Synopsis
Storage of erythrocytes in blood banks is associated with biochemical and morphological changes to the RBC. It has been suggested that these changes have a potential negative clinical effects characterized by inflammation and microcirculatory dysfunction which add to other transfusion related toxicities. However, mechanisms linking RBC storage and toxicity remain unclear. In this study we tested the hypothesis that storage of leukodepleted RBC result in cells that inhibit nitric oxide (NO)-signaling more so than younger cells. Using competition kinetic analyses and protocols that minimized contributions from hemolysis or microparticles, our data indicate that NO-consumption rates increased ~40-fold and NO-dependent vasodilation was inhibited 2-4 fold with 42d old vs. 0d RBC. These results are likely due to the formation of smaller RBC with increased surface area: volume as a consequence of membrane loss during storage. The potential for older RBC to affect NO-formation via deoxygenated RBC mediated nitrite reduction was also tested. RBC storage did not affect deoxygenated RBC-dependent stimulation of nitrite-induced vasodilation. However, stored RBC did increase the rates of nitrite oxidation to nitrate in vitro. Significant loss of whole blood nitrite was also observed in stable trauma patients after transfusion with 1 RBC unit, with the decrease in nitrite occurring after transfusion with RBC stored for >25d, but not with younger RBC. Collectively, these data suggest that increased rates of reactions between intact RBC and NO and nitrite may contribute to mechanisms that lead to storage lesion-related transfusion risk
Keywords: hemoglobin, vasoconstriction, kinetics
Introduction
Transfusion with red blood cells (RBCs1) is the front line therapy for massive blood loss, with ~5 million patients being transfused each year in the US. This figure is increasing at rate of 6% per year. Current blood banking standard allow pRBCs to be stored up to 42 days. However, an association between the storage age of RBC (>14 days) and increased incidence of adverse clinical outcomes has been demonstrated in diverse patient populations including trauma patients[1, 2]. In the latter, transfusion related adverse effects are further compounded with increasing number of RBC units transfused [3]. The potential harm from pRBCs as a function of storage time is referred to as the ‘storage lesion’. Its general concept is that in the presence of an underlying inflammatory stimulus, e.g. from infection (sepsis), surgery or trauma, older RBCs relative to younger RBCs, will provide a second hit that results in dysfunction in tissue perfusion/oxygenation and exacerbation of the inflammatory injury.
Morphological and chemical changes that occur during RBC storage are well documented and include decreases in 2,3-diphosphoglycerate and pH; as well as an increase in potassium, lactate and hemolysis and appearance of echinocytic and smaller more dense RBCs [4]. As a consequence stored RBCs have increased fragility and decreased deformability. How these changes are associated with toxicity remains unclear. Several hypotheses have been forwarded including increased oxidative stress and formation of reactive lipid species, release of chemokines by contaminating leukocytes and loss of RBC chemokine scavenging potential, increased hemolysis and iron release, and formation of microparticles and other soluble mediators that agonize the host immune/inflammatory response[4-8].
Recent studies have demonstrated the association of in vivo toxicity with transfusion of older pRBCs despite leukoreduction [3] suggesting that mechanisms intrinsic to the RBC may also play a role in storage lesion-dependent pathology. In this context, an emerging and unifying hypothesis to explain compromised tissue perfusion and exacerbation in inflammatory responses is that aged pRBCs cause a loss in nitric oxide (NO) signaling. Nitric oxide plays important roles in vascular homeostasis with a decrease in its bioavailability leading to hypertension, coagulation and inflammation. Specific proposed mechanisms for decreased NO-signaling with transfusion of older pRBCs include a storage dependent loss of RBC dependent stimulation of NO-signaling (via loss of S-nitrosohemoglobin or ATP-release) [9-11], and/or increased rates of NO-scavenging by hemoglobin in RBC-derived microparticles or after hemolysis [12, 13]. This is underscored by the similarity between the RBC storage lesion toxicities with the pathogenic effects of acellular hemoglobin based oxygen carriers.
NO-scavenging reactions of hemoglobin discussed above refer to oxy-ferrous heme dependent oxidation of NO to nitrate and deoxyferrous heme binding of NO to form nitrosylhemoglobin (Hb-Fe2+-NO) (Equations 1 and 2 respectively).
| Equation 1 |
| Equation 2 |
With cell-free hemoglobin, both reactions occur with rate constants between 3-8×107M−1s−1 [12, 14-17] With erythrocytic, and hence encapsulated hemoglobin the rate constant for NO-scavenging is decreased by ~500-1000-fold, a property proposed to be key in allowing endothelial derived NO to regulate signaling processes in the vasculature [14, 17-21]. The exact mechanism for the decreased NO-scavenging rate of RBC-hemoglobin vs. cell free hemoglobin remains debated and involves diffusion barriers created by an unstirred layer immediately adjacent to the RBC and/or membrane based structures, which slow down NO reactions. Interestingly, these diffusion barriers are regulated by RBC size, shape and surface area [17], biophysical properties that are known to change during RBC storage. However, the effects of these changes on reactions with NO have not been considered previously and are tested herein.
In addition, we also tested if reactions between RBC and nitrite were altered during storage. Nitrite reactions with hemoglobin are regulated by the hemoglobin fractional saturation such that under oxygenated conditions, nitrite is oxidized to nitrate, but when deoxygenated, nitrite reduction to NO can occur to mediate hypoxic NO-signaling [22-26]. The balance between nitrite oxidation vs. nitrite reduction is important in regulating NO-signaling [27, 28] and could play a role in affecting inflammatory tissue injury since lower nitrite levels predispose to, whereas nitrite supplementation protects, against ischemic and inflammation dependent tissue injury[29]. Since stored RBC have increased P50 and altered membrane properties which could affect nitrite transport, we reasoned that altered nitrite metabolism and NO-formation by stored RBC could also play a role in transfusion related toxicities.
Experimental
Materials
All materials were purchased from Sigma-Aldrich (St Louis, MO) except MahmaNONOate and L-NMMA, which were obtained from Axxora Platform (San Diego, CA). Male Sprague-Dawley rats (200-250 g) were purchased from Harlan (Indianapolis, IN). All animal studies were performed following Institutional Animal Care and Use approved procedures.
RBC collection and storage
Blood was collected from healthy donors (following Institutional Review Board approved protocols), leukodepleted by filtration (Sepacell RZ-200A) and stored according to blood bank protocols (at 4°C) in Adsol. Leukodepletion decreased white blood cells from 12000 ± 2000 cells/ml blood to undetectable levels determined by light microscopy (mean ± SEM, n =3). At 0, 1, 7, 14, 28 and 42 days post storage, aliquots of RBCs were collected and washed 3 times with PBS containing 0.1% BSA by centrifugation (1500g for 10 minutes per wash and the supernatant discarded). Alternatively, leukodepleted pRBCs were collected from segments from pRBCs stored in the UAB blood bank. RBC were washed as described above prior to use.
Light microscopy of RBC
RBC (5μl of packed cells) were smeared onto a glass slide and fixed using methanol and then stained with Wrights stain. Slides were imaged at 40X magnification using a Leica DM600 microscope (Leica Microsystems, Bannockburn, IL). Images were adjusted to control for contrast.
Ex Vivo Aorta Vasodilation
Thoracic aortas were isolated from male Sprague-Dawley rats (200-250g) and divided into approximately eight 5-mm wide sections. Rings were suspended between two hooks connected to a force transducer and placed within a vessel bath chamber containing Krebs-Henseleit (KH) buffer as described previously [27]. After two rounds of KCl-induced contractions followed by washing and 30min equilibration, vessels were equilibrated with 21% or 1% oxygen in KH buffer at 37°C in the presence of 5% CO2. Vessels were pretreated with indomethacin (5 μM) and N-monomethyl-L-arginine (L-NMMA; 100μM) and precontracted with phenylephrine (200nM at 21% oxygen tension and 400nM at 1% oxygen tension) before the addition of RBCs (0.3% hematocrit final concentration). Once vessels had reached a stable tone, vasorelaxation was elicited by the addition of either sodium nitrite (3 and 10 μM) or the NO donor Mahma-NONOate (10nM and 30nM). A dose-dependent protocol was used to limit the time of experiment to <10min post RBC addition; preliminary studies determined that longer durations resulted in significant RBC hemolysis which would preclude assessment of RBC-dependent effects on NO- or nitrite dependent vasodilation. Vasorelaxation was determined in the absence and presence of RBCs, and the percent inhibition of nitrite- or NO-dependent vasodilation by the RBCs calculated using stable tensions at the end of vasodilation. Also, at the end of each experiment, the concentration of cell free and RBC heme in the vessel bioassay chamber was measured.
Heme concentration measurement
Heme was measured by either the Drabkins assay or after deconvolution of visible spectra for oxyhemoglobin, deoxyhemoglobin and methemoglobin as previously described [27].
Fractional saturation calculation
RBCs were lysed in deionized water at a ratio of 1:5 and then diluted to 20μM heme in PBS. This resultant suspension maintained the ratio of hemoglobin to allosteric effectors whilst allowing measurement of visible spectra (450-700nm) without scattering associated with intact RBC. 3ml of hemolysate was deoxygenated in tonometer and equilibrated at 34°C. Wavelength scans were taken after the sequential addition of 1ml of air until complete oxygenation of the hemoglobin was achieved. OxyHb and deoxyHb concentrations were measured by spectral deconvolution as described [27] and deoxyhemoglobin vs. pO2 plotted to calculate P50. P50 values for hemolysates collected from freshly isolated (day 0) RBC were 28.9 ± 1 mmHg (mean ± SEM, n=4), a value which falls within the range of P50 reported for intact freshly isolated RBC at pH7.4, 37°C (27-30mmHg). Validation of the approach used is further provided by the magnitude of storage age dependent decrease in P50 being similar to recent studies in which oxygen binding affinity of stored RBC was determined by a Hemox analyzer[30].
Kinetic analysis of NO scavenging by RBCs
The rate of NO-dioxygenation induced by RBCs was determined using competition kinetics as previously described [20] with slight modifications. Three experimental conditions were used, PBS + 0.5%BSA containing either, oxyHb, oxyHb with spermine NONOate (SpNO, an NO donor), or a suspension of RBC plus oxyHb and SpNO. Final concentrations were 7μM for oxyHb, 7%Hct for RBC (RBC were added after washing (3 times at 1500 × g, 10min) to remove any hemolysis derived products that may have accumulated during storage. Experiments were started by addition of SpNO (10μM, preliminary studies established these conditions to result in linear rates of oxyHb oxidation over 60min, data not shown). Samples were placed in six-well tissue culture plates at room temperature and rocked gently on a rocking platform. Prior to the addition of SpNO samples were taken to assess free hemoglobin concentrations. After addition of SpNO, 0.5mL samples were taken at every 10min for 60min, immediately centrifuged (20 s at 2,000 ×g) to separate the RBCs, and the supernatant collected and concentration of oxyHb and metHb determined by visible spectroscopy. The relative rate of cell-free oxyHb oxidation to metHb by SpNO, in the presence or absence of RBC allows determination of kinetics of RBC-dependent NO-dioxygenation reactions. In separate experiments evaluating the effects of butylated hydroxytoluene (BHT, 100μM), superoxide dismutase (SOD) or SOD + catalase (both 100 U/ml). reagents were added and incubated for 5 minutes prior to addition of SpNO.
Relative rate constants for RBC (kRBC) vs. cell-free oxyHb (kHb) were calculated as described [20] using the following equation 3:
| Equation 3 |
where [metHb]c is the concentration of cell-free metHb in the preparation containing cell-free oxyHb and SpNO (no RBC), Hct is the RBC hematocrit, [oxyHb]RBC is the concentration of total Hb in RBC, kRBC and kHb are the rate constants for RBC and Hb-dependent NO-dioxygenation reactions respectively, [totalHb]ex and [oxyHb]ex are the total cell free and oxyHb concentrations respectively in preparations containing RBC. At each time point sampled, the term (y-axis) was plotted against term (x-axis) and kRBC/kHb determined by the gradient. Initial studies indicated that significant hemolysis occurred during the experiment when assessing RBC stored for more than 14d. The protocol was modified therefore to collect samples every 3 min for 12 min, a time period over which the above described plot remained linear and cell-free hemoglobin concentration changed by <6%.
Microparticle measurement
Stored RBCs were left unwashed (60%Hct) or washed (3 times) and brought to 60% Hct in PBS + 0.1% BSA. RBCs were then incubated with anti-glycophorin A-FITC conjugated antibody (0.17μg/ml) for 30min in the dark at room temperature. Samples were then analyzed by flow cytometry using a Becton Dickinson FACSCalibur (BD Biosciences, Franklin Lakes, NJ) and events acquired using CellQuest software. Approximately 100,000 events were collected per measurement. All analyses were done with FlowJo software (Tree Star, Inc., Ashland, OR).
Nitrite Consumption by RBC
Nitrite consumption by RBC (washed to remove microparticles and cell-free heme) was determined as described [31] at different oxygen tensions in a controlled-atmosphere chamber (Plas Labs, Lansing, MI) using atmospheric gas combined with nitrogen gas to produce 21% or 2% oxygen tensions. RBC suspensions at 5% Hct in Tris-buffer containing 0.1%BSA, pH 7.4 were equilibrated for 30min in six-well tissue culture plates with gentle rocking. To initiate experiments, nitrite (100μM) was added (nitrite stock solutions were prepared in deoxygenated PBS) and aliquots removed at 0, 5 and 15 minutes. Aliquots were taken out of the chamber and immediately centrifuged at 2,000 g for 30 s. The extracellular fraction (supernatant) was collected and vortex mixed with equal volumes of methanol then frozen in liquid nitrogen. In all experiments, parallel incubations of nitrite alone in Tris-BSA buffer were included. Nitrite consumption was determined as the difference between its concentration in the samples with and without RBCs.
Trauma Patient studies
Patients admitted in to the trauma intensive care unit at UAB with orders to receive 1 pRBC unit transfusion were enrolled into the study. Study protocol, patient enrollment, exclusion criteria, and demographics were recently described[32]. Approval for this study was granted by the University of Alabama at Birmingham Institutional Review Board. Blood samples (400μl) were collected immediately before RBC transfusion and 1h after the completion of transfusion and immediately processed for whole blood nitrite measurements by methanol extraction as previously described [33]. Methanolic extracts were stored (-80°C) prior to nitrite measurement.
Nitrite and Nitrate measurements
Methanolic extracts were thawed on ice and in the dark and then centrifuged (15,000 × g, 5 min, 4 °C). The supernatant volume was measured and nitrite and nitrate concentrations determined using triodide based chemiluminesence as previously described on a Sievers NOanalyzer and comparison to respective standard curves[33].
Statistical Analysis
Storage time-dependent changes were analyzed by 1-way repeated measures ANOVA with Tukey’s post-test or by 2-way ANOVA when also assessing effects of pO2. Changes in circulating nitrite levels in trauma patients before and after RBC transfusion were analyzed by unpaired Student’s t-test. P-values less than 0.05 were considered significant. All analyses used GraphPad Prism Software (San Diego, CA, USA.
Results
Validation of storage-dependent changes in RBC
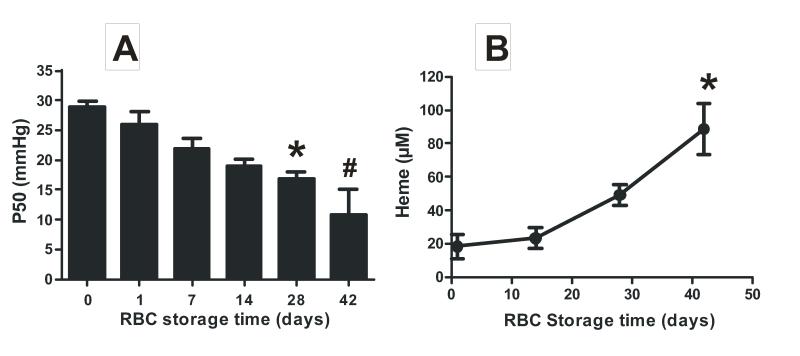
RBC segments of pRBCs of different storage ages were collected from the UAB blood bank or RBCs isolated from healthy volunteers, leukodepleted and stored according to standard blood banking conditions at our institution were used. Initial studies were performed to validate that these RBC preparations exhibited biochemical and morphologic changes that typify the storage lesion. Supplementary Figure 1 shows that RBC shape changed from a normal discoidal form (day 0) to echinocytes characterized by spiculated membranes (day 28 and 42). Furthermore, the RBC P50 decreased and hemolysis increased with storage time (Figure 1) consistent with previous studies [13, 30].
Figure 1. RBC storage increases oxygen affinity.
RBC were collected from healthy volunteers, leukodepleted, stored for the indicated times and P50 (Panel A) and hemolysis (Panel B) measured. For panel A data show mean ± SEM (n=3-4) *P < 0.05 vs day 0; #P < 0.05 vs days 0, 1, 7 by 1-way ANOVA with Tukey’s post test. For Panel B data show mean ± SEM (n=3) *P < 0.01 vs day 1 and 14 by 1-way ANOVA with Tukey’s post test.
RBC storage increases NO scavenging and inhibits NO-dependent vasodilation
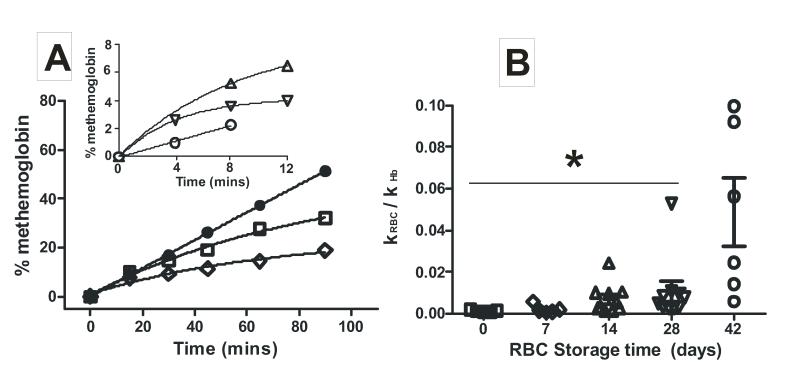
The rate constant for NO-reactions with oxyhemoglobin in RBC can be determined using a competition assay, in which the NO-donor SpNO is added to a mixture of cell-free oxyhemoglobin and RBC, and then following the time dependent formation of cell-free methemoglobin. At non-limiting SpNO concentrations, an increase in the rate of RBC-hemoglobin reactions with NO is reflected as a decrease in the rate of cell-free methemoglobin formation. Figure 2A shows representative kinetic traces and demonstrates that RBC-dependent inhibition of cell-free methemoglobin formation, increases with the length of RBC storage. The transition from linear to curved kinetic traces for metHb formation in the presence of RBC is similar to previous studies [20] and reflects the competition between RBC and cell-free hemoglobin for reaction with NO. Figure 2B plots the calculated ratio of rate constants for NO-dioxygenation reaction between erythrocytic and cell-free hemoglobin (kRBC/kHb) and demonstrates that RBC-dependent NO-scavenging increases ~40-fold over 42 days of storage. Significance by 1-way ANOVA was observed with 42d old RBC relative to all other ages. A trend towards increasing NO-scavenging rates is also noted by 14-28d which was not significant due to variance within any given RBC age especially from day 14 and onwards (likely reflecting donor to donor differences). Exclusion of outlier data (n=1 each from 14d and 28d data set) detected by Grubbs’ test resulted in an observation of a significant increase in NO-scavenging kinetics between both day 14 and 28 RBC relative to day 0 (P < 0.05 by t-test, not shown). Addition of SOD, SOD + catalase or BHT, had no effect on NO-dioxygenation rates by 42d old RBCs (not shown) suggesting that increased superoxide or lipid alkoxyl/peroxyl radicals are not responsible for accelerated NO-consumption.
Figure 2. Effects of RBC storage time on NO-scavenging kinetics.
Panel A shows representative traces for SperNO dependent formation of cell-free metHb from oxyHb (7μM) in the presence and absence of RBC (7% Hct) of different storage ages (●, cell free oxyhemoglobin alone; □, day 0 RBC, ◇, day 7 RBC). Inset shows data with older RBC (△, day 14; ▽, day 28; ○, day 42) where kinetics were determined over shorter times due to hemolysis (as described in methods). Panel B shows scatter plot of calculated ratio of rate constants for NO-dioxygenation by RBC relative to cell-free Hb. RBC of different ages were collected from UAB blood bank. For day 0 samples, RBC were collected from healthy volunteers, leukodepleted and processed as described in methods. Experiments were performed in PBS, pH 7.4 at 20°C. Data represent mean ± SEM, n = 5-10. *P < 0.01 relative to day 42 by 1-way ANOVA (P = 0.0005) and Tukey’s post test.
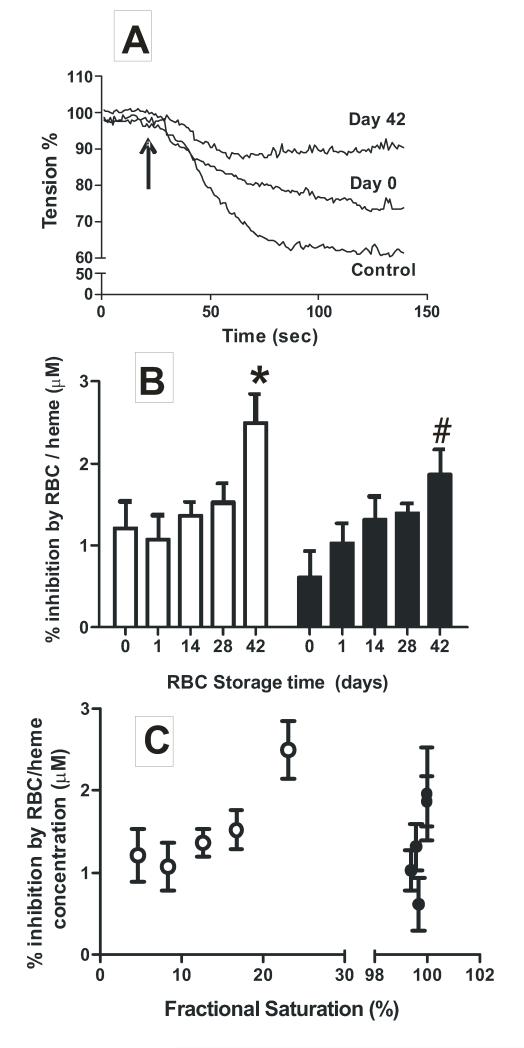
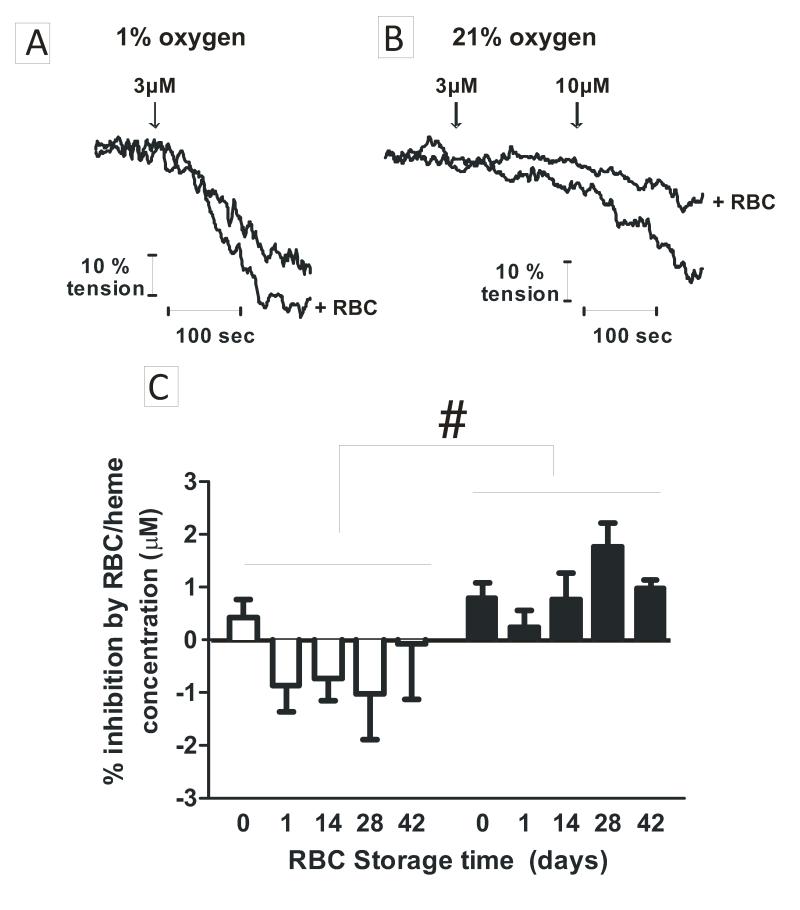
NO-dependent dilation of isolated rat thoracic aorta was used to test if storage dependent increased NO scavenging by RBC translates to a greater degree of inhibition of NO-signaling. Aortas were pre-constricted with PE and L-NMMA, the latter to inhibit endogenous NO-formation from endothelial nitric oxide synthase. RBC of different ages were then added, and followed by addition of MNO (10nM and 30nM). Fig 3A shows representative vessel tension traces showing that MNO (30nM) stimulated ~40% dilation, which was inhibited in the presence of day 0 and day 42 RBCs; the latter having a greater inhibitory effect. Figure 3B shows the percent inhibition of MNO-dependent vasodilation by RBC as a function of storage time. 42d RBCs inhibited MNO-dependent vasodilation at both 21% and 1% O2 consistent with increased NO-scavenging kinetics. No significant effect of oxygen tension (P = 0.1, by 2-way ANOVA) was observed.
Figure 3. Effects of RBC storage time on NO-dependent vasodilation.
RBC (0.3%Hct) of different ages were added to vessel bioassay chambers followed by addition of MNO to rat aortic segments. Experiments were performed at 21%O2 and 1% O2. Panel A shows representative vessel tension vs. time traces (21%O2). RBC were added at time 0 and MNO (30nM) addition indicated by arrow. Panel B shows percent inhibition of MNO-dependent vasodilation by RBC of different storage ages at 1% O2 (□) and 21% O2 (■). Data are normalized to the RBC heme concentration in the vessel bioassay chamber measured at the end of each experiment and are mean ± SEM (n=5-6). *P < 0.05 relative to day 0, 1, 14 and #P < 0.05 relative to day 0 by 1-way ANOVA with Tukey’s post test. Panel C plots percent inhibition of MNO-dependent vasodilation by RBC as a function of calculated fractional saturation. ○, data collected at 1%O2; ●, data collected at 21%O2.
As shown in Fig 1A, RBC P50 decreases with storage time. Since the rate of NO-scavenging by oxyhemoglobin is slightly (~1.5-2-fold) faster compared to deoxyhemoglobin[14, 15, 17, 34], the increased inhibition of NO-dependent vasodilation observed with older RBCs (~2-3 fold for day 0d vs. 42d RBC), could reflect the presence of more oxy- vs. deoxyhemoglobin. This is unlikely however since the rate of NO-consumption by RBC is zero order with respect to hemoglobin[17]. Another possibility is that when deoxygenated, the RBC membrane permeability to NO is increased and thereby contribute in part, to differential NO-scavenging kinetics by RBC compared to cell-free hemoglobin [14]. Inhibition of MNO-dependent vasodilation was replotted as a function of oxygen fractional saturation of RBCs in vessel bioassay chambers (Fig 3C). At 1% O2, fractional saturation was higher for 42d vs. 0d RBC (P<0.05 by 1-way ANOVA with Tukey’s post test) and inhibition of MNO-dependent vasodilation paralleled storage time (Fig 3B) suggesting that higher concentrations of oxyhemoglobin in older RBC mediate increased inhibition of MNO-dependent vasodilation. At 21% O2 however, no difference in fractional saturation was observed (P = 0.21 by 1-way ANOVA) between RBCs of different age. This suggests that increased inhibition of NO-scavenging, and increased rate of NO reactions are due to storage dependent changes in RBC morphology or biochemistry that are independent of oxygen affinity.
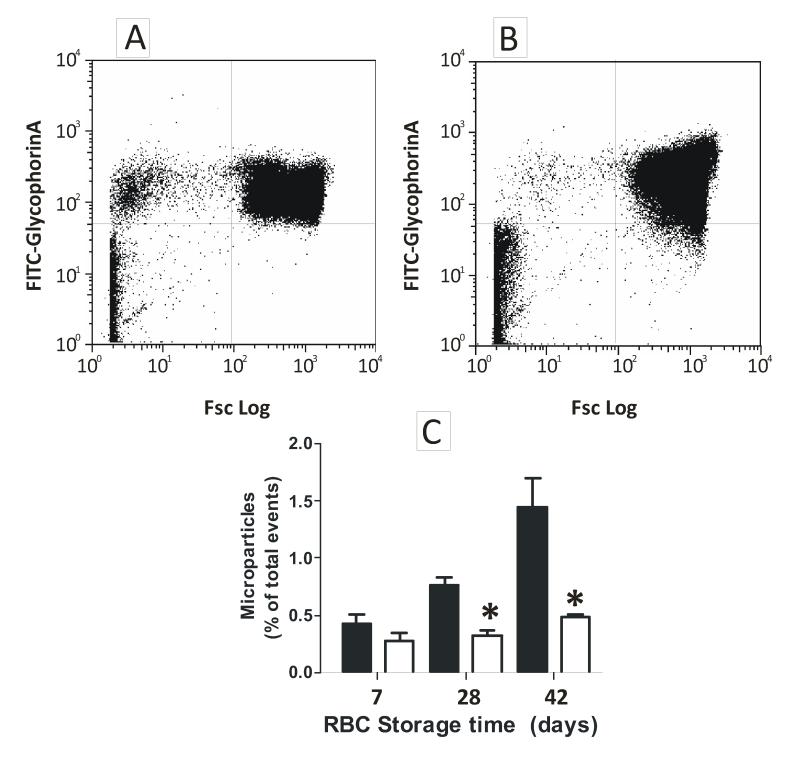
Stored RBCs are more sensitive to hemolysis and produce more hemoglobin containing microparticles, both of which exhibit increased NO-scavenging kinetics [13]. Microparticle formation and hemolysis was measured before and after washing. Microparticles increased with storage age, but washing removed them to ≤ 0.5% of total glycophorin positive events (Fig 4A-C). Moreover, washing also decreased cell-free hemoglobin to <0.5μM (not shown). Finally, and since hemolysis may occur during the time over which vessel relaxation is assessed, cell-free hemoglobin was measured in vessel bioassay chambers at the end of each experiment. No significant correlation between the concentration of cell-free heme and the extent of inhibition of MNO-dependent vasodilation was observed (data not shown). Collectively, these data suggest that using the described protocols, neither microparticles nor cell-free hemoglobin contributed to the enhanced NO-scavenging observed by stored RBCs.
Figure 4. Testing a role for microparticles or cell-free hemoglobin in enhanced stored RBC dependent inhibition of NO-signaling.
Panel A and B show representative histograms for microparticle analysis in 42d RBC by FACS before and after washing respectively. Events in upper left quadrant represent microparticles and upper right quadrant intact RBC. Panel C shows changes in microparticle levels during storage before (■) and after (□) washing. Data are mean ± SEM (n=3). *P<0.02 relative to before washing by t-test.
Effects of RBC storage on nitrite metabolism
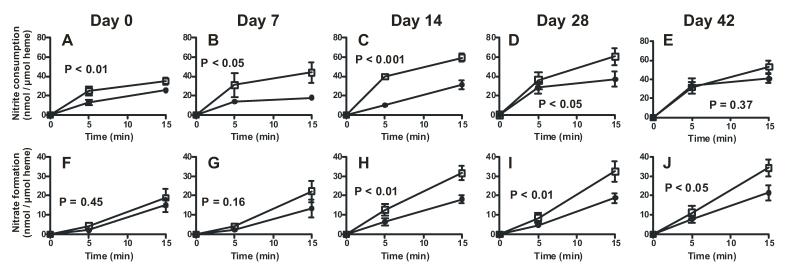
Nitrite was added to RBC of different storage ages pre-equilibrated at either 21% O2 or 2% O2. Fig 5 shows how RBC storage affects nitrite consumption and nitrate formation profiles. Key results from these experiments were i) consistent with our previous data [31], nitrite consumption was faster under deoxygenated compared to oxygenated conditions with freshly isolated (day 0) RBC (Fig 5A), ii) Fig 5A-E show that as a function of storage time, the difference between low and high oxygen-dependent nitrite consumption kinetics increases up to 14d, and then decreases thereafter, with no oxygen-dependent difference evident with 42d old RBCs. iii) comparison of how storage age affected nitrite consumption at each pO2 showed that at low O2, rates of nitrite consumption were higher with RBC stored for 14d or longer compared to 0d RBCs (supplementary figure 2). Similarly, significant increases in rates of nitrite consumption under oxygenated conditions were observed by stored RBCs compared to d0 RBC (supplementary Fig 3 and Fig 6A), iv) Both low and high oxygen conditions resulted in similar rates of nitrate formation from nitrite with d0 RBC (Fig 5F). As a function of storage time however, an oxygen dependent effect became apparent with nitrate formation being greater at the lower relative to the higher oxygen tensions with RBCs stored for 14d or longer (Fig 5F-J). Importantly however nitrate formation increased as a function of storage time at both high and low oxygen conditions; Fig 6B shows rates at 21% O2.
Figure 5. RBC storage age effects on nitrite metabolism.
Nitrite (100μM) was added to RBC (5%Hct) of different storage ages in PBS + 0.1% BSA pre-equilibrated at either 21% O2 (●) or 2% O2 (□). At 5 and 15 min after nitrite addition, RBC were pelleted and nitrite and nitrate levels measured in the extra-erythrocytic fractions. Data show nitrite consumption (Panels A-E) and nitrate formation (Panels F-J) normalized to heme. P-values indicated on each panel demonstrate effects of pO2 on nitrite consumption or nitrate formation rates determined by 2-way ANOVA. Calculated oxygen fractional saturations for 2% O2 condition for storage times 0, 7, 14, 28, 42 were respectively 0.27, 0.46, 0.56, 0.66, 0.69.
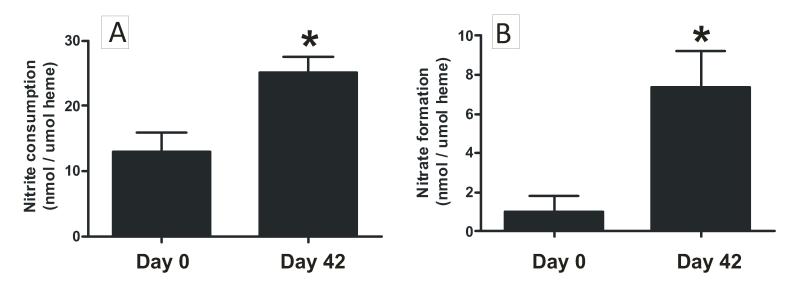
Figure 6. RBC storage increases nitrite consumption and nitrate formation under oxygenated conditions.
Nitrite was added to RBC at 21% O2 as described in Figure 5 legend and nitrite consumption (panel A) and nitrate formation (panel B) measured at 5 min. Data show mean ± SEM (n=4-5). * P < 0.03 by t-test.
Effects of RBC storage on nitrite-dependent vasodilation
Nitrite dependent vasodilation was assessed in the presence and absence of RBCs and at 21% and 1% O2 Fig 7A-B show representative tension vs. time traces. Consistent with previous studies, nitrite alone is a more potent vasodilator at low oxygen tensions [28, 35, 36]. RBCs inhibited nitrite dependent vasodilation when oxygenated, but promoted vasodilation when deoxygenated. Fig 7C shows how storage duration affected RBC-dependent inhibition or potentiation of nitrite-dependent vessel relaxation. Low oxygen tensions resulted in a relative potentiation of nitrite dependent vasodilation compared to high oxygen tensions consistent with a deoxygenation-dependent nitrite-reductase activity of RBCs. This relative effect was not affected by RBC storage age.
Figure 7. Effects of RBC storage on nitrite-dependent vasodilation of rat thoracic aorta.
Nitrite (3 and 10μM) was added to aortic baths containing Krebs buffer with or without RBC (0.3%Hct) of different storage ages and at 1% O2 or 21% O2. Panel A-B show representative vessel tension traces. Arrows indicate nitrite addition. Panel C shows percent change in vasodilation elicited by RBC relative to nitrite alone at 1% O2 (□) and 21% O2 (■) and storage age. Data are normalized to the concentration of RBC heme in each vessel bioassay chamber. A positive value denotes inhibition, and negative value indicates potentiation of nitrite-dependent vasodilation. Data are mean ± SEM (n = 3-6). #P < 0.005 by 2-way ANOVA for effects of oxygen.
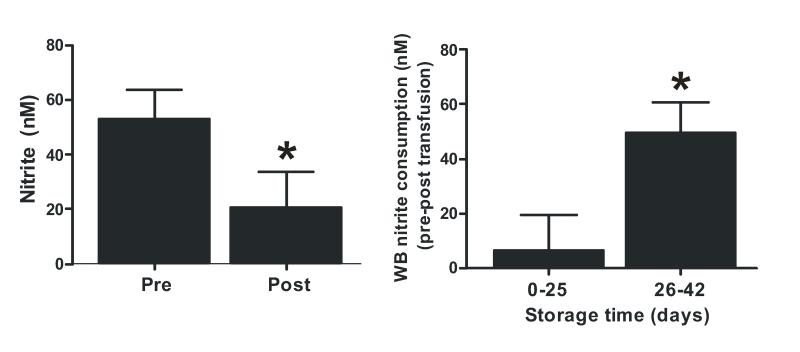
Effects of RBC storage and transfusion on nitrite levels in trauma patients
Fig 8A shows that whole blood nitrite levels decreased significantly after transfusion of stable trauma patients with 1pRBC unit. Each unit was transfused occurred over ~60-90min and resulted in ~60% decrease in blood nitrite levels. Moreover, the decrease in nitrite levels was greater when transfusion occurred with RBC stored for >25d compared to <25d (Fig 8B). Since the same volume (1unit, ~500ml) of RBC were transfused, these data also suggest that decreased nitrite levels are not due to resultant dilution of blood (~10%).
Figure 8. RBC transfusion decreases circulating nitrite levels in stable Trauma patients.
Panel A: Whole blood nitrite was measured pre- and post transfusion with 1 unit of RBC in stable trauma patients. Data show mean ± SEM (n =31), *P<0.01 by paired t-test. Panel B: Data were separated by the storage age of transfused RBC (0-25d, n = 14 or 26-42 d, n = 17) and changes in whole blood nitrite (pre – post transfusion) plotted for these groups. *P<0.02 by unpaired t-test.
Discussion
Decreased NO-signaling has emerged as a central mechanism underlying the RBC storage lesion [37-40]. This hypothesis is further supported by data presented herein, that show RBC-dependent scavenging of NO and nitrite is enhanced during storage. We propose this contributes to an overall deficit in NO-bioavailability and predisposition to circulatory dysfunction and tissue inflammatory injury, two features emblematic of transfusion related toxicity [4, 6, 41].
Decreased RBC dependent stimulation of NO-signaling and increased NO-scavenging (secondary to hemolysis and increased microparticle formation) with stored cells, have been reported to contribute to a deficit in NO-bioactivity [9-11, 13, 42, 43]. How the storage time dependent changes in these processes translate to the clinical manifestation of storage lesion related toxicity is difficult to ascertain directly. Retrospective studies indicate adverse effects are observed with transfusion of RBC stored for >14d [44]. Consistent with this, ATP release and subsequent activation of eNOS is diminished with deficits observed with 7-14d old RBC and increased hemolysis and microparticle formation occurs in a similar storage time dependent manner with significant increases and effects on NO-scavenging evident after ~14-21d (Fig 1 and 4 and [13]). Reported deficits in SNOHb bioactivity occur much faster and within a day of storage [9, 10] leading to suggestions that the RBC lesion occurs even earlier than expected or that there is no direct cause and effect relationship in this case [45]. How intact stored RBC-interactions with NO are affected by storage has received little attention. Data presented herein indicate that significant increases in NO-scavenging occur with RBC stored for 42d but with trends being observed by 14-28d. How intact RBC affect NO-homeostasis is an important consideration since i) biophysical and biochemical properties of the RBC are key in controlling NO-diffusion [12, 19, 20] and slowing NO-scavenging by encapsulated heme, and ii) RBC storage leads to smaller, less flexible cells with greater surface area to volume ratio [4]. The latter is predicted to decrease NO-diffusion barriers and thus increase NO-scavenging rates (see below). To test this hypothesis, we utilized in vitro competition kinetic assays to determine the rate of NO-dioxygenation by RBCs stored for different times. The kRBC/kHb increased ~40-fold over 42 days which was reflected by an enhanced inhibition of NO-dependent vasodilation. Importantly, experimental conditions that allowed exclusion of contributions from storage dependent hemolysis and microparticles were used, although we note that whereas washing decreased microparticles to control levels, complete removal was not achieved precluding a definitive exclusion of these species in contributing to increased NO-dioxygenation kinetics, or inhibited MNO-dependent vasodilation observed. Although the rate constant of RBC-dependent scavenging of NO increased during storage, this remained significantly less than cell-free hemoglobin (by ~20 fold, Fig 2B). In a unit of stored RBC with a 60% Hct however, the concentration of erythrocytic hemoglobin is ~150-fold greater compared to cell-free hemoglobin (~12mM RBC heme vs. ~80μM for cell-free hemoglobin, see Fig 1B). Since the rate of NO-scavenging is the product of the rate constant and concentration, this suggests that intact stored RBCs may effectively contribute to NO-scavenging. According to current blood banking guidelines, at least 75% of transfused RBC are expected to be present in the circulation 24h post-transfusion. This suggests that RBC with enhanced NO-scavenging properties may be persistent for many hours post transfusion. The potential role for intact RBC dependent effects on NO-function is further underscored by the fact that we used leukodepleted RBCs, which may decrease (although not completely prevent) hemolysis and microparticle formation. We also note that at least in trauma patients, evidence for storage lesion dependent toxicity still remains despite the use of leukodepleted pRBCs [46]. It is difficult to ascertain the relative contributions of erythrocytic vs. microparticle vs. cell-free hemoglobin towards inhibited NO-signaling, since steady states of the above species are unlikely in a transfusion setting. Many factors will affect the concentrations of intact RBCs, microparticles and cell free hemoglobin, including RBC turnover, variable hematocrit, number of pRBC units transfused, and the balance between formation of microparticles and hemolysis vs. clearance of these species (e.g. cell-free hemoglobin is cleared by haptoglobin and CD163-dependent pathways)[8, 47, 48]. The calculation presented above also does not account for differences in NO-scavenging that occurs due to the RBC-free zone. Irrespective of the relative contributions, we posit that intact RBCs are important partners with microparticles and cell-free hemoglobin, that collectively inhibit endogenous NO-dependent signaling. In addition our data suggest that therapeutic strategies aimed at limited NO-scavenging should also target intact RBC-dependent NO-reactions.
How storage increases RBC dependent scavenging of NO is unclear. Storage changes RBC size, shape, membrane permeability and extracellular diffusion all of which can regulate the kinetics of NO-scavenging [12, 14, 17-20, 49]. Moreover, the rate limit may be controlled by distinct factors as exemplified by recent experimental and modeling studies that indicate the unstirred layer is the primary component regulating NO-scavenging by RBC, whereas membrane permeability is the key controlling factor for NO-reactions with microparticles [12]. Although it has been proposed that an additional factor is an intracellular diffusion barrier due to the very high hemoglobin concentration [21, 49], this has been challenged on experimental and theoretical grounds [14]. In addition, the fact that NO consumption by RBC is zero order with intracellular hemoglobin concentration (which was actually the initial observation that prompted the concept of an extracellular diffusion barrier) is also inconsistent with this conclusion[17]. During storage the RBC size decreases in certain storage conditions [50-52]. The half life of NO (t1/2) in the presence of RBCs has been modeled previously[17] according to equation 4:
| Equation 4 |
where N = number of cells/ml, DNO = aqueous diffusion constant for NO, and r = cell radius. According to this equation as the cell radius increases; there will be proportional decrease in the NO half life or an increase in RBC scavenging kinetics. This is consistent with the concept of an unstirred layer, where at non-limiting hemoglobin concentrations, the rate of NO-dioxygenation is proportional to the RBC surface area [17]. During storage RBC change from a biconcave disc to echinocytes with spiculated membranes (see supplementary Figure 1). Although the cells become smaller, these morphologic changes are likely to increase the total surface area comparing like volumes of old RBC to young RBC, which would result in increased NO-consumption rates. Another consideration is that the internal cell volume of RBC decreases during storage [4]. However, hemoglobin concentration does not change (not shown) indicating that during storage, hemoglobin packaging is altered. How this may affect the rate of NO-dioxygenation can be derived from equations 5-6.
| (equtaion 5) |
| (equtaion 6) |
where the ratio of internal volume of the cell (Vi) to total volume occupied by all cells (Vt) is equal to the number of cells/ml (N) multiplied by the volume of a sphere (where r is expressed in cm).
Substituting equation 4, the half-life of NO in the presence of RBC with different volumes is shown in equation 7:
| (equtaion 7) |
Thus, if we compare a suspension of RBC of equal total hemoglobin concentration but different sizes similar to our experimental protocol to assess reaction kinetics, the rate of NO consumption by the smaller cells will be greater in proportion to the square of the ratio of the radii. Thus, RBCs with smaller volumes provide another possible explanation for why stored RBC reacted faster with NO. A limitation of this modeling is that it assumes spherical RBC while storage induces a spectrum of cells with different shapes and sizes. Thus, it is likely that individual RBCs possess heterogenous NO-scavenging potential in stored pRBC units.
We also investigated the effects of storage time on reactions between RBCs and nitrite under oxygenated and deoxygenated conditions to test whether deoxyhemoglobin dependent nitrite-reduction and NO-formation is altered. Storage age had no effect on how RBCs modulated nitrite-dependent relaxation. At high oxygen tension, RBCs of all storage ages inhibited, whereas at low oxygen, no change or a potentiation of relaxation was observed reflecting the balance between NO-scavenging by oxyheme and nitrite-reduction to NO by deoxyheme [28]. However, as shown in Fig 3, 42d old RBCs inhibited NO-dependent vasodilation more than 0d RBCs. Since nitrite-dependent vasodilation occurs via NO-formation [28] we speculate that to observe no storage age effect on nitrite-dilation at low oxygen, increased rates of nitrite-reduction must be occurring to counter increased NO-scavenging. Indeed, increased rates of nitrite consumption were observed at low oxygen by RBCs stored for greater than 14d (see supplementary Figure 2). Another consideration in these experiments is that the initial rates of nitrite reduction follow a bell-shape dependence with respect to hemoglobin oxygen fractional saturation, with maximal rates achieved close to the P50 [25, 53]. Due to decreasing RBC P50, calculated oxygen fractional saturations for RBC in nitrite consumption studies (at 2% O2) indicated that maximal rates were observed with 14d RBC which were also closest to the P50 (see Figure 5 legend). These data underscore the fact that the precise RBC oxygen fractional saturation, a product of local pO2 and RBC oxygen affinity will be key determinants of how quickly nitrite may be reduced to NO.
RBC storage also increased rates of nitrate formation under oxygenated conditions (Fig 5F-J), which was paralleled by increased rates of nitrite consumption (Figure 6). A higher concentration of oxyhemoglobin (due to lower P50) may also underlie increased nitrate formation kinetics observed with stored RBC at 2% oxygen (supplementary Fig 3). Oxyhemoglobin mediated nitrite oxidation to nitrate is an autocatalytic reaction that proceeds via intermediate formation of hydrogen peroxide and nitrogen dioxide radical [26]. Therefore antioxidant enzymes or reductants that can scavenge these reactive species slow down nitrite oxidation[26]. Oxidative damage concomitant with loss of antioxidants has been reported in RBCs during storage[54]. Thus the increase in nitrite oxidation to nitrate observed with stored RBCs is likely the result of lower endogenous reductant systems. The lower blood nitrite levels in stable trauma patients transfused with an older vs. younger pRBC unit may reflect increased rates of nitrite consumption by deoxygenated and/or oxygenated RBC. However, since P50 is decreasing with RBC storage (implying greater oxyhemoglobin concentrations) we speculate that RBC-dependent oxidation of nitrite predominates over reduction pathways.
In summary, we show that in addition to increased NO-scavenging by hemolysed cell-free hemoglobin and microparticles, changes to the RBC itself that occur during storage can lead to decreased NO-bioavailability. We posit that the combination of stored RBC dependent increased NO-scavenging and nitrite oxidation dispose tissues to inflammatory stress during and following transfusion and underscores the potential therapeutic benefit for NO-repletion strategies with recent studies [43] showing promise in this concept.
Supplementary Material
Acknowledgements
RPP is a coinventor on a patent for use of nitrite salts for the treatment of cardiovascular conditions. We thank Scott Tanner for assistance with flow cytometry studies.
Funding: This study was supported by grants from the National Institutes of Health (HL095468 to RPP. JW, SB, CA131653 to JRL)
Footnotes
Abbreviations Footnote: RBCs (Red Blood Cells), pRBC (packed red blood cells), Hb (hemoglobin), oxyHb (oxyhemoglobin), metHb (methemoglobin), NO (nitric oxide), P50 (pO2 (mmHg) at which hemoglobin is half saturated with oxygen), PBS (Phosphate buffered saline), BSA (bovine serum albumin), SpNO (spermine nonoate), MNO (mahmanonoate), BHT (butylated hydroxytoluene)
References
- 1.Biffl WL, Moore EE, Offner PJ, Ciesla DJ, Gonzalez RJ, Silliman CC. Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: abrogation by poststorage washing but not prestorage leukoreduction. J Trauma. 2001;50:426–431. doi: 10.1097/00005373-200103000-00005. discussion 432. [DOI] [PubMed] [Google Scholar]
- 2.Zallen G, Offner PJ, Moore EE, Blackwell J, Ciesla DJ, Gabriel J, Denny C, Silliman CC. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg JA, McGwin G, Jr., Griffin RL, Huynh VQ, Cherry SA, 3rd, Marques MB, Reiff DA, Kerby JD, Rue LW., 3rd Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. The Journal of trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. discussion 282-274. [DOI] [PubMed] [Google Scholar]
- 4.van de Watering L. Red cell storage and prognosis. Vox Sang. 2011;100:36–45. doi: 10.1111/j.1423-0410.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 5.Kor DJ, Van Buskirk CM, Gajic O. Red blood cell storage lesion. Bosn J Basic Med Sci. 2009;9(Suppl 1):21–27. doi: 10.17305/bjbms.2009.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandromme MJ, McGwin G, Jr., Weinberg JA. Blood transfusion in the critically ill: does storage age matter? Scand J Trauma Resusc Emerg Med. 2009;17:35. doi: 10.1186/1757-7241-17-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlaar AP, Straat M, Juffermans NP. The relation between aged blood products and onset of transfusion-related acute lung injury. A review of pre-clinical data. Clin Lab. 2011;57:267–272. [PubMed] [Google Scholar]
- 8.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med. 2011;39:2478–2486. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azarov I, Liu C, Reynolds H, Tsekouras Z, Lee JS, Gladwin MT, Kim-Shapiro DB. Mechanisms of slower nitric oxide uptake by red blood cells and other hemoglobin-containing vesicles. J Biol Chem. 2011;286:33567–33579. doi: 10.1074/jbc.M111.228650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280:39024–39032. doi: 10.1074/jbc.M509045200. [DOI] [PubMed] [Google Scholar]
- 15.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 16.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR., Jr. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Samouilov A, Lancaster JR, Jr., Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 19.Joshi MS, Ferguson TB, Jr., Han TH, Hyduke DR, Liao JC, Rassaf T, Bryan N, Feelisch M, Lancaster JR. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci U S A. 2002;99:10341–10346. doi: 10.1073/pnas.152149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocyte consumption of nitric oxide: competition experiment and model analysis. Nitric Oxide. 2001;5:18–31. doi: 10.1006/niox.2000.0328. [DOI] [PubMed] [Google Scholar]
- 21.Sakai H, Sato A, Masuda K, Takeoka S, Tsuchida E. Encapsulation of concentrated hemoglobin solution in phospholipid vesicles retards the reaction with NO, but not CO, by intracellular diffusion barrier. J Biol Chem. 2008;283:1508–1517. doi: 10.1074/jbc.M707660200. [DOI] [PubMed] [Google Scholar]
- 22.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 23.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 24.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantu-Medellin N, Vitturi DA, Rodriguez C, Murphy S, Dorman S, Shiva S, Zhou Y, Jia Y, Palmer AF, Patel RP. Effects of T- and R-state stabilization on deoxyhemoglobin-nitrite reactions and stimulation of nitric oxide signaling. Nitric Oxide. 2011;25:59–69. doi: 10.1016/j.niox.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293:H2565–2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 29.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, and Gladwin, M. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelderman MP, Yazer MH, Jia Y, Wood F, Alayash AI, Vostal JG. Serial oxygen equilibrium and kinetic measurements during RBC storage. Transfus Med. 2010;20:341–345. doi: 10.1111/j.1365-3148.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- 31.Vitturi DA, Teng X, Toledo JC, Matalon S, Lancaster JR, Jr., Patel RP. Regulation of nitrite transport in red blood cells by hemoglobin oxygen fractional saturation. Am J Physiol Heart Circ Physiol. 2009;296:H1398–1407. doi: 10.1152/ajpheart.01303.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberg JA, Maclennan PA, Vandromme-Cusick MJ, Angotti JM, Magnotti LJ, Kerby JD, Rue LW, 3rd, Barnum SR, Patel RP. Microvascular response to red blood cell transfusion in trauma patients. Shock. 2012;37:276–281. doi: 10.1097/SHK.0b013e318241b739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang JD, Jr., Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doyle MP, Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 35.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007;292:H3072–3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 36.Ormerod JO, Ashrafian H, Maher AR, Arif S, Steeples V, Born GV, Egginton S, Feelisch M, Watkins H, Frenneaux MP. The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovasc Res. 2011;89:560–565. doi: 10.1093/cvr/cvq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds JD, Hess DT, Stamler JS. The transfusion problem: role of aberrant S-nitrosylation. Transfusion. 2011;51:852–858. doi: 10.1111/j.1537-2995.2011.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. 2011;51:859–866. doi: 10.1111/j.1537-2995.2011.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011;51:867–873. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 45.Winslow RM, Intaglietta M. Red cell age and loss of function: advance or SNO-job? Transfusion. 2008;48:411–414. doi: 10.1111/j.1537-2995.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 46.Weinberg J, McGwin G, Jr, Griffin R, Huynh V, Cherry S, Marques M, DA R, Kerby JD, Rue L., III Age of transfused blood: An independent predictor of mortality despite universal leukoreduction. Journal of Trauma, Injury, Infection and Critical Care. 2008 doi: 10.1097/TA.0b013e31817c9687. In Press. [DOI] [PubMed] [Google Scholar]
- 47.Alayash AI. Haptoglobin: Old protein with new functions. Clin Chim Acta. 2011;412:493–498. doi: 10.1016/j.cca.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 49.Sakai H, Sato A, Sobolewski P, Takeoka S, Frangos JA, Kobayashi K, Intaglietta M, Tsuchida E. NO and CO binding profiles of hemoglobin vesicles as artificial oxygen carriers. Biochim Biophys Acta. 2008;1784:1441–1447. doi: 10.1016/j.bbapap.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Henkelman S, Dijkstra-Tiekstra MJ, de Wildt-Eggen J, Graaff R, Rakhorst G, van Oeveren W. Is red blood cell rheology preserved during routine blood bank storage? Transfusion. 2010;50:941–948. doi: 10.1111/j.1537-2995.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 51.Lawrence AC, Bevington JM, Young M. Storage of blood and the mean corpuscular volume. J Clin Pathol. 1975;28:345–349. doi: 10.1136/jcp.28.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veale MF, Healey G, Sparrow RL. Effect of additive solutions on red blood cell (RBC) membrane properties of stored RBCs prepared from whole blood held for 24 hours at room temperature. Transfusion. 2011;51(Suppl1):25S–33S. doi: 10.1111/j.1537-2995.2010.02960.x. [DOI] [PubMed] [Google Scholar]
- 53.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr., Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rael LT, Bar-Or R, Ambruso DR, Mains CW, Slone DS, Craun ML, Bar-Or D. The effect of storage on the accumulation of oxidative biomarkers in donated packed red blood cells. The Journal of trauma. 2009;66:76–81. doi: 10.1097/TA.0b013e318191bfe0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.