot” (the most active scalp position for the target muscle), the excitability threshold (measured at the hot spot), the area of motor output representation, the MEP latency, the amplitude-weighted center of gravity, the MEP amplitudes (at rest and sometimes with facilitation), and the MEP recruitment curves (73–75). A thorough review of the physics and measurement characteristics of TMS can been found elsewhere (67–79).
Application to Motor Reorganization After Stroke
Several investigators have examined the correlation between TMS-evoked motor map characteristics after stroke and the extent of motor recovery in humans (80,81). Pennisi et al (82) demonstrated that complete hand paralysis in association with absence of early MEPs (within 48 hours of ictus) predicted poor neurologic recovery at 1 year in 15 subjects after stroke (middle cerebral artery infarct). Conversely, the preservation of TMS-evoked MEPs in the early poststroke period may portend good functional recovery (71,83). Other investigators have reported relationships between the rate and extent of poststroke recovery and changes in presence of MEP, conduction time from cortex to muscle, MEP latency, excitability threshold, and MEP amplitude (71,82,84,85). In mono-hemispheric infarctions, decreased affected hemisphere (AH) motor output area and increased excitability thresholds for paretic muscles have been repeatedly observed in TMS-derived maps performed in poststroke patients during the subacute and chronic phases (77,86). These electrophysiologic changes are presumably related to the motor impairment and may be secondary to neuronal damage, disuse, and unbalanced transcallosal inhibition from the less-affected hemisphere or other unidentified mechanisms (76).
Response to Repetitive Task Practice
Recent work with animal models has suggested that the specificity and difficulty of training may impact the extent of use-dependent cortical plasticity (53,54). Similar findings have been reported in motor recovery in human subjects after stroke. Leipert et al (86) examined the effect of one intensive session of physical therapy in nine subjects 4 to 8 weeks after stroke. Subjects received 1.5 hours of manual dexterity exercises in addition to ongoing standard therapy. TMS mapping of the abductor pollicis brevis (APB) representation was performed 1 week before, immediately before, immediately after, and 1 day after the training session. Measures of motor output area, excitability threshold at the APB hot spot, and center of gravity for the APB muscle (location at which an evoked muscle response greater than 50 μV in amplitude is seen at minimal stimulus intensity) of the AH and unaffected hemispheres (UH) did not significantly change between the two pretraining measures, indicating that no significant changes occurred because of spontaneous recovery or nonspecific training. AH area increased significantly immediately after training but then decreased toward baseline after 1 day. Increased AH motor output area was associated with improved dexterity on a clinical measure (the Nine Hole Peg Test) in seven of the subjects, although the amount of clinical improvement did not correlate with the extent of change in area. The excitability threshold at the hot spot and the center of gravity was unchanged after training, possibly signifying that the AH area increases were attributable to increased excitability at the edges of the map. The rapid change detected in the TMS-derived maps after brief training epochs suggests that functional, rather than structural, mechanisms were involved. Potential mechanisms discussed include the modulation of inhibitory GABA-ergic transmission at the borders of the motor map and alteration in glutamate transmission (86). Classen and coworkers (87) have suggested that the “motor cortex builds up, and then loses, in a short time, memory traces of movements retaining the subject’s recent history of performance.” They (86) postulate that either the short duration of a training effect is a function of the short duration of the training (and, hence on a continuum with long-term storage) or, alternatively, that the primary cortex subserves short-term plasticity, with long-term procedural memory requiring involvement of other cortical and subcortical structures.
Transcranial Magnetic Stimulation Mapping in Constraint-Induced Therapy
Recent studies have used TMS motor mapping to investigate the effect of CI therapy for the more-affected UE. Liepert et al (86) used focal TMS to construct cortical output maps to the APB in six chronic stroke patients before and after 10 days of CI therapy. As noted in prior studies of poststroke subjects, significantly higher motor thresholds, smaller amplitudes, and a smaller area of excitable cortex were observed in the AH. After CI therapy, TMS showed no change in thresholds but significant increases in MEP amplitude and APB motor output area in the AH, possibly indicating increased excitability of surrounding neuronal networks. The UH output areas were smaller after the training period, perhaps because of decreased use of the less-affected UE, normalization of the UH APB representation, or increased transcallosal inhibition of the UH by the AH. Center of gravity shifts were significant (in the mediolateral axis) only for the AH, suggesting possible recruitment of adjacent areas along the motor cortex. All subjects improved significantly in their use of the affected extremity, but scores on the motor activity log (MAL) did not correlate to degree of map change. The Leipert group suggests that “physiotherapy induces use-dependent reorganization which supports recovery-associated plastic changes.” (86, p. 321)
In another study (77), clinical (MAL) and TMS measures were made at multiple time points before and after CI therapy in 13 chronic stroke patients. Neither baseline measure showed appreciable change at 2 weeks and 1 day before CI therapy, suggesting little spontaneous recovery and good test-retest reliability. Again, the AH showed a smaller APB representation area at baseline, with a near doubling of the area after CI therapy.
Motor activity improvements (MAL) were maintained at the later measurement points. However, a return toward baseline AH APB representation area was seen at the 4-week and 6-month TMS sessions, indicating a possible “normalization after therapy-induced hyperexcitability” via improved synaptic efficiency or the relegation of motor function to TMS-inaccessible regions.
Examining Mechanisms to Explain Transcranial Magnetic Stimulation Map Changes
Changes in cortical motor representation areas have been documented in TMS investigations of motor recovery after stroke with and without specific therapeutic intervention. Suggested mechanisms for these map changes can be summarized to include the following: (1) resolution of edema and removal of necrotic tissue after CNS injury (62); (2) restitution of damaged pathways (77); (3) modulation of GABA-ergic intracortical inhibition (77,89,90); (4) changes in synaptic efficacy (77,87); (5) alteration of transcallosal inhibition (77); (6) substitution from ipsilesional parallel pathways (77,91); (7) activation of ipsilateral (contralesional) pathways (88); (8) short-term potentiated responses after terminating repetitive stimulation (87); and (10) long-term potentiation (75,92).
Alternative Explanations for Observed Changes in Map Area
Changes in the excitable surface area derived from TMS motor maps to an individual muscle have been linked to changes in motor function and interpreted as a reflection of alterations in the cortical representation for that muscle. However, the measured surface area of these TMS maps seems to exceed the likely cortical volume that is dedicated to a single muscle representation. Thickbroom et al (93) used excitability curves at each scalp location that elicited responses in the first dorsal interosseous and found that the shapes of the curves remained similar at each scalp site (with a similar slope and saturation level) but were shifted along the intensity axis. This finding suggests that a small population of motor cortical neurons, perhaps deeply situated, may be stimulated by current spread with gradually less responsiveness as the TMS coil is moved away from the epicenter of the representation. Therefore, changes in the surface area may represent increased excitability to current spread, without reflecting a true expansion or contraction of the cortical representation area. The center of the map may be a more stable measure of map change and should be included in future mapping studies. A few recent TMS mapping studies in poststroke subjects have revealed mediolateral shifts in the center of gravity in association with improvements in motor function of the target muscle (77,88).
Considerations Regarding Somatotopic Organization
Some physiologists believe that the motor cortex represents movements rather than individual muscles (87,94). Mosaic patterns of movement representations have been found via intracortical mapping studies in nonhuman primates (54,95). Although many studies involving TMS report motor output maps to a single muscle as a confluent area of excitability, the topography of the human motor cortex also seems to be complex (69,96,97). Wasserman et al (97) found significant overlap in the motor output areas for four different UE muscles in healthy subjects, although there was a clear somatotopic organization of the hot spots for these muscles. Similarly, Mortifee et al (76) identified considerable overlap in the motor output areas for the abductor pollicis brevis and abductor digiti minimi in a group of healthy controls. The hot spots for the two muscles were also discretely positioned.
The extent to which TMS map changes and clinical improvements are attributable to changes in the cortical output to the individual target muscle or changes in activity of multiple agonists and antagonist muscles remains to be elucidated. In the future, intrasubject comparison of TMS-derived motor maps with those achieved through intracortical microstimulation may help to clarify this issue. Multichannel needle electromyography of multiple agonist and antagonist muscles may also yield important information. In this regard, Rossini and Pauri (43) have demonstrated the ability to map up to 12 UE muscles concurrently and display a distributed motor network along the precentral cortical area. This kind of display represents our best alternative to simulating mapping procedures from intracortical stimulation studies in nonhuman primates and may provide the basis for future detailed intrasubject examination of cortical reorganization in response to any intervention.
CONCLUSION
Therapies based on repetitive practice of functionally related tasks to reduce impairment and improve function in the UEs of patients who have sustained a stroke are gaining recognition within the neurorehabilitation community. CI therapy that requires patients to use their impaired UEs intensely is among the approaches for selected patients. This approach is capturing increasing interest and is of fundamental scientific and clinical importance because of its basic scientific and theoretical foundations. Mechanisms to explain the improvements that are seen with CI therapy have exploited emerging technologies, including neurophysiological mapping in nonhuman primates, PET, fMRI, and transcranial magnetic stimulation in humans. TMS has lent some insight into potential explanations for cortical reorganization and represents a viable avenue that will allow additional understanding of physical therapeutic interventions.
CASE HISTORY
A patient presented with a past medical history of hypertension and a left ischemic lacunar infarct in the posterior limb of the internal capsule. Symptoms included a sudden onset of right-sided weakness and slurred speech. Upon admission for rehabilitation, gross motor tests were 5/5 strength in the left extremities, 0/5 in the right UE, and 3+ to 4− in the right lower extremity. She received 19 days of inpatient rehabilitation, including physical, occupational, and speech therapy. At the time of discharge from the center, she was independent with bed mobility but required supervision with transfers and ambulated household distances with a straight cane. She exhibited a predominant flexor synergy in her right UE through approximately three fourths of the range against gravity at the shoulder and elbow, with trace movement at the wrist and finger flexors. At 4 months after stroke, she was independent in all activities of daily living and ambulating without an assistive device and did not exhibit any residual speech deficits. She lived with her daughter but was able to provide some assistance with meal preparation and cleaning. She then began CI therapy. She could move her impaired UE though 90 degrees of shoulder abduction and flexion, full elbow extension, 30 degrees of wrist extension from a fully flexed position, and 20 degrees of finger extension in all digits. She primarily used her left UE for most ADLs, displaying little effort to initiate activity with her impaired limb.
For 14 days, the patient’s less-involved hand was constrained in a mitten for 90% of her waking hours, except for bathing and toileting. During 10 weekdays of that period, the patient participated in 6 hours of supervised practice of functional activities with the more-involved limb. Activities were chosen based on the patient’s interests before the stroke. She took an active role in choosing those tasks, which included grooming, writing, dressing, playing board games, gardening, computer work, eating, and sewing. Each task was subdivided into component movements and gradually progressed in complexity as the patient improved. She was also encouraged to maintain a home diary, documenting compliance wearing the mitt away from the lab as well as the activities attempted at home. Each morning the trainer would evaluate her progress the prior evening, helping her to problem solve ways to accomplish difficult tasks at home. For example, she indicated she had difficulty bringing food to her mouth when she ate dinner. She was given a foam build up to assist with her grip of utensils and consequently was able to independently feed herself by the end of the first week.
After 2 weeks of CI therapy, the patient reported using her more-involved limb at least half as much as she had before the stroke in 25 of 30 daily activities evaluated, compared with only 1 of 30 activities before intervention. She was able to prepare meals for her family independently and began using the sewing machine again with some assistance. Her daughter indicated that she seemed like a different woman, paying more attention to her appearance, interacting more socially, and beginning to drive again. These improvements persisted at 3-month follow-up.
Acknowledgments
This work is supported in part by National Institutes of Health grant HD 37606. We wish to thank the Publications Committee of the Extremity Constraint Induced Therapy Evaluation (EXCITE) clinical trial for their review and Miki DeJean for assistance with typing.
References
- 1.Gordon J. Assumptions underlying physical therapy intervention: theoretical and historical perspectives. In: Carr J, Shepherd R, editors. Movement Science: Foundations for Physical Therapy in Rehabilitation. Rockville: Aspen Publishers; 1987. pp. 1–30. [Google Scholar]
- 2.Horak FB. Assumptions underlying motor control for neurologic rehabilitation: contemporary management of motor control problems. Proceedings of the II Step Conference; Alexandria, VA: APTA; 1991. pp. 11–27. [Google Scholar]
- 3.Shumway-Cook A, Woollacott M. Motor Control: Theory and Practical Applications. Baltimore: Williams and Wilkins; 1995. [Google Scholar]
- 4.Kwakkel G, Kollen BJ, Wagenaar RC. Therapy impact on functional recovery in stroke. Physiotherapy. 1999;85:377–391. [Google Scholar]
- 5.Gelber DA, Josefczyk PB, Herrman D, et al. Comparison of two therapy approaches in the rehabilitation of the pure motor hemiparetic stroke patient. J Neurol Rehabil. 1995;9:191–196. [Google Scholar]
- 6.Wagenaar RC, Meijer OG, van Wieringen PC, et al. The functional recovery of stroke: a comparison between neurodevelopmental treatment and the Brunnstrom method. Scand J Rehabil Med. 1990;22:1–8. [PubMed] [Google Scholar]
- 7.Coote S, Stokes E. Physiotherapy for upper extremity dysfunction following stroke. Phys Ther Rev. 2001;6:63–69. [Google Scholar]
- 8.Dickstein R, Hocherman S, Pillar T, et al. Stroke rehabilitation: three exercise therapy approaches. Phys Ther. 1986;66:1233–1238. doi: 10.1093/ptj/66.8.1233. [DOI] [PubMed] [Google Scholar]
- 9.Ernst E. A review of stroke rehabilitation and physiotherapy. Stroke. 1990;21:1081–1085. doi: 10.1161/01.str.21.7.1081. [DOI] [PubMed] [Google Scholar]
- 10.Logigian M, Samuels M, Falconer J, et al. Clinical exercise trial for stroke patients. Arch Phys Med Rehabil. 1983;64:364–367. [PubMed] [Google Scholar]
- 11.Lord JP, Hall K. Neuromuscular reeducation versus traditional programs for stroke rehabilitation. Arch Phys Med Rehabil. 1986;67:88–91. doi: 10.1016/0003-9993(86)90108-5. [DOI] [PubMed] [Google Scholar]
- 12.Moreland J, Thompson MA. Efficacy of electromyographic feedback compared with conventional physical therapy for upper-extremity function in patients following stroke: a research review and meta-analysis. Phys Ther. 1994;74:534–547. doi: 10.1093/ptj/74.6.534. [DOI] [PubMed] [Google Scholar]
- 13.Basmajian JV, Gowland CA, Finlayson MA, et al. Stroke treatment: comparison of integrated behavioral-physical therapy vs traditional physical therapy programs. Arch Phys Med Rehabil. 1987;68:267–272. [PubMed] [Google Scholar]
- 14.Heckmann J, Mokrusch T, Krockel A, et al. EMG-triggered electrical muscle stimulation in the treatment of hemiparesis after a stroke. Eur J Phys Med Rehabil. 1997;7:138–141. [Google Scholar]
- 15.Kraft GH, Fitts SS, Hammond MC. Techniques to improve function of the arm and hand in chronic hemiplegia. Arch Phys Med Rehabil. 1992;73:220–227. [PubMed] [Google Scholar]
- 16.van der Lee JH, Snels IA, Beckerman H, et al. Exercise therapy for arm function in stroke patients: a systematic review of randomized controlled trials. Clin Rehabil. 2001;15:20–31. doi: 10.1191/026921501677557755. [DOI] [PubMed] [Google Scholar]
- 17.Jongbloed L, Stacey S, Brighton C. Stroke rehabilitation: sensorimotor integrative treatment versus functional treatment. Am J Occup Ther. 1989;43:391–397. doi: 10.5014/ajot.43.6.391. [DOI] [PubMed] [Google Scholar]
- 18.Aisen ML, Krebs HI, Hogan N, et al. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol. 1997;54:443–446. doi: 10.1001/archneur.1997.00550160075019. [DOI] [PubMed] [Google Scholar]
- 19.Feys HM, DeWeerdt WJ, Selz BE, et al. Effect of a therapeutic intervention for the hemiplegic upper limb in the acute phase after stroke: a single-blind, randomized, controlled multicenter trial. Stroke. 1998;29:785–792. doi: 10.1161/01.str.29.4.785. [DOI] [PubMed] [Google Scholar]
- 20.Volpe BT, Krebs HI, Hogan N, et al. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology. 1999;53:1874–1876. doi: 10.1212/wnl.53.8.1874. [DOI] [PubMed] [Google Scholar]
- 21.Wolf SL, Lecraw DE, Barton LA, et al. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 22.Vander Lee J, Wagenaar R, Lankhorst G, et al. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 23.Taub E, Crago JE, Uswatte G. Constraint-induced movement therapy: a new approach to treatment in physical rehabilitation. Rehabil Psychol. 1998;43:152–170. [Google Scholar]
- 24.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 25.Miltner WH, Bauder H, Sommer M, et al. Effects of constraint-induced movement therapy on patients with chronic motor deficits after stroke: a replication. Stroke. 1999;30:586–592. doi: 10.1161/01.str.30.3.586. [DOI] [PubMed] [Google Scholar]
- 26.Kunkel A, Kopp B, Muller G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–628. doi: 10.1016/s0003-9993(99)90163-6. [DOI] [PubMed] [Google Scholar]
- 27.Tower SS. Pyramidal lesions in the monkey. Brain. 1940;63:36–90. [Google Scholar]
- 28.Taub E. Somatosensory deafferentation research with monkeys: implications for rehabilitation medicine. In: Ince LP, editor. Behavioral Psychology in Rehabilitation Medicine: Clinical Applications. Baltimore: Williams and Wilkins; 1980. pp. 371–401. [Google Scholar]
- 29.Taub E, Goldberg IA, Taub P. Deafferentation in monkeys: pointing at a target without visual feedback. Exp Neurol. 1975;46:178–186. doi: 10.1016/0014-4886(75)90040-0. [DOI] [PubMed] [Google Scholar]
- 30.Ostendorf CG, Wolf SL. Effect of forced use of the upper extremity of a hemiplegic patient on changes in function: a single-case design. Phys Ther. 1981;61:1022–1028. doi: 10.1093/ptj/61.7.1022. [DOI] [PubMed] [Google Scholar]
- 31.Taub E, Pidikiti RD, DeLuca SC, et al. Effects of motor restriction of an unimpaired upper extremity and training on improving functional tasks and altering brain behaviors. In: Tool J, Good D, editors. Imaging in Neurologic Rehabilitation. New York: Demos Publications; 1996. pp. 133–154. [Google Scholar]
- 32.Taub E, Wolf SL. Constraint induced movement techniques to facilitate upper extremity use in stroke patients. Top Stroke Rehabil. 1997;3:38–61. doi: 10.1080/10749357.1997.11754128. [DOI] [PubMed] [Google Scholar]
- 33.Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000;31:2984–2988. doi: 10.1161/01.str.31.12.2984. [DOI] [PubMed] [Google Scholar]
- 34.Blanton S, Wolf SL. An application of upper-extremity constraint-induced movement therapy in a patient with sub-acute stroke. Phys Ther. 1999;79:847–853. [PubMed] [Google Scholar]
- 35.Page SJ, Sisto SA, Levine P, et al. Modified constraint induced therapy: a randomized feasibility and efficacy study. J Rehabil Res Dev. 2001;38:583–590. [PubMed] [Google Scholar]
- 36.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation. A clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- 37.Wolf SL, Binder-MacLeod SA. Electromyographic biofeedback applications to the hemiplegic patient: changes in upper extremity neuromuscular and functional status. Phys Ther. 1983;63:1393–1403. doi: 10.1093/ptj/63.9.1393. [DOI] [PubMed] [Google Scholar]
- 38.Winstein CJ. Designing practice for motor learning: clinical implications. Contemporary management of motor control problems; Proceedings of the II Step Conference; Alexandria, VA: APTA; 1991. [Google Scholar]
- 39.Schmidt R. Motor learning principles for physical therapy: contemporary management of motor control problems. Proceedings of the II Step Conference; Alexandria, VA: APTA; 1991. [Google Scholar]
- 40.Morris D, Taub E. Constraint-induced therapy approach to restoring function after neurological injury. Top Stroke Rehabil. 2001;8:16–30. doi: 10.1310/BLJX-M89N-PTPY-JDKW. [DOI] [PubMed] [Google Scholar]
- 41.Carr J, Shepherd R. A Motor Relearning Programme for Stroke. 2. Oxford: Butterworth-Heinemann; 1987. [Google Scholar]
- 42.Lettinga A, Siemonsma P, van Veen M. Entwinement of theory and practice in physiotherapy. Physiotherapy. 1999;85:476–490. [Google Scholar]
- 43.Rossini PM, Pauri F. Neuromagnetic integrated methods tracking human brain mechanisms of sensorimotor areas “plastic” reorganisation. Brain Res Rev. 2000;33:131–154. doi: 10.1016/s0169-328x(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 44.Jones TA, Hawrylak N, Klintsova AY, et al. Brain damage, behavior, rehabilitation, recovery, and brain plasticity. Ment Retard Dev Disabil Res Rev. 1998;4:231–237. [Google Scholar]
- 45.Fisher B, Sullivan K. Activity-dependent factors affecting poststroke functional outcomes. Top Stroke Rehabil. 2001;8:31–44. doi: 10.1310/B3JD-NML4-V1FB-5YHG. [DOI] [PubMed] [Google Scholar]
- 46.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 47.Kwakkel G, Wagenaar RC, Koelman TW, et al. Effects of intensity of rehabilitation after stroke: a research synthesis. Stroke. 1997;28:1550–1556. doi: 10.1161/01.str.28.8.1550. [DOI] [PubMed] [Google Scholar]
- 48.Kwakkel G, Wagenaar RC, Twisk JW, et al. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354:191–196. doi: 10.1016/S0140-6736(98)09477-X. [DOI] [PubMed] [Google Scholar]
- 49.Cohen LG, Bandinelli S, Topka HR, et al. Topographic maps of human motor cortex in normal and pathological conditions: mirror movements, amputations and spinal cord injuries. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:36–50. [PubMed] [Google Scholar]
- 50.Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- 51.Wu CW, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. J Neurosci. 1999;19:7679–7697. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nudo RJ, Milliken GW, Jenkins WM, et al. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:784–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effect of rehabilitative training motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 54.Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabil Neural Repair. 2000;14:187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- 55.Grafton ST, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. J Cogn Neurosci. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- 56.Karni A, Meyer G, Jezzard P, et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 57.Cao Y, D’Olhaberriague L, Vikingstad EM, et al. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–122. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- 58.Weiller C, Chollet F, Friston KJ, et al. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 59.Weiller C, Ramsay SC, Wise RJS, et al. Individual patterns of functional reorganization in the human cerebral-cortex after capsular infarction. Ann Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- 60.Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 61.Binkofski F, Seitz RJ, Hacklander T, et al. Recovery of motor functions following hemiparetic stroke: a clinical and magnetic resonance-morphometric study. Cerebrovasc Dis. 2001;11:273–281. doi: 10.1159/000047650. [DOI] [PubMed] [Google Scholar]
- 62.Chollet F, DiPiero V, Wise RJS, et al. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- 63.Nelles G, Spiekermann G, Jueptner M, et al. Reorganization of sensory and motor systems in hemiplegic stroke patients: a positron emission tomography study. Stroke. 1999;30:1510–1516. doi: 10.1161/01.str.30.8.1510. [DOI] [PubMed] [Google Scholar]
- 64.Seitz RJ, Hoflich P, Binkofski F, et al. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–1088. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- 65.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 66.Levy CE, Nichols DS, Schmalbrock PM, et al. Functional MRI evidence of cortical reorganization in upper-limb stroke hemiplegia treated with constraint-induced movement therapy. Am J Phys Med Rehabil. 2001;80:4–12. doi: 10.1097/00002060-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Barker AT. The history and basic principles of magnetic nerve stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:3–21. [PubMed] [Google Scholar]
- 68.Beric A. Transcranial electrical and magnetic stimulation. In: Devinsky O, Beric A, Dogali M, editors. Electrical and Magnetic Stimulation of the Brain and Spinal Cord. New York: Raven Press; 1993. pp. 29–42. [Google Scholar]
- 69.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 70.Epstein CM, Scwartzberg DG, Davey KR, et al. Localizing the site of magnetic brain stimulation in humans. Neurology. 1990;40:666–670. doi: 10.1212/wnl.40.4.666. [DOI] [PubMed] [Google Scholar]
- 71.Ziemann U. Transcranial magnetic stimulation: its current role in the evaluation of patients post-stroke. Neurol Rep. 2000;24:82–93. [Google Scholar]
- 72.Brasil-Neto JP, McShane LM, Fuhr F, et al. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- 73.Classen J, Liepert J, Wise S, et al. Rapid plasticity of human movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 74.Kaelin-Lang A, Cohen LG. Enhancing the quality of studies using transcranial magnetic and electrical stimulation with a new computer-controlled system. J Neurosci Methods. 2000;102:81–89. doi: 10.1016/s0165-0270(00)00284-3. [DOI] [PubMed] [Google Scholar]
- 75.Liepert J, Classen J, Cohen LG, et al. Task dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- 76.Mortifee P, Stewart H, Schulzer M, et al. The reliability of transcranial magnetic stimulation for mapping the human cortex. Electroencephalogr Clin Neurophysiol. 1994;93:131–137. doi: 10.1016/0168-5597(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 77.Liepert J, Bauder H, Miltner WHR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 78.Cicinelli P, Traversa R, Bassi A, et al. Interhemispheric differences of hand muscle restoration in human motor cortex. Muscle Nerve. 1997;20:535–542. doi: 10.1002/(sici)1097-4598(199705)20:5<535::aid-mus1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 79.Cicinelli P, Traversa R, Oliveri M, et al. Intracortical excitatory and inhibitory phenomena to paired transcranial magnetic stimulation in healthy human subjects: differences between right and left hemisphere. Neurosci Lett. 2000;288:171–174. doi: 10.1016/s0304-3940(00)01216-7. [DOI] [PubMed] [Google Scholar]
- 80.Traversa R, Cicinelli P, Rossini PM, et al. Mapping of motor cortical reorganization after stroke: a brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 81.Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120:1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- 82.Pennisi G, Rapisarda G, Bella R, et al. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999;30:2666–2670. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- 83.Catano A, Houa M, Caroyer JM, et al. Magnetic transcranial stimulation in non-haemorrhagic sylvian strokes: interest of facilitation for early functional prognosis. Electroencephalogr Clin Neurophysiol. 1995;97:349–354. doi: 10.1016/0924-980x(95)00127-7. [DOI] [PubMed] [Google Scholar]
- 84.Rossini PM, Caltagirone C, Castriota-Scanderbeg A, et al. Hand motor cortical reorganization in stroke: a study with fMRI MEG and TCS maps. Neuroreport. 1998;9:2141–2146. doi: 10.1097/00001756-199806220-00043. [DOI] [PubMed] [Google Scholar]
- 85.Rossini PM, Rossi S. Clinical applications of motor evoked potentials. Electroencephalogr Clin Neurophysiol. 1998;106:180–194. doi: 10.1016/s0013-4694(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 86.Liepert J, Graef S, Uhde I, et al. Training-induced changes of motor cortex representations in stroke patients. Acta Neurol Scand. 2000;101:321–326. doi: 10.1034/j.1600-0404.2000.90337a.x. [DOI] [PubMed] [Google Scholar]
- 87.Classen J, Liepert J, Hallet M, et al. Plasticity of movement representation in the human motor cortex. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:162–173. [PubMed] [Google Scholar]
- 88.Liepert J, Miltner WHR, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 89.Schiene K, Bruehl C, Zilles K, et al. Neuronal hyperexcitability and reduction of GABA A receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab. 1996;16:906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 90.Jacobs KM, Donoghue JP. Reshaping the cortical map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 91.Ziemann U, Ishii K, Borgheresi A, et al. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518:895–906. doi: 10.1111/j.1469-7793.1999.0895p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hagemann G, Redecker C, Neumann-Haefelin T, et al. Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Ann Neurol. 1998;44:255–258. doi: 10.1002/ana.410440217. [DOI] [PubMed] [Google Scholar]
- 93.Thickbroom GW, Sammut R, Mastaglia FL. Magnetic stimulation mapping of motor cortex factors contributing to map area. Electroencephalogr Clin Neurophysiol. 1998;109:79–84. doi: 10.1016/s0924-980x(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 94.Georgopoulos AP. New concepts in generation of movement. Neuron. 1994;13:257–268. doi: 10.1016/0896-6273(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 95.Kwan HC, MacKay WA, Murphy JT, et al. Spatial organization of precentral cortex in awake primates, II: motor outputs. J Neurophysiol. 1978;41:1120–1131. doi: 10.1152/jn.1978.41.5.1120. [DOI] [PubMed] [Google Scholar]
- 96.Sanes JN, Donoghue JP, Thangaraj V, et al. Shared neural substrate controlling hand movement in human motor cortex. Science. 1995;268:1775–1777. doi: 10.1126/science.7792606. [DOI] [PubMed] [Google Scholar]
- 97.Wassermann EM, McShane LM, Hallet M, et al. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- 98.Taub E, Burgio L, Groomes T, et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994;61:281–293. doi: 10.1901/jeab.1994.61-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morris DM, DeLuca S, Pidikiti R, et al. Constraint-induced movement therapy for motor recovery after stroke. Neurorehabilitation. 1997;9:29–43. doi: 10.3233/NRE-1997-9104. [DOI] [PubMed] [Google Scholar]
- 100.Kopp B, Kunkel A, Muhlnickel W, et al. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. Neuroreport. 1999;10:807–810. doi: 10.1097/00001756-199903170-00026. [DOI] [PubMed] [Google Scholar]
- 101.Sabari JS, Kane L, Flanagan SR, et al. Constraint-induced motor relearning after stroke: a naturalistic case report. Arch Phys Med Rehabil. 2001;82:524–528. doi: 10.1053/apmr.2001.21857. [DOI] [PubMed] [Google Scholar]
- 102.Liepert J, Uhde I, Graf S, et al. Motor cortex plasticity during forced-use therapy in stroke patients: a preliminary study. J Neurol. 2001;248:315–321. doi: 10.1007/s004150170207. [DOI] [PubMed] [Google Scholar]


