Abstract
The authors examined serial changes in optical topography in a stroke patient performing a functional task, as well as clinical and physiologic measures while undergoing constraint-induced therapy (CIT). A 73-year-old right hemiparetic patient, who had a subcortical stroke 4 months previously, received 2 weeks of CIT. During the therapy, daily optical topography imaging using near-infrared light was measured serially while the participant performed a functional key-turning task. Clinical outcome measures included the Wolf Motor Function Test (WMFT), Motor Activity Log (MAL), and functional key grip test. Transcranial magnetic stimulation (TMS) and functional magnetic resonance imaging (fMRI) were also used to map cortical areas and hemodynamic brain responses, respectively. Optical topography measurement showed an overall decrease in oxy-hemoglobin concentration in both hemispheres as therapy progressed and the laterality index increased toward the contralateral hemisphere. An increased TMS motor map area was observed in the contralateral cortex following treatment. Posttreatment fMRI showed bilateral primary motor cortex activation, although slightly greater in the contralateral hemisphere, during affected hand movement. Clinical scores revealed marked improvement in functional activities. In one patient who suffered a stroke, 2 weeks of CIT led to improved function and cortical reorganization in the hemisphere contralateral to the affected hand.
Keywords: Rehabilitation, Forced-use, Upper limb
Many interventions, including therapeutic exercise, neurofacilitatory methods, electrical stimulation, and biofeedback, have been used to improve motor recovery after stroke. However, little evidence has been provided to demonstrate the efficacy of these approaches for upper extremity recovery after stroke.1
Findings from studies in which nonhuman primates have undergone unilateral forelimb deafferentation2 have served as a catalyst for the development and application of constraint-induced therapy (CIT), a procedure involving restraint of the less impaired upper extremity, forced-use of the affected upper extremity, and use of principles derived from behavioral conditioning procedures,3 motor learning, and skill acquisition literature.4 CIT has been shown to be effective when applied to stroke patients whose affected upper extremity has some movement capabilities.5,6 There is increasing evidence that this type of therapy may induce plasticity or dynamic changes in the pattern of brain activity as well as improve clinical outcome.7–14 Using transcranial magnetic stimulation (TMS), Liepert et al.9 observed increases in motor map size and shift of the center of gravity in motor maps governing the thumb within the affected hemispheres of chronic stroke patients after a 12-day period of CIT. Wittenberg et al.11 reported expansion of the affected side map compared with the less affected side as well as a medial shift of the center of gravity and increased intracortical facilitation in the less affected side after 10 days of CIT. They also demonstrated decreased task-related cortical activation after CIT measured by positron emission tomography (PET). In a functional magnetic resonance imaging (fMRI) study, Schaechter et al.10 observed increased activation of ipsilateral hemisphere with movement of the affected hand in stroke patients after CIT.
TMS, fMRI, and PET have been used most often to evaluate cortical substrates of functional recovery after stroke in the human. Although these methods can assess cortical activity in the human brain, they require a large time allocation for data collection and often impose limitations on the extent of functional activities undertaken by the participant. The steric hindrance of fMRI and the inability to amass multiple time points in PET due to radiation exposure limit the usefulness of these modalities in assessing motor-related behavioral changes following physical intervention. On the other hand, optical topography, using near-infrared light, is gaining recognition as a safe, painless, non-invasive, and relatively quicker method for mapping functional brain activity in awake behaving humans. Optical topography monitors tissue oxygenation and hemodynamics within the brain. Although spatial resolution is not as precise as fMRI, optical topography has advantages such as high temporal resolution, simple construction, and suitable application at the bedside or during comparatively more dynamic functional situations.15 Optical topography has been applied to assess brain activities during language function16–18 and a variety of motor tasks.15,19–21 Similar to fMRI, optical topography has been applied indirectly to measure cortical activity in both normal and stroke populations.18,21,22
This study is the first to explore the relationship between signals produced by a novel brain mapping device, optical topography, and serial changes in clinical and physiologic (TMS mapping of finger extensors and fMRI during active finger movements) measures in a stroke patient performing a functional task (key grasp and turning) throughout the course of CIT.
METHODS
A 73-year-old, right-handed male, who suffered a stroke in the left basal ganglia and internal capsule 4 months previously, was enrolled in this study. Informed consent was obtained in accordance with the local institutional review board. He had received conventional treatment at an acute rehabilitation unit and discharged to his home. Upon examination, he showed right hemiparesis and mild dysarthria. He was able to 1) actively extend his wrist and elevate his thumb and at least 2 fingers from a supported, resting position 3 times within 1 min, 2) independently and safely transfer to the toilet, and 3) stand up and maintain balance for 2 min with support. He had a history of diabetes mellitus, hypertension, and dyslipidemia and had previously undergone a right carotid endarterectomy and coronary artery bypass grafting. He was on medication to control hypertension, diabetes, and dyslipidemia. His blood pressure was stable during the intervention.
CIT Intervention
CIT consisted of restraint of the unaffected upper extremity, while the patient performed task-oriented therapy of the affected upper extremity. For 2 consecutive weeks, the patient wore a padded mitt on the stronger hand during all waking hours and attended therapy sessions 5 days a week for up to 6 hours per day. During therapy, the participant practiced daily activities, combining repetitive task practice and adaptive task practice (shaping) under the guidance of a licensed occupational therapist.23
Pre- and Posttreatment Evaluation
Clinical outcome measures included the Wolf Motor function test (WMFT)24 and Motor activity log (MAL).9 TMS motor maps of the EDC and precision grasping forces were measured before and after 2 weeks of CIT. Using whole-brain fMRI, images were acquired during performance of finger flexion/extension tasks of each upper extremity. The fMRI was performed only once at the conclusion of the intervention for comparison with data obtained from optical topography and TMS mapping.
Optical Topography
Optical topography was performed using a 24-channel near-infrared spectroscopy (NIRS) machine (Hitachi ETG-100, http://www.hitachimed.com/products/optical_top.asp). Near-infrared lights with wavelengths of 780 nm and 830 nm were guided by optical fiber bundles and transmitted into the brain through the cranium. The scattered and reflected light was sampled by receiving probes placed on the scalp 30 mm away from the transmitting probes.
Cortical activity using optical topography was measured at the same time every morning for 10 consecutive days during the intervention. The plastic probe holder (3 × 3 matrix) with 12 measurement channels positioned on either side of the head was securely placed on the scalp over the patient’s bilateral motor areas using Velcro straps. Motor areas were identified as lying approximately between the external auditory meatus of the ear and vertex of the cranium by drawing a line between the ear and the probe holder. As further assurance of motor area specificity, we placed the probes directly overlying the site of the TMS hotspot for the EDC that had been previously measured and marked with a permanent ink marker. To ensure that the probe holder was located consistently over sessions, distances from the probe holder to both external auditory meati and inion of the skull were recorded.
Optical topography measurements were made with the patient in an upright, sitting position. The patient was instructed to extend his right (affected) upper arm and wrist and turn a key in a door lock 180 deg (i.e., 90 deg clockwise and 90 deg counterclockwise).* The experiment was a blocked design with 5 repeated cycles: 15 s movement and 45 s rest. The participant was instructed to repeatedly turn a key in a lock at a comfortable speed during the 15-second movement period and return his hand to his lap and rest comfortably during the 45-second rest period. At all times the participant was told to maintain fixation on a central fixation point located at the keyhole. Electromyographic (EMG) activity in the limbs was not monitored in the present study. But task demands required the participant to relax his left hand during the performance of the key-turning task with his right hand. Therefore, relaxation was constantly monitored by one of the experimenters.
The relative changes in oxy-hemoglobin concentration data were averaged across 5 repetitions for all 24 channels. Active channels in which oxy-hemoglobin concentration during the task period was significantly higher than that of the rest period, and the most active channel, which demonstrated peak oxy-hemoglobin concentration, were identified in each session. The sum of oxy-hemoglobin concentrations during stimulation time in all active channels was then calculated. The 2-dimensional optical topographic image of the average oxy-hemoglobin concentration for each channel was co-registered and superimposed on the 3-dimensional anatomical image of the patient by engineers at Hitachi Medical Corporation.
A laterality index (LI) was computed that describes the contrast in amount of activation between the unaffected and affected cortices. The LI was determined from oxy-hemoglobin concentrations in active channels in each hemisphere as LI = (LH − RH)/(LH + RH), where LH denotes the sum of oxy-hemoglobin concentrations in the active channels of the left hemisphere and RH denotes that of the right hemisphere.18,20 LI values ranged from +1, indicating that all motor cortical activation occurred in the hemisphere contralateral to the moving hand, to −1, indicating that all motor cortical activation occurred in the hemisphere ipsilateral to the moving hand.
For comparison of equivalent brain areas in an undamaged brain, a 71-year-old able-bodied man underwent the same optical topography protocol as the patient for 10 consecutive days.
TMS Motor Mapping of the EDC
Motor-evoked potentials (MEP) were recorded from the extensor digitorum communis (EDC) muscle using closely spaced electrodes (interelectrode distance = 1.5 cm) over the manually isolated EDC as described previously.25 Briefly, the hot spot was identified as the scalp position of maximum response; the resting motor threshold was defined as the stimulus intensity, which produced at least 5 MEPs, the amplitude of which is larger than 0.025 mV, in 10 consecutive trials. Motor maps of the EDC were constructed by acquiring 10 MEPs at a stimulation intensity of 110% resting motor threshold at locations on a 1 cm square grid constructed around the hot spot. The scalp locations, at which greater than 5 MEPs out of 10 trials were elicited, were considered as active. Mapping proceeded until nonactive spots were found along a nonresponse border of the map.11 After finishing the mapping procedure, 2 parameters were used to describe motor system excitability: the motor map area, calculated as the number of active positions, and the center of gravity (CoG, the amplitude weighted center of the map).
Cortical Activation as Measured Using fMRI
Imaging, consisting of a routine sagittal T1-weighted localizer followed by a high-resolution axial T1-weighted acquisition of the entire brain, was performed on a 1.5 Tesla Phillips scanner. The high-resolution images were used both to co-register the optical topography output signal and to spatially normalize the functional scans to a standard space. Functional imaging was performed with an EPI sequence, involving a standard single-shot gradient echo dynamic acquisition with FOV = 24, 64 × 64 matrix, 28 slices, 5 mm thickness, no skip, TR = 3.0 sec, and TE = 40 ms. Images were reconstructed off-line on a remote workstation, and analysis was performed with SPM99. Only significant clusters defined according to extent (at P < 0.05 corrected for multiple comparison according to random field theory, using family-wise error rate)26 were used in the analysis.
The fMRI scanning protocol consisted of 30-second periods of rest alternating with 30-second periods of flexion/extension at the metacarpophalangeal joints, for 4 rest periods and 3 task periods per scan. While wearing a splint designed to position the wrist in a neutral position and to limit the amplitude of movement, the participant repeatedly extended and flexed his fingers at the metacarpophalangeal joints. Only 1 hand was actively moved during a 3-min scanning run. Trials were undertaken at the rate of 1 Hz and also at 70% of the maximum rate the participant could achieve. To check for finger movements (and mirror movements), a fiber optic position-sensing device that linearly transduces bending at a single point was secured at the dorsal base of the 2nd nail bed of each hand (S 720 Shape Sensor, Measurand Inc, Fredericton, NB, Canada).
An LI was computed in the same manner for fMRI as that described for optical topography, except the number of suprathreshold voxels (those greater than P < 0.05 corrected for multiple comparisons) within the primary motor cortex were used in the calculation (LI).10,18 The structural scan was used to outline a single region of interest (ROI) in the primary motor cortex on the basis of anatomic landmarks.27 The extent of activation in the ROI was determined by counting the number of activated voxels. Therefore, during affected hand movement, the LI was calculated by using the formula LI = (L − R)/(L + R), where L and R denote the number of suprathreshold voxels in left (L) or right (R) primary motor cortices.
Kinetic Key Grip Test
In an experimental session separate from the key-turning activity performed during optical topography measurement, grasping forces were measured during precision grip (e.g., thumb and index finger only) and lift task using a key instrumented with a Nano-17 Force-torque transducer (ATI Inc., Garner, NC). The transducer provides a measure of normal (grip) and tangential (load) forces. The patient was instructed to reach and grasp the key using a precision grip and lift and hold it approximately 10 cm above the table for approximately 3 seconds and then return the key to the table. Five trials were performed with the more affected limb prior to and following the CI intervention. Three maximum grip trials, using a precision grip, were collected for the more affected and less affected limb prior to lifting trials. The greatest force achieved during the pre- and postintervention testing sessions was considered the patient’s maximum.
Clinical Outcome Measures
To test motor function, the patient was administered the Motor Activity Log (MAL) and the Wolf Motor Function Test (WMFT). The MAL was designed to assess the patient’s subjective impression of how well and how often movement was observed in the affected arm during daily activities. Patients rated their own performance from 0 to 5.13 The WMFT is a time-based method to evaluate upper extremity performance through single- or multiple-joint motions and functional tasks. It consists of 15 timed tasks and 2 force measurements; its reliability and validity was established in a stroke population.24
RESULTS
Optical Topography
During the 2-week intervention, daily optical topographic images using near-infrared light were measured serially while the participants performed a functional key turning task (Figure 1). Among the total 10 sessions, 1 session (the 7th day) was discarded due to excessive head movement artifact. The remaining 9 test sessions were analyzed. No sessions were discarded for the control participant. Within the 12 possible recording channels per hemisphere, the number of active recording channels fluctuated between 9 and 12.
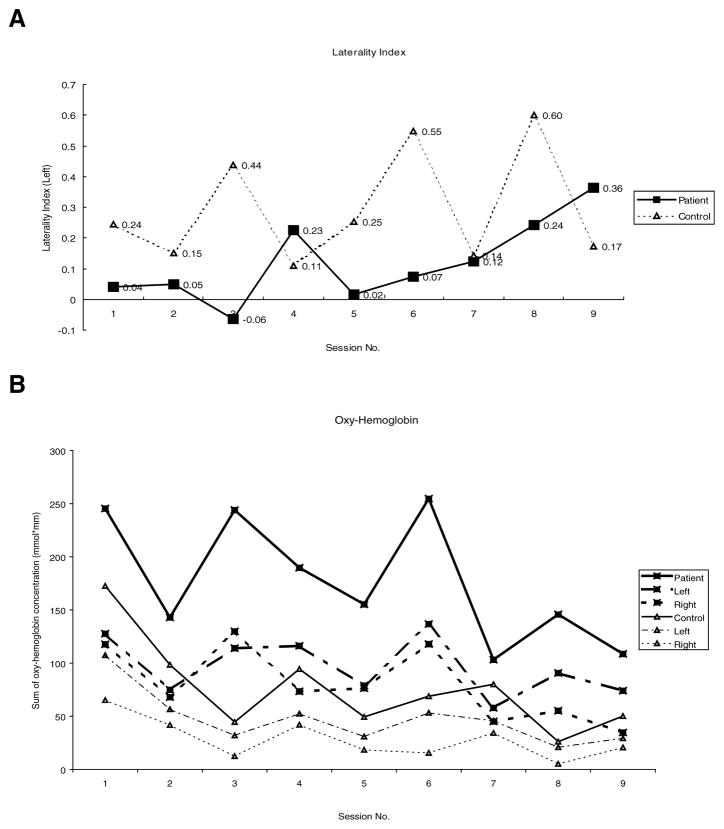
Figure 1.
Changes in optical topography measures over time. A. The laterality index of patient and control subject from baseline (Session 1) to completion of CI therapy (Session 9). B. Sum of oxy-hemoglobin concentrations in active channels of each hemisphere.
There was a trend toward an increasingly positive laterality index from the baseline session 1 (0.04) to session 9 (0.36), suggesting increased neuronal activation in the contralateral hemisphere compared to ipsilateral side when moving the affected (right) hand (Figure 1A). In contrast, the laterality index for the control participant was relatively greater than that seen in the patient and remained positive with less variability throughout all sessions. The sum of oxy-hemoglobin concentration in active channels did fluctuate but showed a decreasing trend by the end of treatment, whereas the control participant showed an initial drop of oxy-hemoglobin concentration after the 1st baseline session and a slight reduction over time as well, but not as profoundly (Figure 1B). This decrease of oxy-hemoglobin concentration was more prominent in the right (unaffected) hemisphere than the left (affected) hemisphere.
The specific location of the most active channels was also variable, but in 6 of the 9 test days, the location of the most active channel was centered on the lower part of the probe holder located on the left hemisphere (i.e., red-colored area depicted in Figure 2A). Numbers and location of active channels did not appear to change in a linear pattern over time. During right-hand key turning, relative increased oxy-hemoglobin concentrations were recorded in a region surrounding the left motor cortex (red area depicted in Figure 2A).
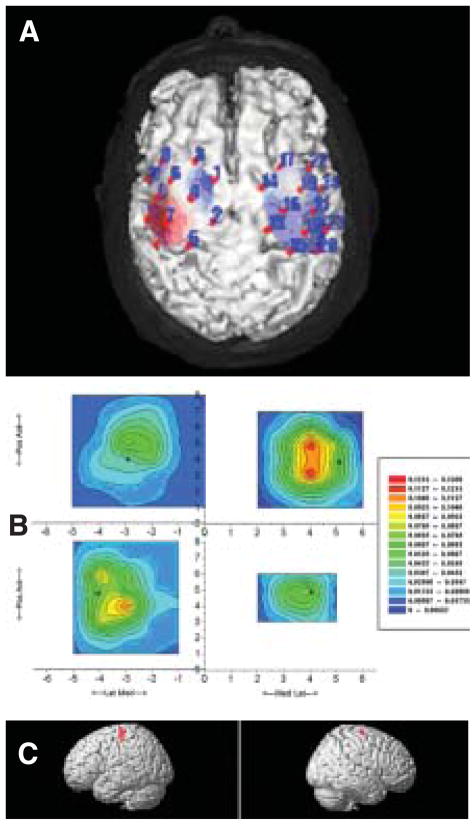
Figure 2.
Example images from the patient. Shown are comparisons of posttreatment cortical activation using 3 different modalities. A. Optical topographic image of oxy-hemoglobin concentration during key turning with (affected) right hand superimposed on a 3-dimensionally reconstructed cerebral cortex of an MRI image of the patient. The red and blue shading in the topographic image indicate an increase and decrease in relative [oxy-Hb], respectively. In the left hemisphere (left side), contralateral to the moving hand, increase and decrease in [oxy-Hb] were observed in the precentral and superior frontal gyri, respectively. The 24 measurement channels are indicated by filled circles and numeric labels. B. The extensor digitorum communis MEP amplitude (mV) is displayed in a contour plot over the scalp surface as viewed from above, with x representing medial-lateral axis and y the anterior posterior axis. Positive x is to the right, y anterior, and the origin (0,0) is the scalp vertex (Cz in the EEG 10–20 system). Red indicates larger average MEP amplitude; blue indicates smaller average MEP amplitude. The top row is Pre-Rx, and the bottom row is Post-Rx. The affected hemisphere is on the left. Asterisks indicate location of hotspot. C. BOLD responses on a rendered brain indicating significant activation during right (affected) finger flexion/extension movements at a rate of 70% maximum compared to rest. In all 3 images the left hemispheric activation is shown in the left column, whereas the right hemispheric activity is depicted in the right column.
TMS
When compared to baseline, the hot spot location in the left (affected) motor cortex shifted laterally and anterior by 1 cm following the intervention (compare asterisk in Figure 2B top-left column with bottom-left column). The center of gravity (CoG) also shifted laterally (0.66 cm) and posterior (0.48 cm) (Fig 2B, left column). In the right hemisphere, the hot spot location shifted medially and anterior by 1.0 cm each, whereas the CoG shifted medially (0.45 cm) and anterior (0.61 cm) (Figure 2B, right column). The overall MEP amplitudes in the right EDC (left brain), which were lower at baseline, became larger than those in the left EDC (right brain) after intervention. The motor map area in the left brain increased, as manifest by the number of active positions increasing from 11 to 15, whereas that of the right brain decreased from 12 to 4 active positions. A more focused CoG was also seen as indicated by 2 apparent CoG locations preintervention and 1 single CoG postintervention. The location of the hot spots corresponded to the upper part of the probe sets in optical topography (area among the channels 2, 4, 5, 7 in the left hemisphere and channels 13, 15, 16, 18 in the right hemisphere, Figure 2A).
fMRI
During right (affected) finger flexion/extension movements at 70% of maximal rate, increased activation appeared in contralateral (left) sensorimotor cortex, basal ganglia, and ipsilateral (right) motor cortex (Figure 2C). During left (unaffected) finger flexion/extension movements, increased activation was observed in contralateral (right) sensorimotor cortex only. Continuous monitoring of finger position during the scan using a fiberoptic position sensing device showed distinct movement of only the hand the patient was instructed to move. Therefore, mirror movement cannot be used to explain the observed results.
The fMRI laterality index for affected hand movement was +0.40 compared to +0.95 for movement of the unaffected hand. A number closer to +1 indicates activation of motor cortical areas in the hemisphere contralateral to the moving arm, whereas lower values indicate greater activation toward the ipsilateral motor cortex.
Kinetic Key Grip Test
For maximum precision grip, there were no changes in strength levels for the less affected limb; 35 N for pre- and posttest. However, there was an increase in maximum grip force for the more-affected limb from a maximum grip force of 27.5 N before CIT to 32.1 N after CIT (Table 1).
Table 1.
Comparison of Functional Activity Measures before and after CIT for 1 Patient
| Pre-CIT | Post-CIT | ||
|---|---|---|---|
| WMFT | Mean score (right arm) | 12.36 | 6.74 |
| Mean score (right-left) | 9.54 | 4.61 | |
| Turn key in lock time (sec) | 20.47 | 6.93 | |
| Grip strength (kgs) | 4.66 | 7.0 | |
| MAL | Mean amount score | 0.6 | 3.1 |
| Mean how well score | 1.1 | 3.0 | |
| Use a key—Amount | 0 | 5 | |
| Use a key—How well | 0 | 4 | |
| Kinetic Key Grip Test | Maximum grip force (N) | 27.5 | 32.1 |
| Optical topography | Laterality index | 0.04 | 0.36 |
| Peak oxy-Hb concentration | 0.58 | 0.12 |
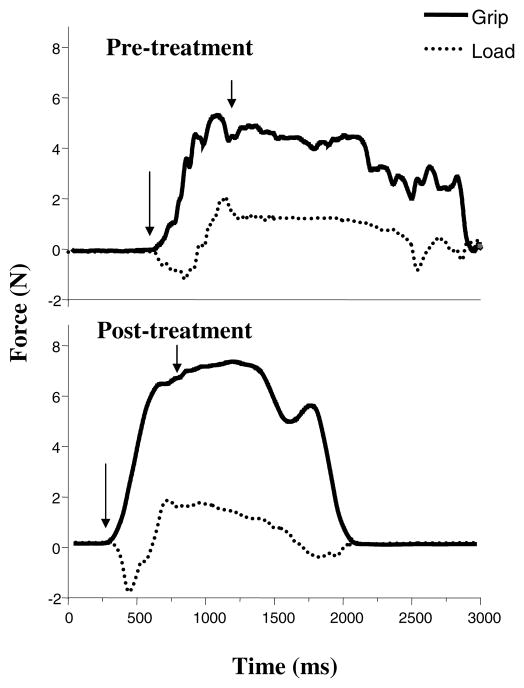
Representative grip and load force profiles during the lift and hold trials are shown in Figure 3. During the pretest, grip force increased in a stepwise fashion until the key was lifted from the table (approximated by 2nd arrow). Prior to lifting the key, the patient appears to exert a downward pushing force on the transducer, hence the immediate negative direction in the load force prior to lifting (Figure 3 top panel). Inspection of postintervention profiles indicates that following CIT there is a smooth monotonic increase in grip force prior to lifting the transducer (Figure 3 bottom panel). However, the patient still exerted a downward pushing force tangential to the transducer.
Figure 3.
Changes in key grip and load forces after 2 weeks of CIT during the “lift and hold” task. Pretreatment forces (N) are shown in the top panel, whereas post-treatment grip forces are depicted in the bottom panel. Arrows indicate starting and ending points of lifting the transducer. See text for further explanation.
Clinical Outcome Measures
The average WMFT scores for the control participant were 1.2 s for both left and right upper extremities, which are compatible with previous reports in able-bodied adults.24 In the patient, the preintervention average WMFT scores were 12.4 s and 2.8 s for the right (more-affected) and left (less-affected) upper extremity, respectively (where lower numbers indicate less time to complete tasks). Following 2 weeks of CIT, scores decreased to 6.7 s and 2.1 s, respectively. All 17 items in WMFT, including key turning and maximum grip strength (similar to tasks performed during optical topography measurement), showed marked improvement. Mean MAL scores also increased from 0.6 and 1.1 to 3.1 and 3.0 in “amount of use” and “how well” scores, respectively (where higher numbers indicate more and better use of the affected arm). Interestingly, the task “Use key amount” score for that MAL activity changed from 0 to 5 in the patient (Table 1). For comparison, an able-bodied participant would have an MAL score of 5.
DISCUSSION
Considerable effort is now being expended by neurorehabilitation investigators and clinicians to learn about mechanisms that might account for changes in the pattern of brain activity following interventions designed to overcome impairments and improve function.10,28,29 In this regard, there is a clear need to exploit those available technologies that will permit associations between function and mechanism. This case study represents an initial effort to associate information derived from optical topography, fMRI, TMS, and kinetics to explain changes in movement behavior following an intensive 2-week intervention.
Optical Topography
The 2 most striking findings are a shift in oxy-hemoglobin concentration toward the affected side and an overall reduction in oxy-hemoglobin concentration following the intervention as recorded with optical topography from both hemispheres. The observed decrease in oxy-hemoglobin concentration toward the end of therapy can represent a global decrease in neural activation in both hemispheres throughout all of the cortical areas measured by the probes due to a reduced requirement of task-related synaptic activity. This observation is consistent with PET findings showing a longitudinal decrease in activation on the affected side in caudate, sensorimotor cortex, medial temporal gyrus, and cerebellar vermis after CI therapy in stroke patients versus volunteers.11 Using functional MRI, Ward et al.30 found that task-related brain activations decreased over time in motor regions of patients recovering from stroke. The prevailing view is that the amount of cortical activation tends to decline as recovery takes place, even though the tasks are unchanged, that is, less “effort” is required by the brain to achieve the same workload as reorganization advances.31 Even in our control participant, oxy-hemoglobin concentration dropped after the initial test session, suggesting a quick acclimation to the task. However, our patient showed a decrease in oxy-hemoglobin concentration only during the last few days of therapy, a finding that we may attribute to an interventional effect.
In previous work using optical topography in poststroke motor recovery, Kato et al.21 reported increased ipsilateral activation during finger tapping in stroke patients compared to normal controls. This finding is consistent with the current study. The study by Kato et al. did not offer any information on serial changes along with rehabilitation. Our study suggests there are changes in the extent of ipsilateral activation according to the state of motor recovery.
By investigating the changes in optical topography signal during each measurement day, we assumed that for adaptive changes to occur, an interventional period of at least 7 consecutive days or more may be required. The laterality index of the patient fluctuated slightly in the 1st week of CIT, ranging from −0.02 to +0.23, suggesting a nearly equal balance of cortical activation or possibly a slight tendency toward the contralateral hemisphere. The laterality index decreased after the 1st weekend during which the patient did not undergo concentrated therapy (i.e., between sessions 4 and 5, see Figure 1A). During the 2nd week of CIT, the laterality index increased steadily and did not decrease but increased further after the 2nd weekend break (between sessions 8 and 9). The distinct increase in the laterality index in the last few sessions is compatible with the finding that the decrease of oxy-hemoglobin concentration became evident in the last 3 sessions. This initial observation will require further investigation.
TMS
We saw a slightly larger map area and a focused CoG location on the contralateral hemisphere following 2 weeks of CIT. The increase in TMS-evoked motor map area and single CoG locus indicates that the left motor cortex showed an increased excitability following a 2-week intervention, suggesting a possible strategy for recovery. Liepert et al7,9 reported similar findings in chronic stroke patients after CIT. Many of the participants had lacunar subcortical lesions that involved the internal capsule. The CoG shift does not seem to be important in our patient because the unaffected hemisphere also showed some degree of shift.
This is inconsistent with some TMS studies that described more ipsilateral activation in poststroke motor recovery. Turton et al.32 examined 21 stroke patients using TMS to assess MEP of both arm and hand muscles. Most of the ipsilateral responses (to the affected hand) from the intact side were found in the group of patients whose recovery was slow and incomplete. They suggested this type of reorganization might not be beneficial to recovery. Netz et al.33 investigated ipsilateral TMS responses in 15 stroke patients and reported similar results, namely, ipsilateral responses were detected in patients with poor recovery at significantly lower thresholds. To further support the contention that activation of the ipsilateral hemisphere is not beneficial to functional outcomes, Werhahn et al.34 observed that TMS of the intact hemisphere resulted in delayed simple reaction times in the contralateral healthy but not in the ipsilateral paretic hand of chronic stroke patients. This suggests that the activity in the intact hemisphere does not contribute greatly to functional recovery. However, the correlation between poor recovery and ipsilateral activation does not necessarily mean that ipsilateral cortical activity inhibits motor recovery after stroke.35 The brain may develop different strategies of recovery according to the lesion site or its capacity for dynamic neurological change in response to injury.36
FMRI
Functional MRI findings recorded following our 2-week intervention revealed ipsilateral activation of motor cortex, though much smaller than the contralateral hemisphere, a finding comparable to the optical topography data showing relatively larger activation in the contralateral hemisphere compared to ipsilateral side when moving the affected (right) hand. Unfortunately preintervention fMRI was unattainable, so the change of laterality cannot be compared to optical topography.
There appears to be a slight difference in the location of cortical activation recorded with optical topography and fMRI, although a direct comparison is difficult because optical topography and fMRI were not performed concurrently (compare Figure 2A and 2C). The accuracy of optical topography measurements for focal hemodynamic changes has been challenged,37,38 making the certainty of what precise neural changes subserve changes in optical topography unclear. However, a combined fMRI and optical topography study of language illustrated fMRI signal changes correlated consistently with changes in oxy-hemoglobin, concluding that the apparent discrepancy with the accepted BOLD theory is caused by the fact that the BOLD theory ignores the effect of capillaries.39 Direct comparison of activated sites using optical topography and fMRI in this study was not possible. Moreover, fMRI activity was recorded during flexion/extention of the MCPs. The task undertaken using each modality was different; a functional key-turning task was performed during optical topography measurement. This is one limitation of the current study design, and future experiment will incorporate MRI-compatible functional lock-key-turning devices. Therefore, caution should be exercised in directly comparing the 2 images. In future studies, we intend to measure concurrent optical topography and fMRI during execution of a key-grasp-and-turn task while in the scanner.
Our study demonstrates consistent increases in activation across modality in the hemisphere contralateral to the affected hand. In patients 3 to 6 months poststroke, with corticospinal tract infarction, using fMRI, Marshall et al.40 observed that the ratio of contralateral to ipsilateral sensorimotor cortex activity increased over time, supporting our finding. Therefore, our patient with a subcortical stroke may have relatively spared cortex in the contralateral hemisphere, manifest as a substrate for utilization when exposed to intensive rehabilitation.36,40,41 This is in agreement with available data indicating recovery is best when there is restitution of activation toward the physiological network over time (see Calautti31 for review). Between the availability of this spared cortex, the intensity of the practice, and the precision in progressively challenging the patient to complete more difficult tasks, improvements in function could have been facilitated much, as has been demonstrated in animal models.42
Many studies show data suggesting a role of ipsilateral activation in poststroke motor recovery. In stroke patients compared to normal participants, some investigators have reported increased ipsilateral activation using fMRI21,36,43 and TMS,32,33 whereas others have shown increased contralateral and bilateral activation.44 Increased ipsilateral activation as a result of CIT has also been suggested.10,14 Others have reported increased contralateral activation following treatment.29,45 One patient in the study of Levy et al.,14 who sustained a right frontoparietal subarachnoid hemorrhage, showed marked increase of ipsilateral cortical activation in fMRI after CIT. Schaechter et al.10 demonstrated fMRI data from 3 poststroke patients who underwent CIT, which showed reduced laterality indices suggesting motor cortical activation occurred in the hemisphere ipsilateral to the moving hand. The lesion sites of the 3 participants were not described precisely. In our case study, the patient had a small subcortical infarction in the left basal ganglia and internal capsule, and the laterality index calculated from optical topography measurements increased as the intervention progressed, suggesting increased activation of motor cortical areas in the hemisphere contralateral to the affected moving arm. Because an LI could not be obtained from fMRI at baseline, we cannot compare/confirm changes in that LI measure.
Kinetic Key Grip
The results from this investigation suggest that CIT increases maximum force production capabilities in a patient following 2 weeks of CIT. It should be noted that the task was not part of the training. Our data suggest that CI therapy can lead to an improvement in the specification of grasping forces and force-timing characteristics. These improvements in force control may contribute to mechanisms responsible for improvements in motor performance. Furthermore, grasping forces were generated and controlled in a more precise manner during a functional grasping and manipulating action.
Clinical Outcomes
CI therapy produced marked improvement in upper extremity motor function (WMFT score) and an increased perceived amount of use of the affected hand (MAL score) in a subacute patient with stroke. These findings complement prior clinical evidence supporting significant clinical improvement after CIT. Many of those studies used the MAL as a clinical outcome measure,7,9,12 and others included WMFT.10,11,14
SUMMARY
This single case study presented clinical and neurophysiological data before and after an intense 2-week CIT intervention in 1 patient who suffered a subcortical stroke 4 months previously. The patient showed behavioral improvements when compared to similar data from an age-matched able-bodied individual. The patient in this study demonstrated marked functional improvement measured by WMFT and MAL as well as improved kinetic measures of key grip. These improvements were accompanied by increased laterality index measured by optical topography, indicating activation occurred in the hemisphere contralateral to the affected moving hand following treatment. Increased excitability of the contralateral (left) motor cortex was also represented by a postintervention increase in TMS motor map area and number of active spots. A single posttreatment fMRI revealed contralateral (left) motor cortex activation with smaller ipsilateral activation. These results suggest that cortical reorganization occurred as illustrated by increased activation of the contralateral hemisphere following 2 weeks of CI therapy. EMG was not recorded, and therefore an alternate interpretation could be put forward that the intervention led to improvement in the patient’s ability to perform focal unilateral movements over time. Further investigation is recommended to reproduce these findings in a larger group and to explore possible underlying mechanisms of cortical reorganization in the affected hemisphere.
Acknowledgments
Support for this study was provided in part by a grant from the Korean National Rehabilitation Center to S. Park. We thank our volunteers for their valuable participation. The assistance of A. Ahmadian, S. Dhruv, J. Ko, H. Mao, and S. Blanton for their help with data acquisition and reduction is gratefully acknowledged. We would like to say a special thanks to Hitachi for providing us with the use of the optical topography machine. We are also grateful to T. Winter, Hitachi Medical Systems America, and T. Kawaguchi, Hitachi Medical Corporation, for their helpful advice on optical topography methodology. This work was supported in part by NIH grants HD 37606 (SLW) and HD 40984 (AJB).
Footnotes
Note that this key-turning task was conducted separately from the Kinetic Key Grip Test described below.
Park S-W, Butler AJ, Cavalheiro V, Alberts JL, Wolf SL. Changes in serial optical topography and TMS during task performance after constraint-induced movement therapy in stroke: a case study. Neurorehabil Neural Repair 2004;18:95–105.
References
- 1.Duncan P. Synthesis of intervention trials to improve motor recovery following stroke. Top Stroke Rehabil. 1997;3:1–20. doi: 10.1080/10749357.1997.11754126. [DOI] [PubMed] [Google Scholar]
- 2.Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–36. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- 3.Taub E, Wolf S. Constraint induced movement techniques to facilitate upper extremity use in stroke patients. Top Stroke Rehabil. 1997;3:38–62. doi: 10.1080/10749357.1997.11754128. [DOI] [PubMed] [Google Scholar]
- 4.Winstein CJ, Wing AM, Whitall J. Motor control and learning principles for rehabilitation of upper limb movements after brain injury. In: Grafman J, Robertson I, editors. Plasticity and rehabilitation. 2. Vol. 9. Amsterdam: Elsevier Science BV; 2003. pp. 77–137. [Google Scholar]
- 5.Wolf SL. From tibialis anterior to Tai Chi: biofeedback and beyond. Appl Psychophysiol Biofeedback. 2001;26:155–74. doi: 10.1023/a:1011395324622. [DOI] [PubMed] [Google Scholar]
- 6.Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy: a new family of techniques with broad application to physical rehabilitation—a clinical review. J Rehabil Res Dev. 1999;36:237–51. [PubMed] [Google Scholar]
- 7.Liepert J, Miltner WH, Bauder H, et al. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neurosci Lett. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- 8.Liepert J, Graef S, Uhde I, et al. Training-induced changes of motor cortex representations in stroke patients. Acta Neurol Scand. 2000;101:321–6. doi: 10.1034/j.1600-0404.2000.90337a.x. [DOI] [PubMed] [Google Scholar]
- 9.Liepert J, Bauder H, Wolfgang HR, et al. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–16. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 10.Schaechter JD, Kraft E, Hilliard TS, et al. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–38. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 11.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 12.van der Lee JH, Wagenaar RC, Lankhorst GJ, et al. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–75. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 13.Wolf SL, Blanton S, Baer H, et al. Repetitive task practice: a critical review of constraint-induced movement therapy in stroke. The Neurologist. 2002;8:325–38. doi: 10.1097/01.nrl.0000031014.85777.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy CE, Nichols DS, Schmalbrock PM, et al. Functional MRI evidence of cortical reorganization in upper-limb stroke hemiplegia treated with constraint-induced movement therapy. Am J Phys Med Rehabil. 2001;80:4–12. doi: 10.1097/00002060-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe E, Yamashita Y, Maki A, et al. Non-invasive functional mapping with multi-channel near infra-red spectroscopic topography in humans. Neurosci Lett. 1996;205:41–4. doi: 10.1016/0304-3940(96)12376-4. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe E, Maki A, Kawaguchi F, et al. Non-invasive assessment of language dominance with near-infrared spectroscopic mapping. Neurosci Lett. 1998;256:49–52. doi: 10.1016/s0304-3940(98)00754-x. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi Y, Takeuchi T, Sakai KL. Lateralized activation in the inferior frontal cortex during syntactic processing: event-related optical topography study. Hum Brain Mapp. 2002;17:89–99. doi: 10.1002/hbm.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennan RP, Kim D, Maki A, et al. Non-invasive assessment of language lateralization by transcranial near infrared optical topography and functional MRI. Hum Brain Mapp. 2002;16:183–9. doi: 10.1002/hbm.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyai I, Tanabe HC, Sase I, et al. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage. 2001;14:1186–92. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- 20.Suto T, Ito M, Uehara T, et al. Temporal characteristics of cerebral blood volume change in motor and somatosensory cortices revealed by multichannel near-infrared spectroscopy. Int Congress Series. 2002;1232:383–8. [Google Scholar]
- 21.Kato H, Izumiyama M, Koizumi H, et al. Near-infrared spectroscopic topography as a tool to monitor motor reorganization after hemiparetic stroke: a comparison with functional MRI. Stroke. 2002;33:2032–6. doi: 10.1161/01.str.0000021903.52901.97. [DOI] [PubMed] [Google Scholar]
- 22.Kennan RP, Horovitz SG, Maki A, et al. Simultaneous recording of event-related auditory oddball response using transcranial near infrared optical topography and surface EEG. Neuroimage. 2002;16:587–92. doi: 10.1006/nimg.2002.1060. [DOI] [PubMed] [Google Scholar]
- 23.Blanton S, Wolf SL. An application of upper-extremity constraint-induced movement therapy in a patient with subacute stroke [see comments] Phys Ther. 1999;79:847–53. [PubMed] [Google Scholar]
- 24.Wolf SL, Catlin PA, Ellis M, et al. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–9. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 25.Butler AJ, Wolf SL. Transcranial magnetic stimulation to assess cortical plasticity: a critical perspective for stroke rehabilitation. J Rehabil Med. 2003:20–6. doi: 10.1080/16501960310010106. [DOI] [PubMed] [Google Scholar]
- 26.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 27.Duvernoy H. The human brain: surface, three-dimensional sectional anatomy and MRI. New York: Springer-Verlag; 1991. [Google Scholar]
- 28.Butefisch CM, Netz J, Wessling M, et al. Remote changes in cortical excitability after stroke. Brain. 2003;126:470–81. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- 29.Johansen-Berg H, Dawes H, Guy C, et al. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–42. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 30.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–96. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–66. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 32.Turton A, Wroe S, Trepte N, et al. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–28. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 33.Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(Pt 9):1579–86. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- 34.Werhahn KJ, Conforto AB, Kadom N, et al. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54:464–72. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- 35.Cramer SC, Bastings EP. Mapping clinically relevant plasticity after stroke. Neuropharmacology. 2000;39:842–51. doi: 10.1016/s0028-3908(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 36.Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–17. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 37.Boas DA, Gaudette T, Strangman G, et al. The accuracy of near infrared spectroscopy and imaging during focal changes in cerebral hemodynamics. Neuroimage. 2001;13:76–90. doi: 10.1006/nimg.2000.0674. [DOI] [PubMed] [Google Scholar]
- 38.Strangman G, Franceschini MA, Boas DA. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage. 2003;18:865–79. doi: 10.1016/s1053-8119(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T, Kato T. Paradoxical correlation between signal in functional magnetic resonance imaging and deoxygenated haemoglobin content in capillaries: a new theoretical explanation. Phys Med Biol. 2002;47:1121–41. doi: 10.1088/0031-9155/47/7/309. [DOI] [PubMed] [Google Scholar]
- 40.Marshall RS, Perera GM, Lazar RM, et al. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–61. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- 41.Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889:278–87. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- 42.Kleim JA, Barbay S, Cooper NR, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 43.Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 44.Nelles G, Jentzen W, Jueptner M, et al. Arm training induced brain plasticity in stroke studied with serial positron emission tomography. Neuroimage. 2001;13:1146–54. doi: 10.1006/nimg.2001.0757. [DOI] [PubMed] [Google Scholar]
- 45.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–88. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]