Abstract
Maintenance of plasma IgM levels is critical for immune system function and homeostasis in humans and mice. However, the mechanisms that control homeostasis of the activated IgM-secreting B cells are unknown. After adoptive transfer into immune-deficient hosts, B lymphocytes expand poorly, but fully reconstitute the pool of natural IgM-secreting cells and circulating IgM levels. By using sequential cell transfers and B cell populations from several mutant mice, we were able to identify novel mechanisms regulating the size of the IgM-secreting B cell pool. Contrary to previous mechanisms described regulating homeostasis, which involve competition for the same niche by cells having overlapping survival requirements, homeostasis of the innate IgM-secreting B cell pool is also achieved when B cell populations are able to monitor the number of activated B cells by detecting their secreted products. Notably, B cell populations are able to assess the density of activated B cells by sensing their secreted IgG. This process involves the FcγRIIB, a low-affinity IgG receptor that is expressed on B cells and acts as a negative regulator of B cell activation, and its intracellular effector the inositol phosphatase SHIP. As a result of the engagement of this inhibitory pathway, the number of activated IgM-secreting B cells is kept under control. We hypothesize that malfunction of this quorum-sensing mechanism may lead to uncontrolled B cell activation and autoimmunity.
Maintenance of plasma IgM levels is critical for innate and adaptive immune system function and homeostasis in humans and mice. Decreased IgM levels result in diminished innate protection against bacterial invasion (1, 2). In humans, splenectomy, a therapeutic measure following trauma, cancer, or autoimmune diseases, results in increased susceptibility to bacterial infections (3); asplenic (Hox-11−/−) or splenectomized mice also show diminished immune responses to bacterial infection (2). Such reduction in the innate protection against bacterial invasion is related to decreases in both natural plasma IgM levels and the number of IgM-secreting cells (2). In contrast, increased IgM titers can be associated with autoimmunity (4, 5). For example, humans develop autoimmune disorders when they have primary immune deficiencies that are characterized by elevated natural IgM levels when B lymphocytes are unable to switch to IgG-producing cells. These deficiencies could be due either to defective T–B cell cooperation, as is the case of CD40/CD40L deficiencies, or to an intrinsic inability of the B cells to perform class switch recombination (4, 5). Similarly, increased IgM levels in mice are associated with several autoimmune disorders (6). For example, mice with activation-induced cytidine deaminase defects that prevent class switch recombination develop hyper-IgM–like syndromes associated with autoimmune diseases (7). Thus, any failure to maintain the homeostasis of IgM-secreting B cells can be deleterious, because it either increases susceptibility to infection or may favor the development of autoimmune disorders; however, the underlying mechanisms are unknown. In most cases, homeostatic regulation is achieved by competition of different cells for the same survival niches (8, 9). It is unclear whether such a mechanism would also apply to IgM homeostasis, as not only must secreting B cell numbers be maintained, but also, the total amount of IgM they produce must be regulated. It is also not clear which mechanisms could limit lymphocyte numbers during immune responses, in situations where resources are not limiting and physiological niches may be disrupted.
In this work, we report an experimental strategy allowing the study of the mechanisms involved in this regulation. In contrast to T lymphocytes, which undergo considerable homeostatic expansion when transferred into immune-deficient mice (10), naive B lymphocytes expand poorly, but reach a stable equilibrium and fully reconstitute the pool of IgM-secreting B cells and circulating IgM levels (11). The size and composition of this pool are tightly controlled, as it remains stable for up to 6 mo after transfer, independently of the number of injected naive B cells (11). Thus, in these host mice, the number of Ig-secreting cells is kept under strict homeostatic regulation as observed in intact mice (8). This model is therefore ideal to study the mechanisms of homeostasis of the number of IgM-secreting B cells, because only an adoptive transfer strategy allows the follow-up of different B cell populations (recognized by different allotypes) in the same mouse. Indeed, by using sequential transfers of B cell populations from several mutant mice, we identified feedback mechanisms regulating the size of the IgM-secreting B cell pool in a B cell–specific manner, that is, excluding side effects induced by the mutations in other nonlymphoid cells. We found that contrary to the previous mechanisms described regulating homeostasis, which involve competition for the same niche by cells sharing overlapping survival signals (8, 9), homeostasis of the innate IgM-secreting B cell pool is also achieved when B cell populations are able to monitor the number of activated B cells by detecting their secreted products. Notably, B cell populations are able to assess their density and limit the number of activated IgM-secreting B cells when they sense the levels of secreted IgG via FcγRIIB, a low-affinity IgG receptor that is expressed on B cells and acts as a negative regulator of B cell activation (12) by a SHIP1-mediated pathway (12, 13). These results reveal a new mechanism of homeostatic regulation, which recalls mechanisms found in procariots that have been collectively named as quorum sensing (14, 15). Importantly, it may explain the development of autoimmune conditions when IgG production is impaired, and the apparent paradox of the beneficial effects of i.v. Ig therapy in several autoimmune disorders (16).
Materials and Methods
Mice and cell transfers
C57BL/6(B6).Ly5bIgHb, B6.Ly5aIgHb, B6.Ly5bIgHa, B6.Ly5aIgHa, B6. Ly5bIgHaIgMs−/− (17), B6.Ly5bIgHbCD3ε−/−, B6.Ly5aIgHaCD3ε−/−, B6.Ly5bIgHbFcγRIIB−/− (12), B6.Ly5aIgHbSHIP1−/− (18), and B6. Rag2−/− mice were purchased from Charles River, Centre Des Techniques Avancées, Centre Nationale de la Recherche Scientifique (Orleans, France), or Taconic (Hudson, NY), or were from our animal facilities at the Institut Pasteur. Lymph node (LN) cells containing ~5 × 106 B cells were injected i.v. into Rag−/− hosts. In experiments involving sequential cell transfers, the injected populations differed by allotype markers (Ly5a or b and IgHa or b allotype). Recipient mice were bled and killed at different times after cell transfer. The number of B cells derived from each cell population was calculated. Experiments were performed according to the Pasteur Institute Safety Committee in accordance with French and European guidelines and the Ethics Committee of Paris 1 (permits 2010-0002,-0003, and -0004).
Flow cytometry and cell sorting
Cells were stained using anti-CD19 (1D3), anti-IgMa (DS1), anti-IgMb (AF6-78), anti-Ly5a (A20), anti-Ly5b (104), anti-CD21 (7G6), anti-CD23 (B3B4), anti-138, anti-Igκ, anti-IgM (R6-60.2), and anti-IgD (11-26c.2a) mAbs from BD Pharmingen coupled with the appropriate fluorochromes. Four/six-color staining used the appropriate combinations of FITC, PE, TRI-color, PerCP, PECy7, biotin, allophycocyanin, AlexaFluor647, and allophycocyanin Cy7-coupled Abs. Biotin-coupled Abs were secondarily labeled with allophycocyanin-, TRI-color (Caltag, San Francisco, CA)–, PerCP (BD Biosciences, San Jose, CA)- or allophycocyanin Cy7 (BD Pharmingen)-coupled streptavidin. Cytoplasmic Ig was detected after fixation using Cytofix/Cytoperm and PermWash buffers (BD Biosciences). Dead cells were excluded based on light scattering. All acquisitions and data analysis were performed with a FACSCanto or LSRFortessa (BD Biosciences) interfaced with the Macintosh FlowJo software. Subsets of follicular (CD21highCD23high), marginal (CD21highCD23low), or activated (CD21lowCD23low) B cells were sorted on a FACSAria (BD Biosciences) cytometer. The purity of the sorted populations varied from 90 to 95%.
ELISA and ELISA spot assay
Sera Ig concentrations were quantified by ELISA. Plates were coated with Abs to total IgM, IgMa, or IgMb and saturated with PBS–1% gelatin. Dilutions of sera were added. After incubation (1 h, 37°C) and washing, peroxidase-labeled goat anti-mouse IgM Abs were added. After incubation and washing, bound Abs were revealed with the substrate O-phenylenediamine and H2O2. The reaction was stopped after 10 min by addition of 10% SDS, and the absorbance was read at 450 nm in a Titertek multiscan spectrometer (Flow Laboratories, Irvine, Scotland). Titration of serum IgM and IgG was performed using purified mouse IgM or IgG as standards (Southern Biotechnology). Ig concentrations were determined by comparing the displacement of the dilution curves in the linear interval between standards at a concentration of 10 mg/ml and the serum samples. Specificity of the reactions for the detection of both IgMa and IgMb was confirmed using serum from B6.IgHa and B6.IgHb donor mice. For autoantibodies, plates were coated with mice mouse tubulin or DNA. To test for oxidized low density lipoprotein (OxLDL), we used the OxLDL-Ab ELISA kit purchased from IMTEC. The quantification of IgM-secreting cells was assayed by ELISA spot assay technique. Briefly, plates were coated with goat anti-mouse IgM Abs or anti-allotypic IgM Abs. After saturating, the cells were distributed into the micro wells in RPMI 1640– 2% FCS. The plates were incubated for 4–6 h at 37°C, 5% CO2 atmosphere. After extensive wash, plates were incubated with goat anti-mouse IgM labeled with alkaline phosphatase. After washing, the revealing substrate was added (2,3 mM 5-bromo-4-chloro-3-indolyl phosphate diluted in 2-amino-2-methyl-1-proprenolol buffer).
Results
Replenishment of the IgM-secreting B cell pool is strictly controlled
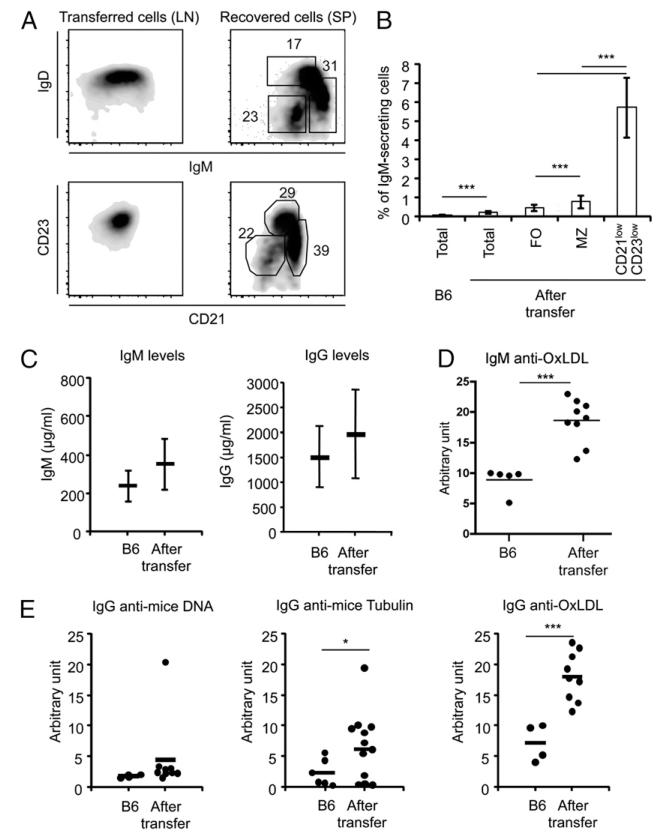
Upon adoptive transfer of LN cells from immune-competent into immune-deficient mice, T cells expanded to reconstitute a considerable fraction of the peripheral T cell pool (10). In contrast, the majority of the IgMlowIgDhighCD21int/highCD23high follicular LN B cells (Fig. 1A) did not proliferate, and therefore, the number of recovered B cells was limited and did not exceed 2 × 106 cells (11), that is, less than the number of injected cells. The size and composition of the pool of persisting cells were regulated independently of the number of injected B cells and remained stable for up to 6 mo after transfer (11). The majority of the persisting B cells were IgMhighIgDlowCD21highCD23low marginal zone (MZ) or IgMlowIgDlowCD21lowCD23low cells (Fig. 1A) expressing increased levels of CD40, CD80, CD86, and I-Ab, and downregulating CD62L expression (data not shown) (11). In the spleen, the frequency of Ig-secreting cells was higher among the CD21lowCD23low B cells (Fig. 1B, Supplemental Fig. 1A). Because quantification of the number of Ig-secreting B cells in the spleen is not a reliable method to evaluate the total size of the IgM-secreting B cell pool, as activated B cells could have migrated to organs (as the liver, gut, or the flat bones bone marrow) where numbers cannot be accurately measured, we chose the serum IgM levels as the most reliable indicator of the state of B cell activation and of the total number of IgM-secreting B cells in the whole mouse (Supplemental Fig. 1). In these host mice, we found that the serum IgM and IgG concentrations were similar to those of control mice, despite reduced B cell numbers (Fig. 1C). Interestingly, the titers of self-reactive Abs were increased compared with donor mice (Fig. 1D, 1E), suggesting that the persisting B cell populations were naturally selected on the basis of their reactivity against self and environmental Ags. These findings indicate that the transfer of mature B cells restored a stable and tightly controlled pool of IgM-secreting B cells in immune-deficient mice, but they do not provide insight into the homeostatic mechanisms that limit their number.
FIGURE 1.
Fate of LN B cells transferred into Rag-deficient hosts. (A) LN cells containing ~5 × 106 B cells were transferred i.v. into immune-deficient hosts. Left dot plots, Show the IgM, IgD (top) and CD21, CD23 (bottom) phenotype of donor LN B cells before transfer (gated on CD19+ cells). Right dot plots, Show the IgM, IgD (top) and CD21, CD23 (bottom) phenotype of the donor B cells recovered from the spleen of the host mice 8 wk after transfer. The relative representation of the different CD21, CD23 subpopulations is displayed. (B) Different populations of B cell follicular (FO; CD21int/high CD23high), MZ (CD21highCD23low), and CD21lowCD23low B cells recovered from the spleen of host mice 8 wk after B cell transfer were purified by cell sorting, and the number of IgM-secreting cells in each B cell subset was detected by ELISPOT. Bars show the mean percentage of IgM-secreting cells among donor B6 spleen B cells and total host spleen B cells as well as between the sorted follicular (FO; CD21int/highCD23high), MZ (CD21highCD23low), and CD21lowCD23low B cells recovered from the spleen of host mice 8 wk after B cell transfer. The frequency of IgM-secreting cells detected by ELISPOT was higher among the CD21lowCD23low B cells. Each column represents the mean of values for three individual mice. Similar findings were obtained in three independent experiments. (C) Shows the IgM (left) and IgG (right) concentrations in the serum of donor B6 mice and of Rag−/− hosts 8 wk after B cell transfer. Each bar represents the mean ± SD of values for eight individual mice. Note that, despite the low number of B cells recovered, Ig concentrations in these hosts were identical or slightly higher than those present in the donor mice. Similar results were obtained in more than seven independent experiments. (D) Titers (expressed in arbitrary units) of self-reactive IgM anti-mouse OxLDL in the serum of individual donor B6 mice or in the serum of Rag-deficient hosts injected with LN cells from WT B6 mice. (E) Titers (expressed in arbitrary units) of self-reactive IgG anti-mouse DNA (left), anti-mouse tubulin (middle), and anti-mouse OxLDL in the serum of individual donor B6 mice or in the serum of Rag-deficient hosts injected with LN cells from WT B6 mice. Bars indicate the mean values. Note the increased titers of self-reactive Abs in the serum of the host mice. Statistically significant differences are indicated (*p < 0.05, ***p < 0.001).
Feedback mechanisms control newly arriving B cell activation and IgM production
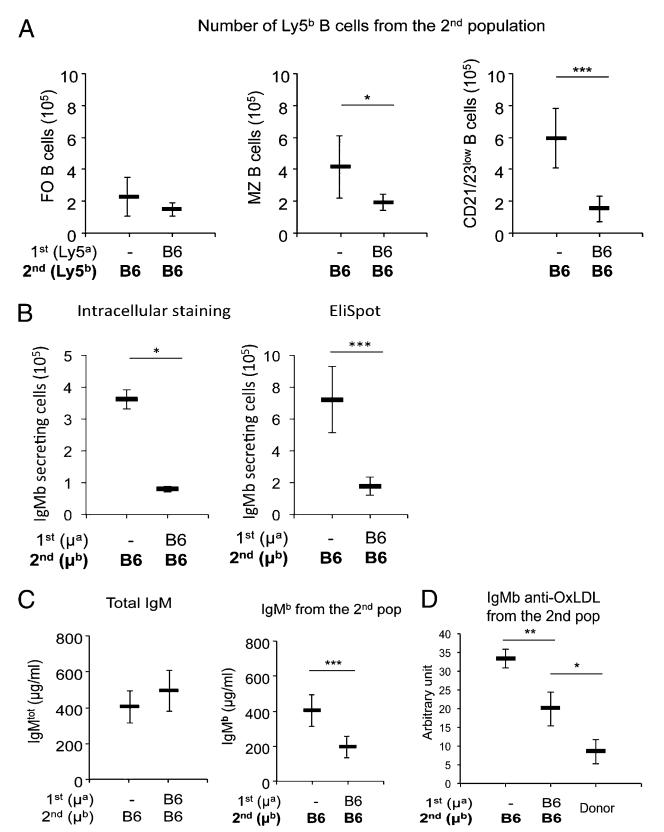
To identify the mechanisms that control the number of the IgM-secreting cells, we investigated whether an established population of B cells could modify the fate of a newly transferred B cell population. To do this, we used a sequential cell transfer strategy, as follows. Rag-deficient murine hosts were first injected with LN cells from Ly5aIgHa donors; second, 4 wk later, the hosts were injected with LN cells from double-congenic Ly5bIgHb mice (Supplemental Fig. 1B). We evaluated the number, phenotype, and function of the B cells recovered 6 wk after the transfer of the second population into the hosts. We found that, among the second population, the number of naive B cells recovered was not significantly altered by the presence of a first B cell population. However, the fraction and number of CD21highCD23low and CD21lowCD23low B cells (Fig. 2A) and the number of IgM-secreting cells (Fig. 2B) were significantly reduced in hosts injected first with B cells from Ly5aIgHa donors compared with controls. Consequently, the amount of IgMb that the second B cell population generated in the Rag-deficient hosts was significantly lower than the amount generated by the same cell population when transferred alone into naive hosts (Fig. 2C). At the same time, total IgM levels were slightly greater in mice receiving both B cell populations (Fig. 2C). More importantly, we observed a selective decrease in the titers of self-reactive anti-OxLDL IgMs produced by the second B cell population when these cells were transferred into mice containing a first set of B cells (Fig. 2D). In summary, we found that in mice injected sequentially with two LN cell populations, B cells from the first population replenished the compartment of activated IgM-secreting cells leading to normal serum levels and used a feedback mechanism to regulate activation of the second population of transferred B cells and, ultimately, their IgM production. Thus, by using this sequential cell transfer strategy, we unraveled feedback mechanisms that maintained the number of the activated IgM-secreting B cells stable. This feedback regulation could result from competition for cellular niches (8, 9), but could also occur in response to soluble factors secreted by the first B cell population.
FIGURE 2.
Sequential B cell transfers. (A) Shows the number of FO CD21int/highCD23high (left panel), MZ CD21highCD23low (middle panel), and CD21lowCD23low cells (right panel) recovered from the second Ly5bIgHb population when injected alone (left) or into mice injected 4 wk before with a first Ly5a IgHa cell population. Each bar represents the mean ± SD of values for nine individual mice. Note that whereas the number of resting B cells was not altered, the number of CD21highCD23low and CD21lowCD23low cells present among the second set of B cells was significantly lower when these cells were transferred into host mice already injected with a first set of B cells. (B) Shows the number of IgMb-secreting cells by the second B cell population when transferred into naive hosts (left) or into a host containing a first B cell population accessed by intracytoplasmic staining (left panel) or by Ig secretion (ELISPOT) (right panel). Each bar represents the mean ± SD of values for four to five individual mice. (C) Shows the quantity of IgMb produced by the second B cell population when transferred into naive hosts (left) or into a host containing a first B cell population. Each bar represents the mean ± SD of values for nine individual mice. Note the significantly reduced IgM production by the second B cell population when these cells were transferred into mice containing a first set of B cells transferred 4 wk before (right). Note that the total IgM concentrations were slightly greater in mice injected with two B cell populations than in the mice injected with the first population only (left). Similar results were obtained in five independent experiments or when the order of injection of the two B cell populations was inverted. Statistically significant differences are shown. (D) Shows the titers (expressed in arbitrary units/unit of IgMb) of self-reactive IgMb anti-mouse OxLDL produced by the second B cell population when transferred into naive hosts (left) or into a host containing a first B cell population (middle). For comparison, the titers of self-reactive IgMb anti-mouse OxLDL in the serum of donor B6 mice are shown (right). The sera of the different mice were normalized so that we can compare the titers of self-reactive IgMs present for the same amount of total IgMb concentration of the different mice. Note the selective reduction of anti-mouse OxLDL self-reactive Abs secreted by the second B cell population when these cells were transferred into mice containing a first set of B cells. Statistically significant differences are shown. *p < 0.05, **p < 0.01, ***p < 0.001.
Plasma IgG regulates the number of activated B cell and IgM production
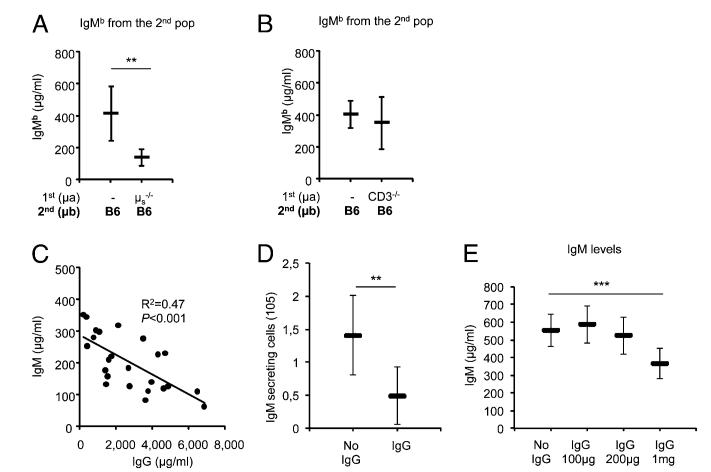
To investigate whether secreted specific B cell products, such as Ig, were involved in the feedback regulation mechanism, we investigated the role of IgM produced by the first B cell population. Rag-deficient mice were injected with a population of Ly5bIgHa LN cells from IgMs−/− mice; the B cells of these donor mice express, but cannot secrete IgM (17). Four weeks later, mice were injected with LN cells from wild-type (WT) Ly5aIgHb mice. As a control, the second WT cell population was also transferred into hosts that were not injected with the first cell population. The two groups of mice were sacrificed 6 wk after transfer of the WT cell population. As shown in Fig. 3A, the amount of IgMb produced by the WT B cell population was markedly lower in mice already hosting cells from IgMs−/− donors than in host mice that received only the WT population (Fig. 3A). We concluded that IgM produced by the first population of B cells was not responsible for the feedback effect, because secretion of IgM was disabled in those cells.
FIGURE 3.
Role of Ig in feedback regulation of IgM production. (A) The IgMb produced by a second Ly5aIgHb B cell population transferred alone into naive mice or into mice preinjected 4 wk before with a first population of cells from Ly5bIgHa μs−/− donor mice. Each bar represents the mean ± SD of values for six mice. Note that, despite the inability of the first B cell population to secrete IgM, the IgM production by the second population was significantly reduced in these hosts as compared with its production in naive hosts. Similar results were obtained in a second independent experiment. (B) The IgMb produced by a second Ly5aIgHb B cell population from normal donors transferred alone into naive mice or into mice preinjected 4 wk before with a first population from Ly5bIgHa CD3ε−/− donors. Each bar represents the mean ± SD of values for six to nine mice. Note that, in this case, the levels of IgM produced by the second B cell population were identical in the two groups of host mice (p = NS). Similar results were obtained in a second independent experiment. (C) Correlation of the levels of IgM produced by the second population with IgG levels present in the sera of 24 host mice pooled from different experiments. The correlation coefficient is shown (r2 = 0.47, p < 0.001). (D) Shows the number of IgMb-secreting cells (ELISPOT) by the B cells of a LN population transferred alone into either untreated or Rag-deficient mice injected i.v. with 1 mg purified soluble mouse IgG (Innovative Research). Each bar represents the mean ± SD of values for four to five individual mice. (E) The IgM produced by the B cells of a LN population transferred alone into either untreated naive mice or mice injected i.v. with 100 μg, 200 μg, or 1 mg purified soluble mouse IgG (Innovative Research) the day before cell transfer and thereafter i.p. every week for 5 wk. Each bar represents the mean ± SD of values for 5–10 mice. Note that in mice injected with the higher dose of IgG, the number of IgM-secreting cells and the IgM production by the transferred B cells were significantly reduced. Similar results were obtained in a second independent experiment. Statistically significant differences are shown. **p < 0.01, ***p < 0.001.
Because B cells from IgMs−/− donors can secrete IgG (17) (Supplemental Fig. 2), we next investigated the role of IgG in feedback inhibition. B cell class–switch recombination and IgG production require T cell help, and consequently, T cell–deficient mice show severely reduced levels of serum IgG (19). Therefore, to prevent strong IgG production by the first population of transferred B cells, Rag-deficient mice were injected with LN cells from Ly5bIgHa CD3ε−/− T cell–deficient mice (Supplemental Fig. 2) and, 4 wk later, with LN cells from Ly5aIgHb WT donors. Of note, B cells from CD3ε−/− mice behave similarly as B cells from WT donors in that the number of recovered cells was identical. We monitored the number of activated B cells and the IgMb produced by the second WT B cell population 6 wk after the second cell transfer. In the quasi absence of IgG secreted by the first population of B cells (Supplemental Fig. 2), there was no inhibition of IgM production by the second B cell population (Fig. 3B).
We had observed that the level of feedback inhibition of the IgM production by the second B cell population was variable in different host mice injected with two WT populations. Considering that the feedback inhibition seems to be determined by the presence of IgG, we compared the IgM levels produced by the second B cell populations with the IgG levels present in individual mice from several different experiments. Notably, we found that the amount of IgM produced by the second B cell population inversely correlated with IgG levels in the host serum (Fig. 3C).
To confirm the role of IgG in the feedback regulation of IgM production, we transferred LN cells into Rag2-deficient hosts that were either untreated (control) or injected with purified mouse IgG. The number of CD21highCD23low and CD21lowCD23low B cells, the number of IgM-secreting cells, and the IgM production were significantly reduced in the mice injected weekly with an IgG dose required to reach and maintain the physiological levels of serum IgG (Fig. 3D, 3E), but not in mice receiving as control soluble IgMs (Supplemental Fig. 3). These findings also suggest that the IgG-mediated inhibitory signals require threshold levels of circulating IgGs because the administration of low doses of soluble IgG did not modify the IgM titers produced by the transferred B cells (Fig. 3E). If these levels are not attained, as it was the case in mice receiving lower doses of IgG or injected with B cells from CD3−/− origin, B cell activation and IgM production are not inhibited.
In conclusion, our findings clearly demonstrate that an active IgG-dependent suppressive mechanism can regulate IgM production. In the absence of IgG secretion by the first B cell population, IgM production by the second B cell population was not inhibited, and the amount of IgM produced by the second B cell population was inversely correlated with the IgG level in the host serum. More importantly, they indicate a presence of a yet nondescribed mechanism of homeostatic control. It has been proposed that homeostasis of Ig-secreting cells is due to competition for a common niche of restricted size (8, 9) and in some cases due to FcγRIIB-induced apoptosis of plasma cells (20). We now show that, in the absence of competing populations, passively administered IgG reduced activation and further IgM production by subsequently introduced B cells.
The inhibitory FcγRIIB is required for IgG-mediated feedback regulation
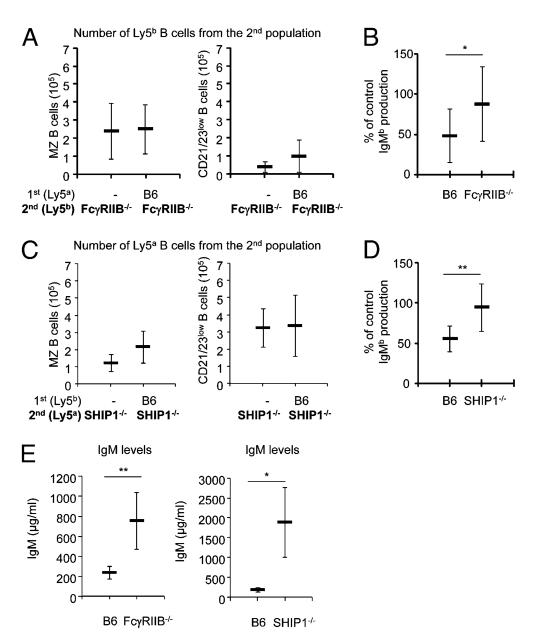
To investigate the mechanism of action underlying the IgG-dependent control of IgM production, we first injected Rag-deficient mice with LN cells from Ly5aIgHa WT mice; 4 wk later, we injected them with a second population of LN cells from either WT or FcγRIIB−/− Ly5bIgHb donors. FcγRIIB is a low-affinity IgG receptor expressed on B cells that acts as a negative regulator of B cell activation (12). We found that the first (WT) B cell population did not suppress the second B cell population that lacked FcγRIIB (Fig. 4A, 4B), but was able to suppress 50% of the IgM production by a population of WT B cells (Fig. 4B). Inhibition of B cell activation may require FcγRIIB cross-linking, which can be achieved in the presence of either immune complexes or Ig aggregates (21, 22). Notably, as mentioned, we found higher titers of self-reactive Abs in the sera of cell transfer host mice than in donor mice (Fig. 1D). These autoantibodies might form immune complexes that could either cross-link FcγRIIB or coaggregate BCR with FcγRIIB and generate negative signals (21) by enabling tyrosyl phosphorylation of FcγRIIB and recruitment of SHIP (13). To test this hypothesis, we injected Rag-deficient mice first with Ly5bIgHa WT cells and then, 4 wk later, with a population of cells from either WT or SHIP1−/− Ly5aIgHb donors (18). We found that WT B cells, although inhibiting a second population of WT B cells, failed to reduce the number of activated SHIP1−/− Ly5a B cells or their IgMb production (Fig. 4C, 4D). Because these findings were obtained upon adoptive cell transfer into lymphopenic hosts, their validity could be questioned, as the new environment would not reflect that of an intact mouse. Nevertheless, the size and composition of the IgM-secreting B cell pool remain stable for up to 6 mo after transfer, indicating that its homeostasis mimics the rules observed in intact mice. By using the strategy of sequential transfer of B cell populations from several mutant mice, we were able to study the mechanisms regulating the size of a stable IgM-secreting B cell pool. Notably, our findings obtained using adoptive cell transfers were consistent with the observation that both intact nonmanipulated FcγRIIB−/− and SHIP1−/− mice had IgM serum levels that were significantly higher than those of control littermates (Fig. 4E). Additionally, the cell transfer strategy demonstrated that these defects are B cell specific and not induced by the lack of expression of the FcRIIB or SHIP1 on other nonlymphoid host cells. Taken together, these results show that there are feedback mechanisms that regulate the number of activated B cell and plasma IgM levels and that these mechanisms involve plasma IgG- and FcγRIIB-mediated, SHIP1-dependent negative signals (Fig. 5, Supplemental Fig. 4).
FIGURE 4.
Role of the FcRγIIB and SHIP1 in feedback regulation of IgM production. (A) The number of MZ CD21highCD23low (left panel) and CD21lowCD23low (right panel) B cells recovered from the second FcRγIIB−/− Ly5b IgHb cell population when injected alone or into mice injected 4 wk before with a first Ly5aIgHa WT population. Each bar represents the mean ± SD of values for 12–14 mice. Note that the number of CD21highCD23low and CD21lowCD23low cells present among the second set of B cells was not modified by the presence of a first set of B cells. (B) The IgMb titers produced by a second population of WT (left) or FcRγIIB-deficient (right) B cells transferred into Rag-deficient mice injected 4 wk before with cells from WT IgHa donors expressed as a percentage of the control levels obtained after their transfer into naive Rag-deficient hosts. Note that the IgMb production by FcRγIIB-deficient B cells was not inhibited in the presence of a first population of WT B cells. Each bar represents the mean ± SD of values for 12–14 mice. Data shown were pooled from two independent experiments. (C) The number of MZ CD21highCD23low (left panel) and CD21lowCD23low (right panel) B cells recovered from the second SHIP1−/− Ly5aIgHb population when injected alone (left) or into mice injected 4 wk before with a first Ly5bIgHa population. Each bar represents the mean ± SD of values for 12–14 mice. Note that the number of Ly5a CD21highCD23low and CD21lowCD23low cells present among the second set of B cells was not modified by the first set of B cells. (D) IgMb levels produced by a second population of WT (left) or SHIP1-deficient (right) B cells transferred into Rag-deficient mice injected 4 wk before with cells from WT IgHa donors expressed as a percentage of the control levels obtained after their transfer into naive Rag-deficient hosts. Note that the IgMb production by SHIP1-deficient B cells was not inhibited in the presence of a first population of WT B cells. Each bar represents the mean ± SD of values for 12–14 mice. Data shown were pooled from two independent experiments. (E) The left panel shows the IgM concentrations present in the serum of age-matched WT littermates and FcRγIIB−/− mice. Each bar represents the mean ± SD of values for eight mice. IgM titers in the mutant mice were significantly higher. The right panel shows the IgM concentrations present in the serum of age-matched WT littermates and SHIP1−/− mice. Each bar represents the mean ± SD of values for four mice. IgM titers in the mutant mice were significantly higher. *p < 0.05, **p < 0.01.
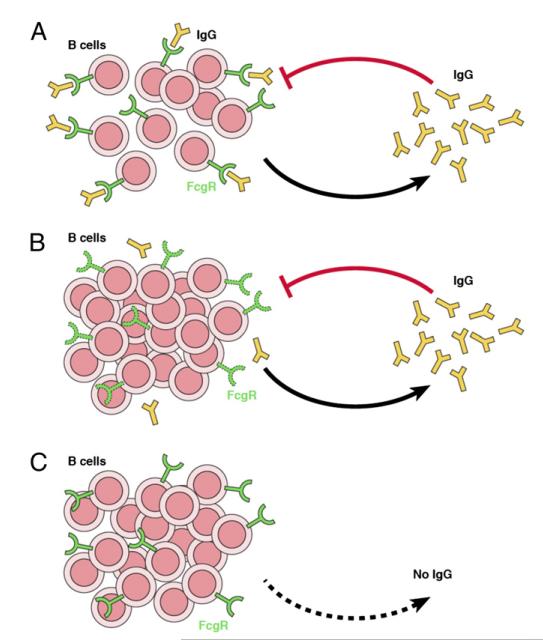
FIGURE 5.
(A) Quorum sensing: the presence of soluble IgG and the ability of the B cells to detect its levels are crucial to the homeostasis of the immune system. Quorum sensing: the IgG secreted by activated B cells is detected (sensed) by the inhibitory FcγRIIB expressed by B cells that prevents further B cell activation. That is to say: overall B cell populations adapt their behavior according to the sensing of the quantities of IgG produced. (B) Failure of quorum sensing by defective sensor molecule: the inability to detect soluble IgG because of defects in the FcγRIIB expression (in FcγRIIB−/− mice) or signaling (in SHIP1−/− mice) leads to hyper-IgM syndromes and autoimmune disease. (C) Failure of quorum sensing by absence of the sensed molecule IgG also leads to hyper-IgM syndromes and autoimmune pathology.
Discussion
The strategies to approach autoimmune disorders displaying elevated Ig (IgM) levels in the absence of obvious immunization are yet unclear. These disorders include hyper-IgM syndromes and other pathological conditions such as systemic lupus erythematosus (SLE). In some cases, therapeutic intervention with the administration of i.v. Igs was shown to have beneficial effects (16), but it appears paradoxical that in steady-state conditions in which Ig levels are already increased and induce pathology, further increases in circulating Ig could still have any beneficial effects. Above and beyond the possible anti-inflammatory effects of the administered Igs (23), the present findings showing that there are feedback mechanisms that regulate the number of activated B cells and plasma IgM levels and that these mechanisms involve plasma IgG- and FcγRIIB-mediated, SHIP1-dependent negative signals, may provide another explanation to this phenomenon.
It has been long known that passively administered Ag-specific IgG can suppress or enhance specific primary thymus-dependent (24, 25) and thymus-independent IgM Ab responses (26). It is generally accepted that IgG-mediated suppression is Ag specific (24). The suppressive effects could either be due to epitope masking and Ag sequestration by the high-affinity IgG preventing initiation of new IgM responses (25), or be mediated by an Fcγ-dependent regulation of B cell activation [for review, see (24)]. Trials on whether F(ab’)2 fragments kept the suppressive activity of intact IgG have given contradictory results (27). It has also been reported that IgG suppressed specific Ab responses as efficiently in FcγR-deficient mice as in wild-type controls (28, 29), questioning the role of this inhibitory receptor in the control of immune responses. However, FcγRIIB-deficient mice downregulate late responses to immune complexes (30) or during inflammation (31, 32), suggesting that these receptors may act to prevent overextended immune responses (27). It should be pointed out that studies that investigated the in vivo role of IgG-mediated regulation of B cell activation and immune responses have all used actively immunized mice, under conditions in which high titers of Ag-specific Abs were produced (24, 27). The novelty of our approach resides on the fact that we performed our studies in the absence of an Ag-specific immune response. In these novel conditions, our results show that circulating IgG, constitutively present in nonimmunized mice, may have a fundamental role in controlling natural activated B cell numbers and the IgM they produce. They seal a hiatus and provide new valuable information as they demonstrate that FcγRIIB-dependent negative regulation controls B cell activation without any intentional immunization.
We show that regulation of IgM production engages a FcγRIIB-mediated, SHIP1-dependent inhibitory feedback loop in which B cells themselves sense the number of activated B cells by detecting their secreted IgG. When there are enough activated B cells, the high titers of IgG and self-reactive Abs that they produce can lead either to FcγRIIB-mediated aggregation by immune complexes, possibly inducing activated B cell apoptosis (33, 34), or to coaggregation of BCR and FcγRIIB, which dampens B cell activation (21). Either mechanism may prevent the accumulation of activated IgM-secreting B cells. However, whereas direct FcγRIIB cross-linking may inhibit B cells independently of their antigenic specificity and affect a large fraction of the cells, BCR and FcγRIIB coaggregation will preferentially inhibit self-reactive B cells. Considering the stronger effects observed using SHIP1-deficient B cells, it is possible that alternative FcγRIIB-independent inhibitory pathways may also participate in the control of the IgM-secreting cell pool. SHIP was indeed shown to control activation/proliferation signals triggered by a variety of receptors that activate PI3K (13). Once IgG levels are re-established normal B cell activation can proceed in response to new antigenic stimulation.
Our current findings might provide an evolutionary explanation for the expression of inhibitory Fcγ receptors by B cells. By simultaneously allowing detection of plasma IgG levels and inhibiting B cell activation, these receptors contribute to control the number of all Ig-secreting B cells (IgM and IgG), and thus may prevent autoimmune disorders. They indicate that self-reactive B cells can expand and secrete autoreactive Igs if not held in check by sufficient IgG in their environment. This notion is supported in part by our observation that self-reactive IgM titers produced by a B cell population were selectively reduced in the presence of a first B cell population (Fig. 2D) and by other previously published observations. The inability of B cells to detect plasma IgG because of defects in FcγRIIB expression or signaling leads to increased Ig levels and autoimmune disease (12, 31, 35–37). Altered human FcγRIIB signaling in B cells resulting from the I232T polymorphism (38, 39) as well as decreased FcγRIIB expression in memory B cells (40) have been reported in SLE patients. Conversely, partial restoration of FcγRIIB expression in the B cells of FcγRIIB−/− mice (41) or FcγRIIB overexpression in B cells reduces SLE incidence (42), findings that indicate the B cell phenotype is likely to be due to FcγRIIB deficiency rather than to other genetic variation (43). Finally, the failure to produce IgG either because of early thymectomy in toads (44), inappropriate T–B cell cooperation in humans (5) (Fig. 5), or the intrinsic inability of activation-induced cytidine deaminase–mutant B cells to switch to IgG production in both mice and humans (4, 7) results in hyper-IgM syndromes and autoimmune pathology. They may also be of relevance to the perception of the proposed hygiene hypothesis (45). Indeed, increase incidence of infections in the developing countries may lead to higher serum titers of IgG, and thus help to prevent development of autoimmune and allergic diseases.
Our findings may also suggest an as yet unsuspected physiological role for natural self-reactive Abs. Specifically, it has never been clear why natural self-reactive Abs should be allowed to exist; but our observation of IgG-mediated feedback regulation in normal nonimmunized mice suggests that natural self-reactive Abs, which make up a significant fraction of the natural plasma Ig (46) by facilitating FcγRIIB aggregation, provide a warning signal that reflects B cell density. Thus, natural self-reactive Abs might participate in activated B cell homeostasis under physiological steady-state conditions.
In conclusion, these results identify a new mechanism of homeostatic control. To date, in mammals, homeostatic control was believed to be due to competition of cellular populations for a common niche of restricted size, defined by the ensemble of cellular interactions and trophic factors required for cell survival (8, 47). However, in situations where resources are not limiting, that is, immune responses, excess of self-Ags or cytokines, it is not clear which mechanisms could limit expanding lymphocyte numbers. Thus, the question is as follows: how do lymphocyte populations count the number of their individuals and how do they know when to stop growing? We now show that, in absence of competing cell populations, passively administered IgG reduced activation and IgM production by subsequently introduced B cells. Thus, we propose that control of lymphocyte numbers could also be achieved by the ability of lymphocytes to perceive the density of their own populations (47). Such mechanism would be reminiscent of the primordial quorum-sensing systems used by some bacteria in which a bacterium senses the accumulation of bacterial-signaling metabolites secreted (by the same or other cells), allowing the bacterium to sense the number of cells present in a population and adapt their growth accordingly (14, 15). In summary, these quorum-sensing mechanisms allow bacteria to coordinate their gene expression according to the density of their population (14, 15). Quorum sensing can play a critical role in lymphocyte homeostasis with the proviso that lymphocytes have the capacity to assess the number of molecules they interact with and can mount a standard response once a threshold number of molecules is detected. The situation described in this work seems to support this hypothesis. We found that homeostasis of the innate IgM-secreting B cell pool is achieved when total B cell populations are able to monitor the number of activated B cells by detecting products secreted by some of their members (Fig. 5A). The ability of B cells to detect plasma IgG levels through such a quorum-sensing mechanism may be crucial to immune system homeostasis, providing a critical checkpoint mechanism that may prevent excessive B cell activation and autoimmunity. We speculate that malfunction of this quorum-sensing mechanism may lead to uncontrolled B cell activation and autoimmune disease (Fig. 5B, 5C). It is possible that similar quorum-sensing mechanisms may work to maintain the homeostasis of other lymphocyte populations (47, 48) or control organ size during development.
Supplementary Material
Acknowledgments
We thank Drs. B. Rocha for critical assessment of the manuscript and G. Laval for help with statistics. We also thank M.-P. Mailhé for technical assistance and the Plateforme de Cytométrie de Flux for cell sorting.
This work was supported by grants from the European Research Council, Agence Nationale pour la Recherche, Agence pour la Recherche sur le Cancer, Institut Pasteur, Centre National pour la Recherche Scientifique, and INSERM. C.M., Y.H., and E.G. were supported by Direction Recherche Enseignement et Technologie; T.F. by Fundación Instituto Mediterraneo para el Avance de la Biomedicina y la Investigación Biosanitaria (Málaga, Spain) and Fondation de le Recherche Médicale; and W.G.K. by National Institutes of Health Grants RO1 HL72523 and R01 101748 and Paige Arnold Butterfly Run.
Abbreviations used in this article
- LN
lymph node
- MZ
marginal zone
- OxLDL
oxidized low density lipoprotein
- SLE
systemic lupus erythematosus
- WT
wild-type
Footnotes
Disclosures The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waghorn DJ. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J. Clin. Pathol. 2001;54:214–218. doi: 10.1136/jcp.54.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durandy A, Revy P, Imai K, Fischer A. Hyper-immunoglobulin M syndromes caused by intrinsic B-lymphocyte defects. Immunol. Rev. 2005;203:67–79. doi: 10.1111/j.0105-2896.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 5.Lougaris V, Badolato R, Ferrari S, Plebani A. Hyper immunoglobulin M syndrome due to CD40 deficiency: clinical, molecular, and immunological features. Immunol. Rev. 2005;203:48–66. doi: 10.1111/j.0105-2896.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 6.Vyse TJ, Kotzin BL. Genetic susceptibility to systemic lupus erythematosus. Annu. Rev. Immunol. 1998;16:261–292. doi: 10.1146/annurev.immunol.16.1.261. [DOI] [PubMed] [Google Scholar]
- 7.Hase K, Takahashi D, Ebisawa M, Kawano S, Itoh K, Ohno H. Activation-induced cytidine deaminase deficiency causes organ-specific auto-immune disease. PLoS One. 2008;3:e3033. doi: 10.1371/journal.pone.0003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu. Rev. Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 9.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu. Rev. Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 10.Rocha B, Dautigny N, Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur. J. Immunol. 1989;19:905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- 11.Agenès F, Freitas AA. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 1999;189:319–330. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 13.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 14.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 15.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 16.Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29:608–615. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JW, Howson JM, Ghansah T, Desponts C, Ninos JM, May SL, Nguyen KH, Toyama-Sorimachi N, Kerr WG. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 2002;295:2094–2097. doi: 10.1126/science.1068438. [DOI] [PubMed] [Google Scholar]
- 19.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KG. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 21.Daëron M. Fc receptor biology. Annu. Rev. Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 22.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 23.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 2000;18:709–737. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 25.Moller G, Wigzell H. Antibody synthesis at the cellular level: antibody-induced suppression of 19s and 7s antibody response. J. Exp. Med. 1965;121:969–989. doi: 10.1084/jem.121.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J. Exp. Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyman B. Feedback regulation by IgG antibodies. Immunol. Lett. 2003;88:157–161. doi: 10.1016/s0165-2478(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson MC, Getahun A, Heyman B. FcgammaRIIB in IgG-mediated suppression of antibody responses: different impact in vivo and in vitro. J. Immunol. 2001;167:5558–5564. doi: 10.4049/jimmunol.167.10.5558. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson MC, Wernersson S, Diaz de Ståhl T, Gustavsson S, Heyman B. Efficient IgG-mediated suppression of primary antibody responses in Fcgamma receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 1999;96:2244–2249. doi: 10.1073/pnas.96.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wernersson S, Karlsson MC, Dahlström J, Mattsson R, Verbeek JS, Heyman B. IgG-mediated enhancement of antibody responses is low in Fc receptor gamma chain-deficient mice and increased in Fc gamma RII-deficient mice. J. Immunol. 1999;163:618–622. [PubMed] [Google Scholar]
- 31.Takai T. Roles of Fc receptors in autoimmunity. Nat. Rev. Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 32.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J. Exp. Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 34.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity. 1999;10:753–760. doi: 10.1016/s1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- 35.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc (gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 36.Pritchard NR, Cutler AJ, Uribe S, Chadban SJ, Morley BJ, Smith KG. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr. Biol. 2000;10:227–230. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 37.Westhoff CM, Whittier A, Kathol S, McHugh J, Zajicek C, Shultz LD, Wylie DE. DNA-binding antibodies from viable motheaten mutant mice: implications for B cell tolerance. J. Immunol. 1997;159:3024–3033. [PubMed] [Google Scholar]
- 38.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, Lehner PJ, Ouwehand WH, Allen JM, Watkins NA, Smith KG. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat. Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 39.Kono H, Kyogoku C, Suzuki T, Tsuchiya N, Honda H, Yamamoto K, Tokunaga K, Honda Z. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum. Mol. Genet. 2005;14:2881–2892. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 40.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J. Exp. Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 42.Brownlie RJ, Lawlor KE, Niederer HA, Cutler AJ, Xiang Z, Clatworthy MR, Floto RA, Greaves DR, Lyons PA, Smith KG. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J. Exp. Med. 2008;205:883–895. doi: 10.1084/jem.20072565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bygrave AE, Rose KL, Cortes-Hernandez J, Warren J, Rigby RJ, Cook HT, Walport MJ, Vyse TJ, Botto M. Spontaneous autoimmunity in 129 and C57BL/6 mice: implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2004;2:E243. doi: 10.1371/journal.pbio.0020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss N, Horton JD, Du Pasquier L. The effect of thymectomy on cell surface associated and serum immunoglobulin in the toad, Xenopus laevis (Daudin) In: Panijel J, Liacopoulos P, editors. L’étude Phylogénique et Ontogénique de la Réponse Immunitaire et Son Apport à la Théorie Immunologique. INSERM; Paris: 1973. pp. 165–174. [Google Scholar]
- 45.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin. Exp. Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton. Immunol. Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 47.de Freitas AA. Tractus Immuno-logicus: a Brief History of the Immune System. Landes Bioscience; Austin, TX: 2009. [Google Scholar]
- 48.Almeida AR, Amado IF, Reynolds J, Berges J, Lythe G, Molina-París C, Freitas AA. Quorum-sensing in CD4(+) T cell homeostasis: a hypothesis and a model. Front Immunol. 2012;3:125. doi: 10.3389/fimmu.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.