Introduction
Cerebral venous thrombosis (CVT) is a multi-causal disorder that affects the venous aspect of the neurovascular tree and is related to certain hereditary and acquired predispositions. The incidence, clinical presentation, imaging findings and clinical outcome of CVT are therefore variable and will relate to various risk factors that are predominant in different age groups1,2,3,4. CVT can also be a part of the dynamic progression of patients with intracra-nial dural arteriovenous fistulas as well as being associated with the syndrome of pseudotu-mor cerebri.
No firm treatment methods for CVT have been established. However, anticoagulation, although not completely scientifically proven, is regarded as a first line treatment4,5,6,7. On the contrary, the interventional neuroradiological procedures such as transvenous thrombolysis 8,9,10 or mechanical thrombectomy11,12 are usually reserved for patients who do not show clinical response to heparin therapy.
The purpose of this article is to discuss the present knowledge of the etiology, clinical presentation, diagnosis and management of this potentially devastating disease from the perspectives of the interventional neuroradiolo-gist.
Incidence and Etiology
The incidence of venous thrombosis between the ages of 1 month and 18 years was 0.7 per 100,000 per year in a Canadian stroke registry and 0.6 per 100,000 in a Dutch hospital discharge registry 1. In the first year of life venous thrombosis frequently occurred in association with indwelling venous catheters or as renal vein thrombosis. Following a peak in the first year the incidence becomes very low during the next ten years and then gradually increases with age. In children, multiple risk factors for venous thrombosis can often be identified and are apparently required to produce symptomatic venous thrombosis. There are numerous causes or conditions that can predispose to CVT (table 1). The risk factors that have become known to be associated with venous thrombosis are tissue damage and stasis (trauma, surgery and immobilization) as well as congenital or acquired abnormalities associated with the hemostatic system2,13.
Table 1.
Risk Factors for Cerebral Venous Thrombosis (CVT)
| Infection | Paranasal sinusitis Intracranial infection: abscess, meningitis |
| Trauma | Head trauma Neurosurgical interventions Internal jugular catheter |
| Medical/surgical conditions | Dehydration Pregnancy and puerperium Coagulation disorders: Factor V Leiden (Activated protein C resistance), Protein C deficiency, Protein S deficiency, Antithrombin III deficiency, Hyperhomocysteinemia, Antiphospholipid syndrome Hematologic disorders: Polycythemia, Sickle cell disease, Thrombotic thrombocytopenic purpura, Polycythemia, Paroxysmal nocturnal hemoglobinuria Malignancies, Inflammatory bowel disease, Nephrotic syndrome, Dehydration, Liver cirrhosis, Collagen vascular diseases including Systemic lupus erythematosus, Wegener's granulomatosis and Behcet syndrome Previous surgical procedures |
| Medication | Oral contraceptives, hormone replacement therapy, L-asparagenase, epsilon aminocaproic acid, Corticosteroid |
Deficiency of protein C, protein S, antithrombin and carriership of factor V Leiden or resistance to activated protein C are estimated to account for 25-35% of all occurrences of venous thrombosis1,14. Hypercoagulable states related to the use of oral contraceptives, preg-nancy/puerperium and malignancy represent another large group known to be associated with increased risk for venous thrombosis.
The use of oral contraceptives (including third generation) is associated with a four to seven fold increase in the risk for venous thrombosis15. The absolute risk remains low with the base-line risk of less then one per 10,000 being increased to three to four per 10,000 person-years during the time when oral contraceptives are used. The risk for CVT increases 20 fold if the females who have an inherited disorder of the hemostatic system such as a mutation in the prothrombin gene use oral contraceptives16 or 35 fold in women who were heterozygous for factor V Leiden13.
Pregnancy and puerperium also increase the risk of venous thrombosis by as much as four fold.
This risk is much greater shortly after delivery than during pregnancy, the incidence of thrombosis is 25 fold higher in the postpartum period than during pregnancy1.
The chance in a female below the age of 40 who presents with a cerebral ischemic event for it to be caused by venous thrombosis is two fold higher than for it to be arterial in origin. However, about 20% of the cases do not show any known risk factors3,7 emphasizing the importance of follow-up investigations.
Clinical Presentation and Natural History
The clinical presentations of patients presenting with CVT (table 2) will depend on the location and extent of the thrombosis (superior sagittal sinus, transverse sinus, straight sinus,cortical versus deep venous system). The clinical impact of the venous thrombosis varies according to the patient's venous anatomical disposition such as collateral pathways. Frequently, the symptoms and signs of CVT are nonspecific such as headaches and vomiting lasting hours or days.
Table 2.
Clinical presentation of Cerebral Venous Thrombosis (CVT)
| Symptoms | Headache Double vision Blurred vision Altered consciousness Nausea, vomiting Seizures |
| Signs | Papilledema Focal neurologic deficit Cranial nerve palsies Nystagmus |
|
* Clinical presentation of CVT is dependent on the location and extent of the thrombosis. | |
These most likely reflect global increased venous pressure related to early dural sinus thrombosis. Such presentation in association with new onset of seizures, papilledema, decreased mentation and focal neurological deficit should raise a strong suspicion of venous occlusive disease affecting the cortical venous system. Seizures may be focal or generalized but they occur in about half of the patients.
The progression of CVT is slow and stepwise. Clinically, it is expressed as accumulative symptoms and signs sometimes extending over days or even weeks. So, crescendo type progression of clinical presentations from non-specific symptoms to seizure or focal neurological deficit over a few days is almost characteristic of the diagnosis and should lead to rapid management. Simultaneous venous occlusive disease involving other body parts is not rare and pulmonary embolism has been demonstrated in up to 11% of patients with dural sinus thrombosis17.
Historically, CVT was believed to be a disease with a poor prognosis. According to the placebo arms of the two prospective data 5,18, about 41% of patients showed poor outcome defined as a death or Barthel index <15. However, recent advances in imaging and increased clinical awareness resulted in earlier medical therapy and showed the mortality rates to be less than 10% 3,19. Despite its relatively more benign clinical course than previously assumed, a small subgroup reveals rapid clinical progression leading to status epilepticus, progressive loss of consciousness and a comatose state, which without treatment is associated with 69% mortality18.
Imaging
Clinical suspicion of the CVT will greatly facilitate the radiological interpretation of subtle abnormalities that may be shown in the early stage of the disease.
Vice versa, the radiological findings interpreted with awareness of this clinical condition may result in an early diagnosis despite nonspecific clinical symptoms.
Computerized tomography (CT), because of its easy access, is probably used in most instances as the first imaging modality. It may demonstrate variable findings depending on the localization of the venous thrombosis and its impact on the surrounding brain. Clot may be demonstrated within the dural sinus as an elongated area of high attenuation in the transverse or straight sinus on plain CT or as a triangular filling defect (empty delta sign) in the superior sagital sinus or torcular on contrast enhanced CT. Thrombus in cortical veins can be visualized on non-enhanced CT as high attenuated tubular structures (so called string sign or the cord sign). Bilateral parasagittal low attenuations may be demonstrated in superior sagittal sinus thrombosis.
In case of deep cerebral venous thrombosis such as straight sinus thrombosis, the basal ganglia and thalamus show bilateral low attenuated lesions 20. Unilateral low attenuated lesion involving the temporal lobe can be shown in transverse sinus thrombosis. The low attenuations on plain CT may reflect vasogenic edema resulting from venous outflow disturbances 21. If the venous cerebral ischemia is prolonged, hemorrhagic conversion occurs and it becomes readily visible on plain CT. Recently developed thin-section helical CT venography is also a very useful tool to diagnose cerebral venous thrombosis22.
Magnetic resonance imaging (MRI) has proven to be the modality of choice and has surpassed cerebral angiography in its ability to demonstrate dural sinus thrombosis and venous ischemia 23.
Conventional MRI sequences can demonstrate clot within the dural sinuses with different signal characteristics depending on the age of the clot, various degrees of brain edema and/or intracerebral hemorrhage. Additionally, MRI can reveal cortical venous thrombosis without dural sinus occlusion (figure 1). In our experience, even in the presence of significant and extensive changes on CT and MRI, these radiologic changes may resolve after active medical and endovascular management and result in good clinical outcome. Recently, gadolinium enhanced MR angiography showed its potential to depict the components of intracranial arteriovenous shunts especially intracranial arteriovenous malformation 24 and MR venography can demonstrate the normal intracranial venous system 25.
Figure 1.
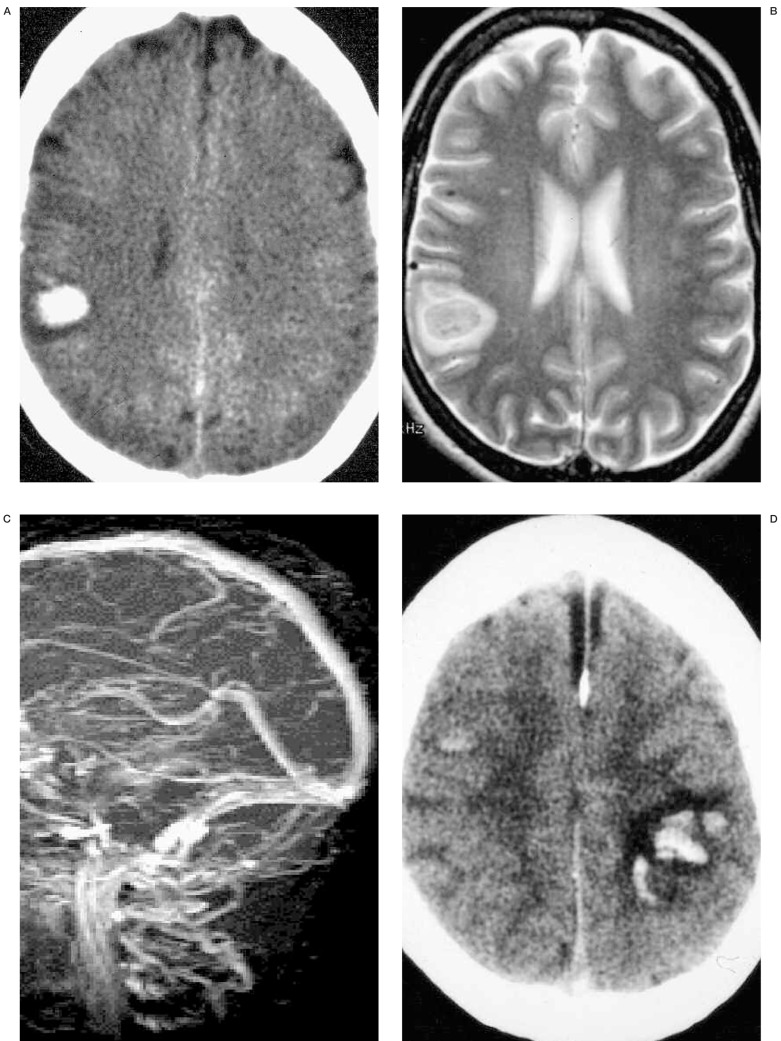
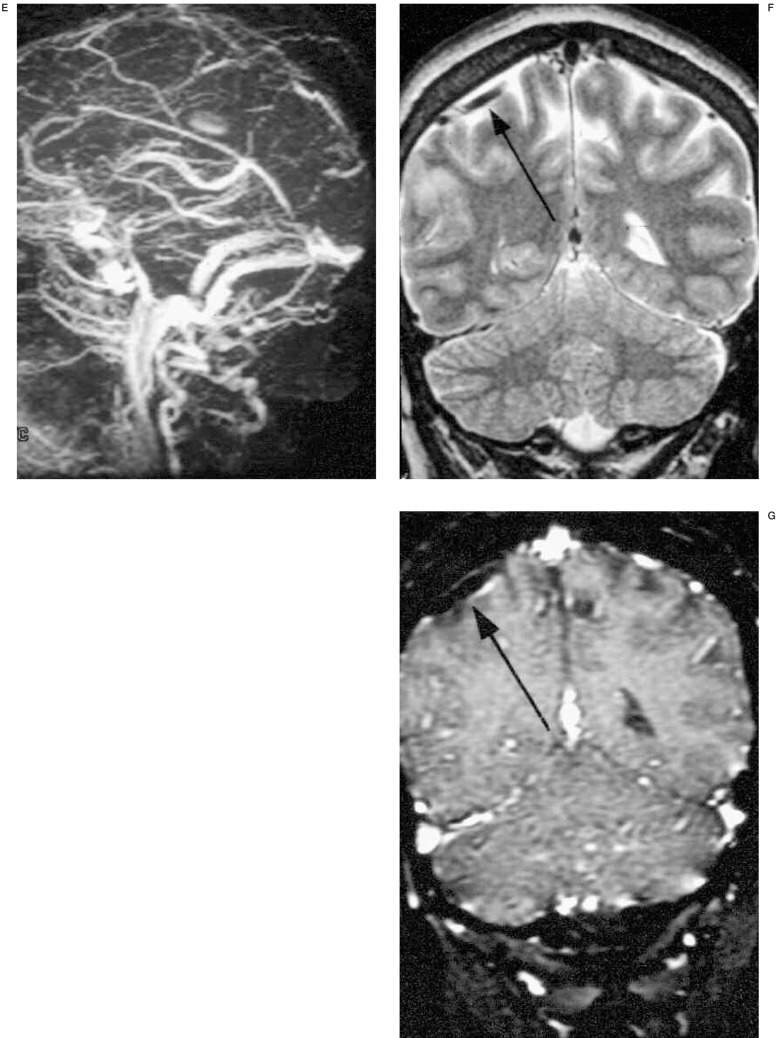
A 32-year-old woman developed generalized seizure at postpartum 2 weeks. Initial non-enhanced brain CT (A) reveals focal right parietal intracranial hemorrhage. T2 weighted axial MR image (B) shows right parietal subacute hematoma and MR venogram (C) does not demonstrate dural sinus occlusion. Because there was no radiologic evidence of CVT, the patient was not given any treatment for 1 week. Follow-up non-enhanced CT (D) shows bilateral intracerebral hemorrhage, dense superior sagittal sinus and subdural fluid collections. MRV (E) demonstrates total occlusion of the superior sagittal sinus. Partial occlusion of distal straight sinus and mid-portion of right transverse sinus are also revealed. Retrograde evaluation of initial MRI (T2 weighted coronal) (F) and MRV source image (G) shows right parietal cortical vein thrombosis without dural sinus involvement. On T2 weighted coronal image (F), thrombosed cortical vein (arrow) shows low signal intensity, so it is difficult to differentiate it from normal vascular signal voids. However, on MRV source image (G), it turns to high signal intensity (arrow) suggesting acute thrombosed cortical vein.
These techniques may obviate the need for conventional diagnostic angiography. However,certain limitations of MRI have to be kept inmind as they need correct interpretations. For example, the presence of hyperacute cerebral hemorrhage and the demonstration of thrombosis in cortical veins associated with a patent adjacent dural sinus are still somewhat difficult to discern on MRI. Careful analysis of the MRI (including the source images) is required to decode various signal characteristics of the clots within the venous channels, the developing venous congestion and infarction. Although the experiences in cerebral venous ischemia with diffusion and perfusion weighted imaging (DWI and PWI) are still limited, early results have shown the potential to predict whether brain tissue is still viable and active intervention is recommended 26.
Cerebral angiography is nowadays rarely used for the diagnostic purpose of CVT. However, angiography can provide very important additional information on intracranial circulations. It can demonstrate individual host response to the presence of venous thrombosis. For instance, it may reveal alteration of the venous phase of brain circulation including so called pseudo-phlebitic patterns 27 suggesting venous congestions and can show alternate (collateral) venous pathways being used if it was developed. These physiological responses of venous thrombosis may explain and provide some clues to the individual differences (clinical presentation and outcome) of CVT patients. Moreover, the information shown at angiography cannot be demonstrated on CT or MRI.
Treatment
Proper management of CVT patients is mainly dependent on our ability to make a clinical suspicion and radiological confirmation of this diagnosis, which continues to be our biggest challenge. Timely diagnosis is critical for effective treatment.
Once the diagnosis is made, medical therapy that includes systemic anticoagulation, hydration and anticonvulsant therapy is started. For 15 years, anticoagulation therapy has been used routinely for the treatment of intracranial venous thrombosis.
To investigate the scientific evidence of heparin's benefit for CVT treatment, two randomized trials and one meta-analysis have been conducted. The first trial, a randomized, blinded placebo-controlled study in 20 aseptic dural sinus thrombosis patients, concluded that anticoagulation with dose-adjusted intravenous heparin was an effective treatment and associated intracranial hemorrhage was not a contraindication for its use 18.
The trial was stopped after 20 patients because of the dramatic difference in outcome between the heparin and placebo group. A statistically significant beneficial effect of heparin treatment was seen after three days of therapy and confirmed at every subsequent examination up to three months after the start of treatment. However, this trial was challenged28 with respect to the use of the non-validated outcome assessment method, the delay in treatment starting point and failure to clarify the duration of heparin treatment and the utilization of warfarin.
A second randomized trial5 investigating the role of low molecular heparin in the management of cerebral venous thrombosis demonstrated the safety of anticoagulant therapy, even in the presence of hemorrhagic lesions on CT. Patients in this trial were treated at an earlier stage of the disease and their clinical conditions were less severe as compared to the first trial. However, a statistically significant improvement in clinical outcome was not demonstrated. A meta-analysis 5 of the above two trials showed a mortality reduction of 14.3% with heparin treatment and 15.5% risk reduction for the combined outcome including “death or dependence”.
Again, although this result showed an improvement in the clinical outcome of heparin treatment, no statistically significant improvement was demonstrated.
Therefore, we still do not have a proven statistically significant clinical improvement of he-parin therapy for CVT over placebo. Nevertheless, the unpredictable clinical outcome, known safety of heparin therapy and favorable trends of clinical results shown in previous trials, are assumed to reinforce the use of heparin as a first line treatment for CVT7.
However, the effect of heparin that can usually be seen within the first day of treatment may be too slow to help a special subgroup of CVT patients. Patients with rapidly progressing thrombosis that involves large parts of the cerebral venous system, with diffuse brain swelling and multiple hemorrhages are considered to have a poor prognosis. Therefore, it is this subgroup that should be considered for thrombolytic therapy as it represents the only possible treatment method, which may prevent catastrophic outcome. It has been our practice to wait for the impact of anticoagulation therapy for at least 24 hours. But if clinical worsening continues during that time period, then we consider thrombolytic therapy (figure 2).
Figure 2.
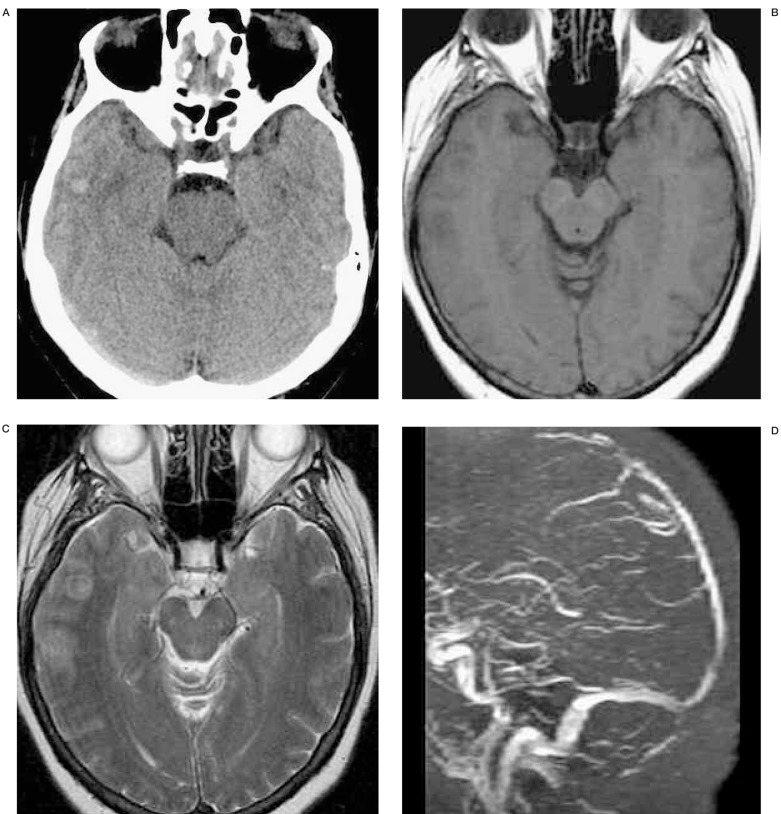
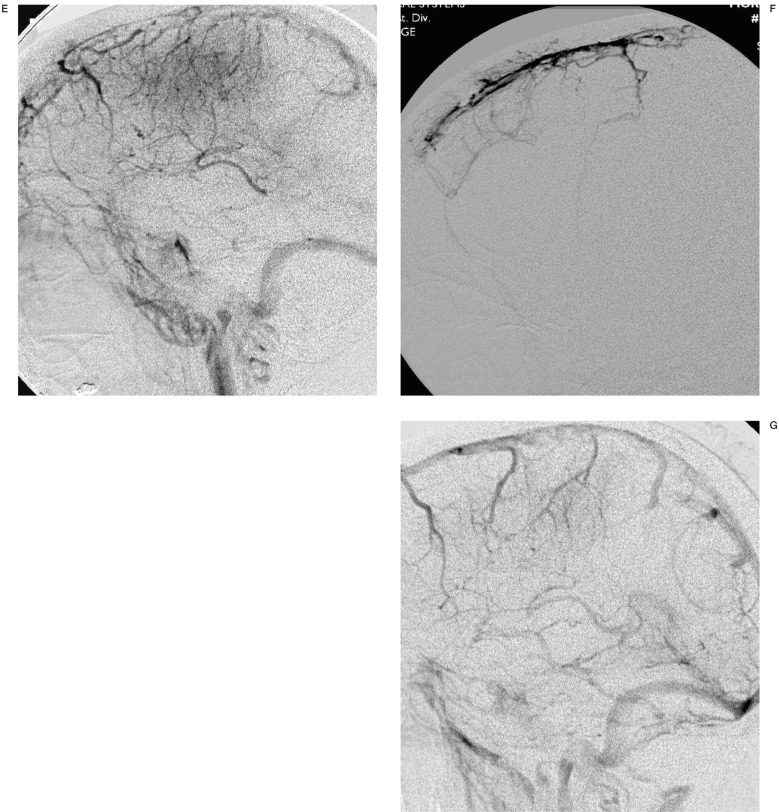
A 62-year-old woman with acute lymphocytic leukemia was treated with heparin due to dural sinus thrombosis. However, the patient's consciousness level deteriorated despite anticoagulation (INR=1.28). Initial non-enhanced CT (A) shows subtle increased attenuation (arrow) on right temporal lobe and right transverse sinus suggesting parenchymal hemorrhage and dural sinus thrombosis, respectively. T2 (B) weighted axial MR demonstrate sulcal effacement and acute parenchymal hemorrhage (arrow) on right temporal lobe. MR venography (C) and venous phase of right internal carotid angiogram (D) reveal nonvisualization of proximal to mid portion of superior sagittal sinus (SSS) and straight sinus. Note there is a lack of parenchymal staining on occipital and temporal lobes on right internal carotid angiogram. Superselective SSS angiography (E) show multifocal filling defects suggesting sinus thrombosis. After infusion of 30mg of t-PA, right internal carotid angiography (F) demonstrates complete re-canalization of SSS and partial recanalization of straight sinus. Three months follow-up MR venography (G) shows patent SSS and straight sinus.
Vines and Davis 29 were the first to propose thrombolytic therapy (urokinase) in the management of CVT, followed by Di Rocco et Al30 who used intravenous Urokinase in combination with heparin treatment. Treatment with local urokinase infusion into the dural sinus was first performed in 198831. Subsequently several reports demonstrated the feasibility and use of thrombolytic agents infusion into the thrombosed dural sinus via direct percutaneous or retrograde transvenous approach 9,11,32,33. With improved catheter technology, it also became possible to access and infuse thrombolytic agents into the deep venous system via the straight sinus 10,34,35. The potential benefit of local administration of thrombolytic therapy is that it can avoid the systemic hemorrhagic effects caused by high dose intravenous anticoagula-tion therapy.
The dose, method and type of thrombolytic therapy have changed over the past decade from prolonged infusions of low dose Urokinase therapy to more rapid pulse spray techniques using tissue type plasminogen activator (tPA). The duration and dose has ranged from 2 to 244 hours to infuse 0.5 to 20 million Units of Urokinase and from 2 to 43 hours to administer 50 to 300 mg of tPA8,32,33,35,36. Local injection of tPA in combination with heparin appears to carry a higher risk for worsening the intracerebral hemorrhage than heparin alone in patients with pretreatment hemorrhagic infarction. Additionally, there are still some technical points which need to be clarified such as the dose of thrombolytic agents, duration of treatment and concomitant use of anticoagulant4.
However, at least in the CVT patients without obvious pretreatment hemorrhage, endovascular treatment showed safety, rapid flow restoration and improvement of clinical outcome 6. While endovascular thrombolysis is being explored, the important fact to keep in mind is that we do not have a clearly defined end point of this therapy. Although most interventional neuroradiologists prefer total absence of clot in the dural sinuses at the end of the procedure, this may in fact not be necessary. In our experience, the reestablishment of antegrade flow is often sufficient to facilitate clinical improvement. After reestablishment of antegrade flow, continued anticoagulation will prevent propagation of clot and in time the lumen of the sinus will become fully reestablished.
Other techniques to re-open the venous channels more rapidly have been explored incombination with thrombolytic therapy. These include mechanical disruption using guidewires, rheolytic thrombectomy catheters, balloon thrombectomy with fibrinolysis, transluminal balloon angioplasty with or without stenting and surgical thrombectomy 36,37,38,39,40 The role of endovascular reopening of the dur-al sinuses using transvenous thrombolytic therapy as well as retrograde transvenous angio-plasty and stent placement is currently being explored for those patients demonstrating a relentless, progressive neurological deterioration and has shown promising results in selected cases41,42.
Conclusions
CVT has extremely variable clinical presentations, imaging findings and unpredictable clinical outcome. While there is still no scientifically proven treatment, heparin treatment appears to be safe and has shown a trend to result in clinical improvement. Endovascular treatments including transvenous thrombolysis combined with intravenous heparin therapy, mechanical thrombectomy and angioplasty with or without stenting are promising, but their exact indications will need to be further established and validated.
References
- 1.Rosendaal FR. Thrombosis in the young: epidemiology and risk factors. A focus on venous thrombosis. Thromb Haemost. 1997;78:1–6. [PubMed] [Google Scholar]
- 2.Manco-Johnson MJ, Nuss R, et al. Combined thrombolytic and anticoagulant therapy for venous thrombosis in children. J Pediatr. 2000;136:446–453. doi: 10.1016/s0022-3476(00)90006-4. [DOI] [PubMed] [Google Scholar]
- 3.Ameri A, Bousser MG. Cerebral venous thrombosis. Neurol Clin. 1992;10:87–111. [PubMed] [Google Scholar]
- 4.Benamer HT, Bone I. Cerebral venous thrombosis: anticoagulants or thrombolyic therapy? J Neurol Neurosurg Psychiatry. 2000;69:427–430. doi: 10.1136/jnnp.69.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30:484–488. doi: 10.1161/01.str.30.3.484. [DOI] [PubMed] [Google Scholar]
- 6.Frey JL, Muro GJ, et al. Cerebral venous thrombosis: combined intrathrombus rtPA and intravenous heparin. Stroke. 1999;30:489–494. doi: 10.1161/01.str.30.3.489. [DOI] [PubMed] [Google Scholar]
- 7.Bousser MG. Cerebral venous thrombosis: nothing, heparin, or local thrombolysis? Stroke. 1999;30:481–483. doi: 10.1161/01.str.30.3.481. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Suh JH. Direct endovascular thrombolytic therapy for dural sinus thrombosis: infusion of alteplase. Am J Neuroradiol. 1997;18:639–645. [PMC free article] [PubMed] [Google Scholar]
- 9.Horowitz M, Purdy P, et al. Treatment of dural sinus thrombosis using selective catheterization and urokinase. Ann Neurol. 1995;38:58–67. doi: 10.1002/ana.410380112. [DOI] [PubMed] [Google Scholar]
- 10.Holder CA, Bell DA, et al. Isolated straight sinus and deep cerebral venous thrombosis: successful treatment with local infusion of urokinase. Case report. J Neurosurg. 1997;86:704–707. doi: 10.3171/jns.1997.86.4.0704. [DOI] [PubMed] [Google Scholar]
- 11.Higashida RT, Helmer E, et al. Direct thrombolytic therapy for superior sagittal sinus thrombosis. Am J Neuroradiol. 1989;10:S4–S6. [PMC free article] [PubMed] [Google Scholar]
- 12.Novak Z, Coldwell DM, Brega KE. Selective infusion of urokinase and thrombectomy in the treatment of acute cerebral sinus thrombosis. Am J Neuroradiol. 2000;21:143–145. [PMC free article] [PubMed] [Google Scholar]
- 13.Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. 2001;344:1222–1231. doi: 10.1056/NEJM200104193441607. [DOI] [PubMed] [Google Scholar]
- 14.de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood. 1999;93:1271–1276. [PubMed] [Google Scholar]
- 15.Vandenbroucke JP, Rosing J, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527–1535. doi: 10.1056/NEJM200105173442007. [DOI] [PubMed] [Google Scholar]
- 16.Martinelli I, Sacchi E, et al. High risk of cerebral-vein thrombosis in carriers of a prothrombin-gene mutation and in users of oral contraceptives. N Engl J Med. 1998;338:1793–1797. doi: 10.1056/NEJM199806183382502. [DOI] [PubMed] [Google Scholar]
- 17.Diaz JM, Schiffman JS, et al. Superior sagittal sinus thrombosis and pulmonary embolism: a syndrome rediscovered. Acta Neurol Scand. 1992;86:390–396. doi: 10.1111/j.1600-0404.1992.tb05106.x. [DOI] [PubMed] [Google Scholar]
- 18.Einhaupl KM, Villringer A, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338:597–600. doi: 10.1016/0140-6736(91)90607-q. [DOI] [PubMed] [Google Scholar]
- 19.Brucker AB, Vollert-Rogenhofer H, et al. Heparin treatment in acute cerebral sinus venous thrombosis: a retrospective clinical and MR analysis of 42 cases. Cerebrovasc Dis. 1998;8:331–337. doi: 10.1159/000015876. [DOI] [PubMed] [Google Scholar]
- 20.Brown JI, Coyne TJ, et al. Deep cerebral venous system thrombosis: case report. Neurosurgery. 1993;33:911–913. doi: 10.1227/00006123-199311000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Keller E, Flacke S, et al. Diffusion and perfusion-weighted magnetic resonance imaging in deep cerebral venous thrombosis. Stroke. 1999;30:1144–1146. doi: 10.1161/01.str.30.5.1144. [DOI] [PubMed] [Google Scholar]
- 22.Casey SO, Alberico RA, et al. Cerebral CT venography. Radiology. 1996;198:163–170. doi: 10.1148/radiology.198.1.8539371. [DOI] [PubMed] [Google Scholar]
- 23.Tsai FY, Wang AM, et al. MR staging of acute dural sinus thrombosis: correlation with venous pressure measurements and implications for treatment and prognosis. Am J Neuroradiol. 1995;16:1021–1029. [PMC free article] [PubMed] [Google Scholar]
- 24.Farb RI, McGregor C, et al. Intracranial arteriovenous malformations: real-time auto-triggered elliptic centric-ordered 3D gadolinium-enhanced MR angiography-initial assessment. Radiology. 2001;220:244–251. doi: 10.1148/radiology.220.1.r01jn15244. [DOI] [PubMed] [Google Scholar]
- 25.Cure JK, Van Tassel P, Smith MT. Normal and variant anatomy of the dural venous sinuses. Semin.Ultrasound CT MR. 1994;15:499–519. doi: 10.1016/s0887-2171(05)80019-8. [DOI] [PubMed] [Google Scholar]
- 26.Manzione J, Newman GC, et al. Diffu. Am J Neuroradiol. 2000;21:68–73. [PMC free article] [PubMed] [Google Scholar]
- 27.Willinsky R, Goyal M, et al. Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: correlations with presentation, location, and MR findings in 122 patients. Am J Neuroradiol. 1999;20:1031–1036. [PMC free article] [PubMed] [Google Scholar]
- 28.Stam J, Lensing AW, et al. Heparin treatment for cerebral venous and sinus thrombosis. Lancet. 1991;338:1154. doi: 10.1016/0140-6736(91)92020-3. [DOI] [PubMed] [Google Scholar]
- 29.Vines FS, Davis DO. Clinical-radiological correlation in cerebral venous occlusive disease. Radiology. 1971;98:9–22. doi: 10.1148/98.1.9. [DOI] [PubMed] [Google Scholar]
- 30.Di Rocco C, Iannelli A, et al. Heparin-urokinase treatment in aseptic dural sinus thrombosis. Arch Neurol. 1981;38:431–435. doi: 10.1001/archneur.1981.00510070065011. [DOI] [PubMed] [Google Scholar]
- 31.Scott JA, Pascuzzi RM, et al. Treatment of dural sinus thrombosis with local urokinase infusion. Case report. J Neurosurg. 1988;68:284–287. doi: 10.3171/jns.1988.68.2.0284. [DOI] [PubMed] [Google Scholar]
- 32.Barnwell SL, Higashida RT, et al. Direct endovascular thrombolytic therapy for dural sinus thrombosis. Neurosurgery. 1991;28:135–142. doi: 10.1097/00006123-199101000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Tsai FY, Higashida RT, et al. Acute thrombosis of the intracranial dural sinus: direct thrombolytic treatment. Am J Neuroradiol. 1992;13:1137–1141. [PMC free article] [PubMed] [Google Scholar]
- 34.Spearman MP, Jungreis CA, et al. Endovascular thrombolysis in deep cerebral venous thrombosis. Am J Neuroradiol. 1997;18:502–506. [PMC free article] [PubMed] [Google Scholar]
- 35.Smith TP, Higashida RT, et al. Treatment of dural sinus thrombosis by urokinase infusion. Am J Neuroradiol. 1994;15:801–807. [PMC free article] [PubMed] [Google Scholar]
- 36.Chaloupka JC, Mangla S, Huddle DC. Use of mechanical thrombolysis via microballoon percutaneous transluminal angioplasty for the treatment of acute dural sinus thrombosis: case presentation and technical report. Neurosurgery. 1999;45:650–656. doi: 10.1097/00006123-199909000-00045. [DOI] [PubMed] [Google Scholar]
- 37.Ekseth K, Bostrom S, Vegfors M. Reversibility of severe sagittal sinus thrombosis with open surgical thrombectomy combined with local infusion of tissue plasminogen activator: technical case report. Neurosurgery. 1998;43:960–965. doi: 10.1097/00006123-199810000-00144. [DOI] [PubMed] [Google Scholar]
- 38.Malek AM, Higashida RT, et al. Endovascular recanalization with balloon angioplasty and stenting of an occluded occipital sinus for treatment of intracranial venous hypertension: technical case report. Neurosurgery. 1999;44:896–901. doi: 10.1097/00006123-199904000-00133. [DOI] [PubMed] [Google Scholar]
- 39.Scarrow AM, Williams RL, et al. Removal of a thrombus from the sigmoid and transverse sinuses with a rheolytic thrombectomy catheter. Am J Neuroradiol. 1999;20:1467–1469. [PMC free article] [PubMed] [Google Scholar]
- 40.Söderman M, Johnsson H, et al. Acute-sinus thrombosis in a child with antibodies against cardiolipins. Interventional Neuroradiology. 1996:143–148. doi: 10.1177/159101999600200207. [DOI] [PubMed] [Google Scholar]
- 41.Kollar C, Parker G, Johnston I. Endovascular treatment of cranial venous sinus obstruction resulting in pseudotumor syndrome. Report of three cases. J Neurosurg. 2001;94:646–651. doi: 10.3171/jns.2001.94.4.0646. [DOI] [PubMed] [Google Scholar]
- 42.Vilela P, Willinsky R, Terbrugge K. Treatment of intracranial venous occlusive disease with sigmoid sinus angioplasty and stent placement in a case of infantile multifocal dural arteriovenous shunts. Interventional Neuroradiology. 2001;7:51–60. doi: 10.1177/159101990100700108. [DOI] [PMC free article] [PubMed] [Google Scholar]