Abstract
Endothelin-2 [ET-2; also known as vasoactive intestinal contractor (VIC), in rodents] differs from endothelin-1 (ET-1) by only two amino acids, and unlike the third isoform, endothelin-3 (ET-3), it has the same affinity as ET-1 for both ETA and ETB receptors. It is often assumed that ET-2 would mimic the actions of the more abundant ET-1 and current pharmacological interventions used to inhibit the ET system would also block the actions of ET-2. These assumptions have focused research on ET-1 with ET-2 studied in much less detail. Recent research suggests that our understanding of the ET family requires re-evaluation. Although ET-2 is very similar in structure as well as pharmacology to ET-1, and may co-exist in the same tissue compartments, there is converging evidence for an important and distinct ET-2 pathway. Specifically is has been demonstrated that ET-2 has a key role in ovarian physiology, with ET-2-mediated contraction proposed as a final signal facilitating ovulation. Furthermore, ET-2 may also have a pathophysiological role in heart failure, immunology and cancer. Comparison of ET-2 versus ET-1 mRNA expression suggests this may be accomplished at the level of gene expression but differences may also exist in peptide synthesis by enzymes such as endothelin converting enzymes (ECEs) and chymase, which may allow the two pathways to be distinguished pharmacologically and become separate drug targets.
LINKED ARTICLES
This article is part of a themed section on Endothelin. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2013.168.issue-1
Keywords: cardiovascular system, ovarian, immunology, cancer, endothelin-2, endothelin-1, ETA, ETB, vasoactive intestinal contractor
Introduction
The existence of a peptidic endothelium-derived constricting factor was originally proposed in 1985 by Hickey et al. (Hickey et al., 1985) but was identified and named endothelin (ET) by Yanagisawa and colleagues in 1988 (Yanagisawa et al., 1988). The peptide was renamed ET-1 to reflect the subsequent discovery a year later of human genes encoding two further members of the family, ET-2 and ET-3 (Inoue et al., 1989). The ET peptides act by activating two G-protein couples receptors, ETA and ETB. (Davenport, 2002; Alexander et al., 2011) ET-3 is the only endogenous isoform that distinguishes between the two receptors, having the same affinity at ETB as ET-1 and ET-2 but, at physiological concentrations, has much lower or little affinity for ETA. The established dogma is that ET-2 has the same affinity for both ETA and ETB receptors as ET-1. It is often assumed that ET-2 would mimic the actions of the more abundant peptide, and current pharmacological interventions used to inhibit the ET system would also block the actions of ET-2. These assumptions have focused research on ET-1, leaving the ET-2 isoform much less studied. Recent research suggests that our understanding of the ET family requires re-evaluation. Evidence is emerging that ET-2 has a key role in ovarian physiology, as ET-2-mediated contraction has been proposed as a final mechanical signal facilitating ovulation (Ko et al., 2006; Palanisamy et al., 2006). The peptide may also have a pathophysiological role in heart failure, immunology and in cancer. Furthermore, ET-2 knockout mice display a phenotype distinct from that of ET-1 or ET-3 (Chang et al., 2009). Our aim is to discuss the mechanisms whereby ET-2 mediates its actions in separate spatiotemporal ‘compartments’ to ET-1, and to review the evidence that suggests a third and separate signalling pathway to the established ET-1/ETA and ET-3/ETB pathways.
ETA/ET-1 pathway and disruption in knockout mice
ET-1 is the most abundant isoform in the human cardiovascular system and widely expressed, for example in endothelial cells lining every blood vessel (Plumpton et al., 1996). The peptide is continuously released to cause vasoconstriction, by predominantly interacting with ETA receptors present on smooth muscle, contributing to the maintenance of normal physiological vascular tone in men and mice (Haynes and Webb, 1994; Yanagisawa et al., 1998). In contrast, mRNA encoding ET-3 has not been detected in human endothelial cells, and there is low expression of ETB receptors on underlying smooth muscle (Davenport et al., 1995; Maguire and Davenport, 1995). Genetically modified mice demonstrated the importance of ET-1/ETA/ECE pathway in cardiovascular and craniofacial development. ET-1-deficient homozygous mice die at birth of respiratory failure secondary to severe craniofacial and cardiovascular abnormalities. In agreement, ETA receptor and ECE-1 knockout mice have similar morphological abnormalities (Hosoda et al., 1994; Clouthier et al., 1998; Yanagisawa et al., 1998).
ETB/ET-3 pathway and disruption in knockout mice
ET-1 does not cross the blood–brain barrier under normal physiological conditions (Johnstrom et al., 2005) and the presence of ET-1 and ECE have been detected in some neurons, glial cells and vascular endothelium indicating synthesis within the CNS (Davenport et al., 1998). However, ET-3 is the major isoform in the rat brain (Matsumoto et al., 1989) mainly restricted to neurons and glia, and being particularly abundant in the neostriatum, hypothalamic nuclei, hippocampus and Purkinje cells of the cerebellum and medulla oblongata (Giaid et al., 1991). The ETB subtype accounts for about 90% of the ET receptors in human normal cerebral cortex. The receptor is localized to neuronal regions particularly levels III and IV of the cortex, consistent with ET-3 functioning as a neuropeptide (Harland et al., 1995). The ET-3/ETB pathway also has a crucial role in development, but importantly homozygote ETB knockout mice exhibit a different and non-overlapping phenotype to ETA-deficient animals. ETB knockout mice survive for up to 8 weeks but display aganglionic megacolon as a result of absence of ganglion neurons, together with a pigmentary disorder in their coats. This is caused by the failure of enteric nervous system precursors and neural crest-derived epidermal melanoblasts to colonize the intestine and skin. ET-3 knockouts display an identical phenotype (Kurihara et al., 2001).
Effect of ET-2 deletion in knockout mice
A global knockout of ET-2 in mice revealed a third, distinct phenotype to the phenotype of ETA/ET-1 and ETB/ET-3 knockout mice (Chang et al., 2009). ET-2-deficient mice exhibited growth retardation with changes in energy homeostasis characterized by hypoglycaemia and ketonaemia. Mice were hypothermic leading to juvenile lethality. Changes in lung morphology and function were observed suggesting a key role in the development of the pulmonary system. This may be significant since the major current clinical application of ET receptor antagonists such as bosentan and ambrisentan is in the treatment of pulmonary arterial hypertension.
ET-2 and vasoactive intestinal contractor (VIC)
ET-2 differs from ET-1 by two amino acids with Trp6 and Leu7 substitutions for Leu6 and Met7, as shown in Figure 1. But this usually results in little or no effect on the binding affinity to ET receptors when compared with ET-1. This sequence difference is comparatively small and the solution structure of ET-2 is similar to ET-1 (Arvidsson et al., 1998). Other mammals including monkeys, cats, dogs and cattle have the same sequence for ET-2 as humans do. An exception are mice and rats where the equivalent peptide to ET-2 is also called VIC, so named because of its contractile activity in the ileum and was originally identified in the mouse genome (Saida et al., 1989; Bloch et al., 1991). VIC differs from ET-1 by three amino acids (Asn4, Trp6 and Leu7), as shown in Figure 1 (Ishida et al., 1989; Saida et al., 1989; 2002). These authors describe a synthetic pathway for VIC that resembles that of ET-2, from a 175 amino acid precursor protein (prepro-VIC) via a 38 amino acid big VIC, which is cleaved to give the mature peptide and a C-terminal fragment (Saida et al., 2000). Only limited studies have been carried out using VIC. Where detailed comparisons have been made VIC is reported to have the same affinity for both ETA and ETB receptors. For example, the ability of ETs to compete for [125I]-VIC binding to ETB receptors present on guinea pig longitudinal smooth muscle was compared, and no difference was found between unlabelled VIC, ET-1, ET-2 or ET-3. In addition, all four peptides were equally potent with a similar contractile response (Yoshinaga et al., 1992). VIC was equipotent with ET-1 and ET-2 in other ETB preparations (Cozza et al., 1992; Lin and Lee, 1992) as well as predominantly ETA tissues (Douglas and Hiley, 1990; Schoeffter and Randriantsoa, 1993). However, discrepancies have been reported in cultured human smooth muscle cells where VIC had a lower potency than ET-1 (Iwashima et al., 1997).
Figure 1.
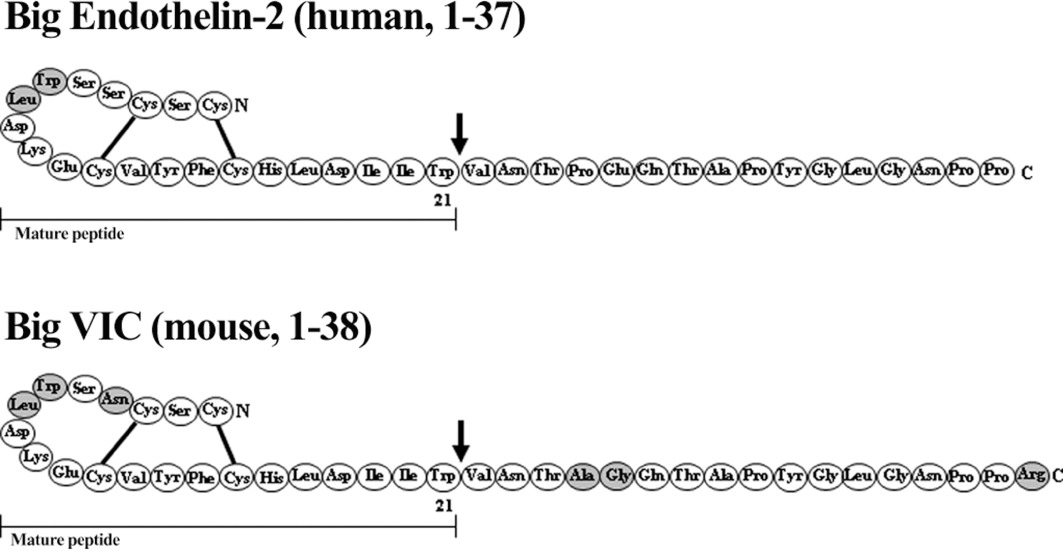
Amino acid sequence of Big ET-2, Big VIC and their respective mature peptides with disulfide bridges (—). Differences in amino acid sequence to Big-ET-1 and ET-1 are shaded in grey.
Mouse and rat genome databases retain the use of EDN2 for the gene encoding mouse VIC. This nomenclature is adopted by the majority of authors reporting on studies in rats and mice and is used in this review.
To date, the physiological and pathophysiological role of ET-2 has been less extensively studied than ET-1 partly because of the difficulty in distinguishing between the structure and function of the two peptides. Although not as ubiquitous as ET-1, mRNA encoding ET-2 has been detected in a range of human tissues (Table 1). Both ET-2 mRNA (O'Reilly et al., 1993a) and Big ET-2 have been detected in the cytoplasm of endothelial cells (Howard et al., 1992). In functional assays, ET-2 is also as potent a vasoconstrictor as ET-1 is (Maguire and Davenport, 1995), implying that on release from the endothelium, ET-2 may also contribute to physiological tone. The biologically inactive precursor Big ET-2 circulates in human plasma at a higher level than Big ET-1 does (Matsumoto et al., 1994), although the level of the mature peptide at ∼0.9 pmol·L−1 is about one-fifth that of mature ET-1 level (Davenport and Maguire, 2006).
Table 1.
ET-2 distribution in humans
| Distribution in humans | Form | Technique: | Reference |
|---|---|---|---|
| Heart | |||
| Left atria, left ventricle | ET-2 | radioimmunoassay, reverse phase high performance liquid chromatography | Plumpton et al. (1993) |
| Left ventricle | ET-2 mRNA | rt-PCR | O'Reilly et al. (1993b) |
| Left ventricle | ET-2 mRNA | rt-PCR | Plumpton et al. (1996) |
| Lung | |||
| Submucosal glands | proendothelin-2 | elisa, immunohistochemistry | Marciniak et al. (1992) |
| Renal | |||
| Medulla, cortex | ET-2 mRNA | rt-PCR | Karet and Davenport (1996) |
| Vasculature | |||
| Saphenous vein, mesenteric artery/vein, internal mammary artery | proendothelin-2 | elisa, immunohistochemistry | Howard et al. (1992) |
| Reproductive organs | |||
| Endometrium, myometrium, placenta | ET-2 mRNA | rt-PCR | O'Reilly et al. (1992; 1993b) |
| Endometrium | ET-2 | radioimmunoassay, reverse phase high performance liquid chromatography | Cameron et al. (1993) |
| Ovarian follicles | mRNA | Northern blot with hybridization using 32P-labeled cDNA probes | Palanisamy et al. (2006) |
| Corpus luteum | mRNA | rt-PCR | Klipper et al. (2010) |
| Gastrointestinal | |||
| Colon | preproET-2 mRNA | rt-PCR | McCartney et al. (2002) |
Regulation of ET genes
Regulation of ET peptide gene EDN1 has been reviewed in detail by Stow et al. (2011). The complexity of ET-1 regulation is a testament to the significant role of ETs in homeostasis. Briefly, most studies suggest that ET-1 regulation occurs at the transcription level, as various factors such as red wine, leptin and hypoxia act through transcription factors that interact with cis-acting elements in the EDN1 promoter regions (Lee et al., 1990; Oliver et al., 1991; Corder et al., 2001; Chao et al., 2007; Belaidi et al., 2009). In addition, post-transcriptional modification, microRNA regulation and epigenetics may also explain tissue specific regulation of ET-1 expression (Vallender and Lahn, 2006; Rodriguez-Pascual et al., 2008; Yeligar et al., 2009). In contrast, very little is known about EDN2, except that it is mapped to chromosome 1p34 (Arinami et al., 1991). Although the evidence is sparse and incomplete, there are distinct mechanisms that regulate ET-2 production. For example, Tokito et al. (1991) demonstrated an inhibitory effect of epidermal growth factor in the production of ET-2 but not ET-1 in renal adenocarcinoma cells. Lambert et al. (1998; 2000) explored compounds regulating expression of ET-2 mRNA and secretion of the peptide in human renal adenocarcinoma cell lines. TNF-α as well as forskolin and dibutyryl cAMP (cell-permeable cAMP analog) stimulated concentration-dependent increases in ET-2 mRNA synthesis and release. The authors showed these were two distinct signalling pathways via TNF receptor-1 linked induction and a cAMP mechanism. As will be discussed in the following themed sections, various factors such as hypoxia and progesterone have been shown to affect ET-2 expression. However, the transcriptional control and mature peptide conversion underlying these factors are far from clear.
Does ET-2 have a separate synthesis from other ET peptides?
In addition to ET receptor antagonists, a second developing therapeutic strategy is to block the detrimental actions of ETs in pathophysiological conditions by inhibition of the ET converting enzymes (ECE-1 and ECE-2), which have been established as the major synthetic pathway for ET-1. As the affinity of ET-2 is similar to ET-1 for both subtypes, it is important to understand whether this therapeutic strategy is also likely to block synthesis of ET-2 or if alternative synthetic pathways exist.
The ET-2 synthetic pathway is initially similar to that of ET-1 and ET-3, as shown in Figure 2. The first product of EDN2 gene transcription is preproendothelin-2. Following the removal of the signal sequence, the resulting proendothelin is cleaved by the enzyme furin, to yield the 37 amino acid peptide, Big ET-2. It is now well established that ET-1 is synthesized from Big ET-1 by an unusual hydrolysis of Trp21-Val22 bond, rather than the more frequent Arg-Arg or Arg-Lys as in other peptide precursors (Turner and Murphy, 1996). Synthesis is catalysed by a membrane-bound metalloprotease, ECE-1. ET-1 is also synthesized by ECE-2, a second metalloprotease with 59% homology with bovine ECE-1 but with an acidic pH for optimum activity in cleaving Big ET-1 to the mature peptide compared with a neutral range for ECE-1 (Emoto and Yanagisawa, 1995). ECE-2 also cleaves other peptides, including the vasodilator bradykinin. At least four isoforms exist for both enzymes that differ in their cellular and tissue distribution, possibly reflecting different functions.
Figure 2.
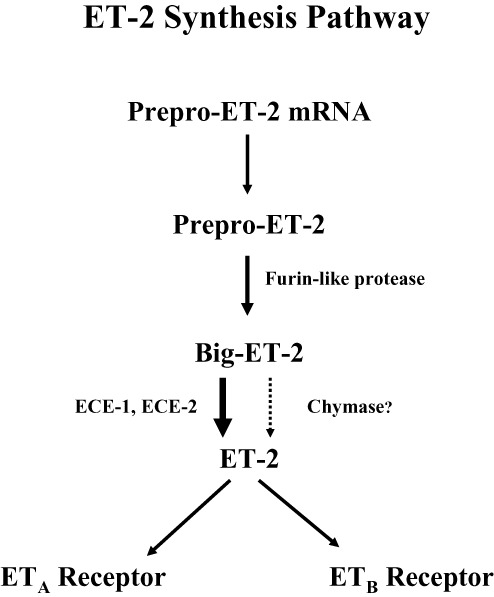
Synthesis pathway of ET-2 from mRNA through intermediates to mature peptide.
Controversy currently exists in the literature about the efficiency of Big ET-2 conversion by ECE in vitro compared with in vivo studies. Although Big ET-2 shares the same scissile bond (Trp21-Val22) with Big ET-1, the rate of conversion of Big ET-2 by ECE-1 and ECE-2 was less than 10% of Big ET-1 when compared in the same assay (Emoto and Yanagisawa, 1995).
Although there are exceptions, a pattern is emerging using purified, cloned or expressed enzyme that Big ET-2 is poorly converted (in contrast to native enzyme) with no activity against Big ET-3 (D'Orléans-Juste et al., 1991; Telemaque and D'Orléans-Juste, 1991; Shimada et al., 1994; Turner and Murphy, 1996). The reason for this difference in specificity is that the scissile bond and the terminal sequence in Big ET-3 are different from the other big ET precursors. A zinc-dependent endopeptidase, Kell, with sequence similarity to ECE-1, efficiently cleaved Big ET-3 at Trp21-Val22, suggesting the existence of a specific converting enzyme for the synthesis of ET-3 (Lee et al., 1999; Clapéron et al., 2005). Big ET-l and Big ET-2 also differ in key residues 27–29, which are crucial for efficient ECE-1 and ECE-2 activity. However, these results contrast with functional studies using native tissues that suggest Big ET-2 is converted mainly by ECE, and can be blocked as expected by dual inhibitors of ECE and neutral endopeptidase (NEP). Big ET-2 caused contraction of human isolated bronchus comparable with Big ET-1 and with the maximal response equivalent to that of ET-2, demonstrating full conversion of this precursor peptide (Yap et al., 2000). The authors suggested ECE activity within human bronchi either has equal specificity for both precursors, or that there are multiple enzymes within this tissue, at least one having the same specificity for Big ET-2. In support of this hypothesis, CGS 26393 and CGS 26303 non-peptide dual ECE and neutral NEP inhibitors with equivalent activity on ECE-1 and ECE-2 inhibits contractions to all three Big ETs (Yap et al., 2000).
Detailed studies in chronically instrumented rats in vivo have shown that Big ET-2 is efficiently converted to produce cardiovascular actions in a similar way to Big ET-1, and can be blocked as expected by phosphoramidon (Gardiner et al., 1992). These regional haemodynamic studies were carried out in conscious, chronically-instrumented rats to avoid the confounding effects of anaesthetics. Gardiner et al. (1992) demonstrated that administration of Big ET-2 in rats causes dose-dependent pressor, bradycardic, renal and, particularly, mesenteric vasoconstrictor effects. Phosphoramidon given as a 10 µmol·kg−1 i.v. bolus completely abolished the pressor, bradycardic and hindquarters vasoconstrictor actions of Big ET-2. Phosphoramidon had no direct effects on the actions of ET-2, consistent at least in rats with Big ET-2 being converted by a phosphoramidon-sensitive enzyme such as ECE. Similar results were obtained in the anaesthetized, ganglion-blocked rat, where intravenous bolus doses of Big ET-2 were compared with Big ET-1. The onset of the vasopressor effect was more rapid (5–6 min) for the latter than that of Big ET-2 (10–13 min) but the actions of both peptides were blocked as expected by phosphoramidon but not thiorphan (Mattera et al., 1993). In renal adenocarcinoma cells producing high levels of ET-2, the presence of ECE was confirmed by sequencing (Yorimitsu et al., 1992; 1995).
In the anaesthetized rabbit, ETA antagonist BQ-123 markedly reduced the pressor responses to ET-1, Big ET-1, ET-2 and Big ET-2, whereas blockade of ETB receptors with BQ-788 sharply potentiated the responses to ET-1, ET-2 and Big ET-1, but not to Big ET-2 (Gratton et al., 2000). Systemically administered Big ET-1, but not Big ET-2, induced a phosphoramidon-sensitive increase in immunoreactive ET. The authors suggested that ETB receptors played a significant limiting role on the pressor responses to Big ET-1. This receptor does not modulate the haemodynamic properties of Big ET-2 as it is poorly converted in the pulmonary or systemic circulation in anaesthetized rabbits. This is supported by human in vivo studies done by Bohm et al. (2003), which showed that ETB functions as a clearance receptor and may modulate ETA-mediated vasoconstriction by altering the plasma concentration of ET-1.
An alternative pathway for ET synthesis has been proposed, which involves chymase, a serine protease present in mast cells within the blood vessel wall that can cleave the Tyr31–Gly 32 peptide bond of both Big ET-1 (Maguire et al., 2001; Fecteau et al., 2005; D'Orléans-Juste et al., 2008) and Big ET-2 (Takai et al., 1998) to generate the intermediate peptides ET-1(1–31) and ET-2(1–31), which is in turn converted to the mature peptides by an as yet uncharacterized mechanism. Interestingly, Takai et al. (1998) found the conversion of Big ET-2 could be blocked by chymase inhibitors but not that of Big ET-1 in monkeys. Because the number of mast cells increases with cardiovascular disease (Marone et al., 1995) and chymase is highly expressed in these cells, it remains unclear if this synthesis pathway of either peptide is of pathological significance. Degranulation, which can occur under pathophysiological conditions, may release chymase into interstitial spaces with the potential to convert Big ETs.
The clinical strategy for inhibition of ECE is represented by SLV-306 (Daglutril), an orally active mixed enzyme inhibitor of both ECE and NEP. Therefore SLV-306 has the added advantage of blocking the NEP dependent breakdown of beneficial vasodilator atrial natriuretic factor. A Phase II trial was completed in 2010 by Solvay for the treatment of essential hypertension and congestive heart failure (Bayes et al., 2003; Tabrizchi, 2003; Dickstein et al., 2004). In agreement with animal studies of Gardiner et al. (1992), oral administration of SLV 306 to healthy volunteers resulted in a significant dose-dependent rise in circulating Big ET-1 levels. In addition, there was an expected parallel increase in the C-terminal fragment as mature ET-1 is also metabolized by NEP (Seed et al., 2012). Because Big ET-2 circulates in human plasma at higher levels than Big ET-1 (Matsumoto et al., 1994), it would be crucial to know whether SLV-306 also inhibits conversion to ET-2.
Distribution of ET-2 mRNA and mature peptide
Genetic techniques such as RT-PCR can provide robust assays for distinguishing between mRNA encoding ET-1 and ET-2 but a limitation is that tissue must be homogenized and it is not usually possible to identify cell types. Furthermore, the presence of mRNA in a specific tissue merely confirms that the tissue is capable of synthesizing mRNA. It does not shed light on post-transcriptional modification, storage and functional secretion or give information about peptide levels. The majority of immunoassays (RIA, elisa) that detect mature ET cross-react with both ET-1 and ET-2. To overcome this problem, HPLC can be used to separate the mature isoforms and their precursors, followed by RIA with antisera raised to the carboxy-terminal hexapeptide ET (15–21). This terminal is common to all three isoforms and detects the levels of the mature peptides but not the precursors in the resulting fraction. This method also relies on homogenates. A third strategy for cellular localization has been to use antisera raised against the variable C-terminal sequence of the precursor, Big ET-2(31–37), which does not cross react with Big ET-1 or Big ET-3. These antisera have been used to identify cells expressing Big ET-2-like immunoreactivity, and therefore these cells have the potential to synthesize and release the mature peptide.
In rats quantitative RT-PCR revealed expression of mRNA encoding ET-1 in all of 16 organs examined from rats (Uchide et al., 2000). In contrast, ET-2 expression was more restricted, and had an organ specific distribution being detected in heart, lung, ovary, stomach and intestine. Particularly high levels were in ovary (but not testes) and all regions of the intestine (duodenum, jejunum, ileum, colon and rectum) (De La Monte et al., 1995; Takizawa et al., 2005). In contrast, the levels in cerebellum and cerebrum were below the level for mRNA detection (Saida et al., 2002). Interestingly, ET-2 mRNA measured by QPCR was much higher than ET-1 mRNA in rat and mice pituitary gland and medulla oblongata (Masuo et al., 2003). Moderate to low levels of ET-2 mRNA expression were observed in other brain regions compared with ET-1 suggesting a possible role in the pituitary. Similar to its wide distribution in rats, ET-2 expression in humans has been localized to different tissues, as shown in Table 1. ET-2 has been identified in human heart, lung, kidney, vasculature, intestine and ovaries (Howard et al., 1992; Marciniak et al., 1992; Plumpton et al., 1993; Karet and Davenport, 1996; Palanisamy et al., 2006).
Unexplained ET-2 pharmacology
Saturation binding studies comparing radiolabelled [125I]-ET-2 with [125I]-ET-1 provide ‘gold standards’ and accurate measures of affinity constants (KD) and receptor densities (Bmax). In cultured rat aortic smooth muscle cells that express ETA receptors, the KD values of [125I]-ET-2 and [125I]-ET-1 were identical at 0.1 nM and their Bmax values were 46 and 54 fmol per million cells respectively. Saturation curves were monophasic with the same association rate constants (Kobs) of 0.01 min−1 for [125I]-ET-2 with [125I]-ET-1 and similar slow dissociation rates (Roubert et al., 1991). Intriguingly however, while [125I]-ET-2 and [125I]-ET-1 bound with the same affinity at ETA receptors in Swiss 3T3 fibroblasts, ET-2 dissociated at a dramatically faster rate than ET-1 did, which may be important in determining pharmacological responses to ET-2 in vivo (Devesly et al., 1990). It is not yet known whether kinetics of binding varies between cell types and can distinguish responses to the two isoforms in vivo.
Consistent with the results from transformed cultured cells, saturation experiments on tissue sections from the vascular smooth muscle layer of human aorta and coronary artery yielded similar constants (Bacon and Davenport, 1996). These experiments demonstrated monophasic binding with Hill slopes close to unity with KD values for [125I]-ET-2 of 0.71 ± 0.17 nM and 0.43 ± 0.10 nM for [125I]-ET-1. No differences were detected in competition binding studies using the ETA selective antagonists FR139317. Inhibition of [125I]-ET-2 and [125I]-ET-1 binding in both arteries was monophasic with KD values in the same range. In the human vasculature at least, there is no evidence for any differences in competition by antagonists such as BQ123 for labelled ET-2, and this is supported by vasoconstrictor studies in human isolated vessels (Maguire and Davenport, 1995) as well as animal preparations such as rat portal vein (Guimaraes et al., 1992).
However, in other functional studies, there are reports of possible differences in the pharmacology of ET-2 compared with ET-1, which have not been explained and warrant further investigation. Bertelsen et al. (1992) reported in spinal cord of the rat using ligand binding assays, inhibition constants for ET-1 and ET-2 were found to be similar whereas in the cerebral cortex the values of ET-2 were found to be at least 6 times higher than ET-1. This suggests that in the rat brain, ET-2 has a higher affinity for ET receptors. In agreement, a proportion of neurones cultured from embryonic rat cerebral cortex responded selectively to ET-2 with an increase in intracellular calcium (Morton and Davenport, 1992). It is unclear whether these responses are due to differences in affinity compared with ET-1 or other factors such as the stability of peptides in this assay. Both ET-1 and ET-2 induced concentration-dependent tonic contraction of the human isolated oviduct by acting on ETA receptors with similar EC50 values (Jankovic et al., 2009). However, ET-1 also caused a concentration-dependent inhibition of rhythmic contractions of the oviduct, with a similar potency whereas surprisingly ET-2 had no effect.
Neutrophils are important effector cells of tissue injury in several pathological conditions such as immune complex induced inflammation and tissue injury. In immune complex stimulated neutrophils, ET-1 but not ET-2 potentiated production of leukotriene B4 (Paulino et al., 2006). Similarly, ET-2 had differential actions to ET-1 on neutrophil migration and intracellular calcium (Elferink and de Koster, 1996). At low concentrations, ET-2 caused a chemotactic stimulation of migration whereas ET-1 induced a chemokinetic stimulation of migration. At higher concentrations, ET-2 inhibited N-formyl-methionyl-leucyl-phenylalanine (fMLP)-activated migration with both actions dependent on extracellular Ca2+. Unlike ET-1, which caused an increase in cytosolic free Ca2+ at a concentration that stimulated migration, ET-2 caused a measurable increase of cytosolic free Ca2+ at a concentration that did not stimulate migration (Elferink and de Koster, 1996). Comparisons have also been made between ET-2 and other agonists such as sarafotoxin 6b (S6b), where Gardiner et al. (1990) showed S6b is a more potent vasoconstrictor than ET-2.
ET-2 in cardiovascular system
ETs were originally discovered as a vasoconstrictor peptide produced by endothelial cells (Yanagisawa et al., 1988). Not only is ET-1 the most studied isoform in cardiovascular disease, but it is one of the most powerful vasoconstrictors (Poli et al., 1991b; Davenport and Maguire, 2006). But studies suggest ET-1 may not be the only ET isoform that regulates the vasculature. Genetic studies have identified a specific EDN2 polymorphism that is associated with essential hypertension (Sharma et al., 1999) whereas other studies have shown associations between rare ET-1 and ET-2 polymorphisms with lower diastolic blood pressures (Brown et al., 2000). In the kidney, human ET-2 (hET-2) transgenic rat studies suggests that overexpression of ET-2 contributes to the development of glomerulosclerosis (Hocher et al., 1996). Apart from its effects on the vasculature, ET-2 is also a strong positive inotrope on the human myocardium (Poli et al., 1991a). Although the benefits of ET antagonism in initial experimental models of heart failure showed promise, clinical trials have thus far not demonstrated the desired outcomes (Sakai et al., 1996; Borgeson et al., 1998; Kalra et al., 2002; Packer et al., 2005). More encouragingly, ET antagonism with bosentan and ambrisentan have been shown to improve exercise tolerance and slow down clinical deterioration in pulmonary hypertension (Channick et al., 2001; Rubin et al., 2002; Barst et al., 2006). Most of the biological evidence that led to these trials stemmed from knowledge in ET-1 signalling. There are, however, notable differences between ET-1 and ET-2 expression in the cardiovascular system. In rat left coronary artery ligation cardiac failure models, ET-1 expression in the failing heart is almost four times higher than in control hearts (Kakinuma et al., 1999). In contrast, ET-2 expression is 88% lower in the failing heart compared with control hearts. Interestingly, this reciprocal differential expression is reversed in hypoxic rat cardiomyocyte cell cultures, where ET-1 expression is decreased by 38% and ET-2 expression is increased by almost fourfold compared with controls. Recently, hET-2 rat studies showed that elevated ET-2 expression increases myocardial fibrosis, particularly in the presence of diabetes (Liefeldt et al., 2010). Long-term ET-2 over expression in diabetic rats also resulted in marked coronary artery wall hypertrophy compared with diabetic wild-type rats. However, Liefeldt et al. (2010) acknowledges that in their experiments, ET-1 levels were higher in hET-2 transgenic rats compared with wild-type, thus ET-1 may be mediating, at least in part, the remodelling seen in hET-2 rats.
In addition to its effects on the myocardium, ET-2 may also modulate cardiac conduction. In a single centre trial with a small number patients, Nagai et al. (2007) showed that a significant association of the A985G polymorphism of the ET-2 gene with increased incidence of atrial fibrillation (the most common cause of tachyarrhythmia) in patients with hypertrophic cardiomyopathy. The functional consequences are not yet known of the A985G polymorphism but the authors speculate that transcription and translation of EDN2 could be altered. The cumulative evidence from these studies suggests ET-2 may modulate vascular tone and also exert control on vascular tissue morphology and remodelling.
ET-2 in ovarian physiology
The ET pathway has been shown to play an important role in ovarian physiology (Meidan and Levy, 2007). Most studies have focused on ET-1 as an important regulator of follicular development and terminating luteal phase (Haq et al., 1996; Flores, 2000; Shirasuna et al., 2006). However, expression of ET-2 is actually higher than ET-1 expression in ovaries (Uchide et al., 1999). Over the last 5 years, there has been growing interest in the role ET-2 may have in ovulation (Ko et al., 2006). Using a rat ovarian gene expression database and subsequent RT-PCR, Ko et al. (2006) found that both ECE-1 and ET-2 were transiently expressed in rat ovaries during ovulation. In particular, ET-2 is expressed in the granulosa cells of periovulatory follicles and not during other stages of follicular development. In mice, induced superovulation results in a dramatic increase in ET-2 mRNA expression (Palanisamy et al., 2006). At 11 h after gonadotropin was given, ET-2 expression surged and quickly declined by 13 h, which coincided with the time of follicular rupture. However, ET-2 expression seems to be different in bovine ovaries compared with rat or mice ovaries (Klipper et al., 2010). Using gonadotropin induced superovulation, Klipper et al. (2010) found that follicular ET-2 mRNA expression transiently increased at 4 h post-gonadotropin, although this result was not statistically significant. Otherwise, ET-2 expression remained low prior to follicular rupture. Interestingly, a surge in ET-2 expression occurred at 60 h post-gonadotropin, corresponding to early corpus luteum phase, which was not previously demonstrated in rat or mice studies. How do we reconcile the differences between bovine and rats/mice ovarian ET-2 expression? Firstly, the discrepancy may be due to interspecies differences. Secondly, it was not clear if the investigators in the rat/mice studies measured ET-2 expression beyond follicular rupture. Thirdly, the difference in approaches used to achieve superovulation induction may account for the differences in ovulation mechanism. This leads to the next question: what causes the alteration in ovarian ET-2 expression?
Studies on progesterone receptor knockout (PRKO) mice have shown that progesterone signalling is critical in ovulation, as PRKO mice not only failed to ovulate but also resulted in accumulation of mature unruptured follicles (Lydon et al., 1995; Chappell et al., 1997). PRKO mice lose the transient ET-2 expression that wild-type mice have when superovulation is induced by gonadotrophin (Palanisamy et al., 2006; Kim et al., 2009). In cultured granulosa cells, forskolin and phorbol 12-myristate 13 acetate (PMA) stimulation has been used to mimic and model the in vivo pre-ovulation LH surge that normally results in increased progesterone receptor expression. Palanisamy et al. (2006) found that forskolin and PMA stimulation in cultured granulosa cells also resulted in increased ET-2 expression 12 h post-exposure, similar to the in vivo gonadotropin challenge. Blocking progesterone receptor function in these cells inhibits this ET-2 expression response to forskolin and PMA. However, a subsequent in vivo study found that indomethacin and a PR antagonist, RU-486 had no effect on ET-2 expression in superovulated mice (Na et al., 2008). This study found that it was hypoxia that ultimately determines follicular ET-2 expression in the peri-ovulatory phase. Continuing on this theme, it has been suggested that signal transduction between progesterone receptor and ET-2 expression is due to a set of hypoxia-inducible factors (HIFs) that regulate cell transcriptional responses to hypoxia (Kim et al., 2009). Blocking progesterone induced HIF gene expression with echinomycin results in reduced ET-2 expression in granulosa cells. The conflicting results from different studies may be explained by the inadequate and inaccurate modelling of in vivo conditions with cultured cells, but may also be a reflection that progesterone signalling and hypoxia reflect different convergent pathways on ET-2 expression during ovulation (Na et al., 2008).
Although the mechanism of follicular rupture and ovulation are still undetermined, ET-2 has been shown to cause contraction of the smooth muscle layer surrounding each follicle (Ko et al., 2006). Intriguingly, this smooth muscle layer is absent at the site of impending follicular rupture, which suggests that the follicle may rupture at this point because of the relatively weak surface tension during muscle contraction (Walles et al., 1990; Ko et al., 2006). The concept that mechanical contraction is a key step in ovulation is relatively new. But this is consistent with the finding that tezosentan, a mixed ET receptor antagonist, reduces ovulation and results in mature unruptured follicles despite gonadotropin superovulation. This is reminiscent of the PRKO mice phenotype, supporting the idea that ET-2 expression is a downstream signal of the progesterone pathway. ET-2-mediated contraction may play a key role in follicular rupture, without which normal ovulation cannot proceed. In particular, it has been shown that ETB antagonism with BQ-788 alone results in 85% reduction in ovulation in mice PMSG/hCG induced superovulation (Palanisamy et al., 2006). This may be because ETB expression is much higher than ETA expression in the ovary. However, these in vivo results differ from in vitro isometric tension experiments done by Bridges et al. (2010), which has shown that blocking ETA with BQ123 reduces the rat ovarian contractile response to ET-2. In addition, these authors have not been able to repeat the same reduction of ovulation with ET antagonists (BQ123, BQ788) in rats as those observed from earlier mice studies.
While the ET pathway in ovarian physiology remains to be elucidated, it is clear that ET-2 has a temporal and spatial role in ovulation. Furthermore, ET-2 has also been shown to be a potent oviductal constrictor (Al-Alem et al., 2007). Better understanding of ET signalling in ovulation is exciting as this is a potential drug target that may ultimately treat causes of infertility such as luteinized unruptured follicle syndrome.
ET-2 in immunology
ET-2 has recently been recognized as a chemokine and modifier of leukocyte function, which may have far reaching implications for cancer and inflammatory diseases (Wright et al., 1994; Elferink and de Koster, 1996; Grimshaw et al., 2002a). It was first shown that ET-2 is a chemoattractant for neutrophils at low concentrations, but inhibits neutrophil migration at high concentrations (Elferink and de Koster, 1996). Subsequent studies demonstrated that ET-2 is also chemotactic for macrophages and the effects are mediated via ETB receptors (Grimshaw et al., 2002b). Using Boyden chambers, Grimshaw et al. (2002b) showed that the chemotactic response of macrophages to ET-2 is as strong as their response to CCL2, a well-established inflammatory chemokine. Moreover, they found that ET-2 exerts chemotactic response in a similar way to CCL2, through the MAPK pathway (Yen et al., 1997; Grimshaw et al., 2002b). But unlike CCL2, ET-2 exerts no chemotactic effects on monocytes. Interestingly, chemotaxis of macrophages towards both CCL2 and ET-2 are inhibited by hypoxia as it induces MAPK phosphatase 1 resulting in dephosphorylation of MAPK (Negus et al., 1998; Turner et al., 1999; Grimshaw and Balkwill, 2001; Grimshaw et al., 2002b). In addition to its role as a chemoattractant, ET-2 increases the activation of macrophages (Grimshaw et al., 2002b). As discussed in the following cancer section, the specific effects of ET-2 on macrophage function may have particular consequences for cancer pathogenesis and represents a tangible target for cancer therapy.
ET-2 in cancer pathogenesis
There is growing evidence that ETs are involved in the pathogenesis of many types of cancer (Nakamuta et al., 1993; Kojima and Nihei, 1995; Bagnato et al., 1999; 2011; Eberle et al., 2002). ET-1 has been the main ET studied in cancer, as it has been shown to affect multiple stages of disease development including proliferation, angiogenesis, migration, invasion and apoptosis (Grant et al., 2003; Bhalla et al., 2009). But actually, it was ET-2 and not ET-1 that was first isolated in renal adenocarcinoma cell lines (Ohkubo et al., 1990). More recently, growing interest in ET-2 within cancer pathogenesis started when it was reported that ET-2 expression was increased in human breast cancer (Grimshaw et al., 2002a). ET-2 is not normally expressed in breast tissue, but Grimshaw et al. (2002a) found that hypoxic areas of breast tumour from HTH-K mice were particularly associated with increased ET expression. To elucidate this further, they simulated hypoxic conditions in 0.1% oxygen for 24 h in HTH-K mice cell culture and human breast tumour cell lines, and found that ET-2, ETA and ETB expressions were all increased. As ET-1 and ET-3 expression were unaffected by hypoxia, it is likely that ET-2 is the main subtype responsible for the increased ET expression in hypoxic tumours. Elevated ET-2 is also reported in other cancers such as those found in the skin, where ET-2 expression is three times higher in basal cell carcinoma compared with normal skin (Tanese et al., 2010). Moreover, the authors showed that ET-2 expression was a downstream effect of hedgehog pathway signalling.
But what is the significance of enhanced ET-2 expression in cancer? In hypoxic conditions, addition of an ETB antagonist, BQ788 decreases the survival of HTH-K mice and human breast cell lines (Grimshaw et al., 2002a). Moreover, addition of ET-2 to these cells under hypoxia increases cell survival rates, but the protective effect are reversed by BQ-788. Consistent with these in vitro studies, HTH-K mice given 5 days of BQ-788 had reduced tumour size and increased necrotic area in breast tumour compared with controls.
In addition to regulating cancer cell apoptosis, ET-2 has been shown to affect invasive and metastatic potential (Grimshaw et al., 2004). Similar to the chemotaxis of macrophages towards ET-2 discussed earlier, cancer cells are also chemotactic and more invasive in the presence of ET-2. Using the matrigel invasion assay, Grimshaw et al. (2004) showed that breast cancer cell lines show a chemotaxis in a dose response relationship towards ET-2. This effect was dependent on activation of both ETA and ETB receptors since BQ-123 (ETA antagonist) or BQ-788 (ETB antagonist) when used on their own reduced invasion of cells but did not abolish it completely. Again, similar to macrophage chemotaxis, the chemotactic response of cancer cells is mediated via the MAPK pathway downstream of ETB, and inhibition of MEK1/2 blocked this response. Interestingly, co-culture of breast tumour cells with macrophages increased the invasive potential of tumour cells when compared with tumour cells alone (Grimshaw et al., 2004; Hagemann et al., 2004). This effect was further accentuated when ET-1 or ET-2 was added to the culture. Importantly, the increased invasiveness of ET-2 was shown to be due to increases in expression of MMP-2 and MMP-9 by macrophages and a marked increase in invasion of tumour cells. Up-regulated ET-2 expression in hypoxic tumours may explain why these tumours have been shown to have higher invasive potential and worse prognosis than their well-oxygenated counterparts (Watanabe, 1975). However, these results were not replicated in BCC cell cultures, where ET-2 and ET antagonism with BQ-123 or BQ-788 had no effects on cell proliferation and invasiveness (Tanese et al., 2010). It is tempting, however to infer from in vitro studies that show ET-2 having an effect on regulating apoptosis and invasion, as these fit conveniently to in vivo studies where there are observed increases of ET and ETB expression from in situ carcinoma to metastatic cancer cells, coinciding with the increasing invasiveness of progressive cancer stages.
How do we incorporate the factors of hypoxia, ET-2 expression, cancer cell apoptosis, chemotasis and invasion? The trigger may well start with hypoxia, which is a consequence of the tumour cells reaching a critical volume where diffusion becomes the limiting factor for growth (Harris, 2002). This can be interpreted as a switch for the cancer to move on to the next stage of development, leading to increased ET-2 expression, which prevents tumour apoptosis and increases their ET receptor expression. In the meantime, the tumour may release chemokines that attract monocytes and macrophages to the tumour site (Grimshaw et al., 2002b). Once in the tumour, areas of hypoxia expressing ET-2 attract macrophages to the site, and further migration is inhibited. These macrophages are activated and express matrix metalloproteinases to prepare for local tissue invasion. In the presence of activated macrophages, tumour cells with up-regulated ET receptors then chemoattract towards ET-1 and ET-2, and invades tissue and metastasize distally. In terms of therapeutics, the difference between ET-1 and ET-2 may ultimately not matter if the targets are the ET receptors that are under investigation in trials such as a Phase III randomized placebo controlled trial on Zibotentan in prostate cancer. Nonetheless, it is still important to appreciate the different contributions of the ET subtype on cancer pathogenesis as it may provide novel targets for upstream intervention.
Conclusion
The three ET genes are thought to have evolved from a common ancestor by exon and gene duplications (Bloch et al., 1989; Landan et al., 1991; Saida et al., 2000) with ET-1 and ET-3 mediating their actions particularly in development through separate pathways. Although ET-2 is very similar in structure as well as pharmacology and may co-exit in the same tissue compartments as ET-1, there is converging evidence for an important and distinct ET-2 pathway. It is clear from knockout mice and studies in cardiovascular and ovarian system that ET-2 does not simply amplify or duplicate the physiological actions ET-1 and may have a separate pathophysiological role in conditions such as cancer. Comparison of ET-2 versus ET-1 mRNA suggests this may be accomplished at the level of gene expression, but differences may also exist in synthesis by enzymes such as ECE and chymase, which may allow the two pathways to be distinguished pharmacologically and become separate drug targets.
Acknowledgments
We thank the British Heart Foundation for their support: Grant Number PG/09/050/27734.
Glossary
- ECE-1
endothelin converting enzyme 1
- ECE-2
endothelin converting enzyme 2
- ELISA
enzyme-linked immunosorbent assay
- HIFs
hypoxia-inducible factors
- MEK1/2
mitogen-activated protein kinase kinase
- MMP-2
matrix metallopeptidase 2
- MMP-9
matrix metallopeptidase 9
- NEP
neutral endopeptidase
- PRKO
progesterone receptor knockout
- PMA
phorbol 12-myristate 13 acetate
- VIC
vasoactive intestinal contractor
Conflicts of Interest
None.
References
- Al-Alem L, Bridges PJ, Su W, Gong MC, Iglarz M, Ko C. Endothelin-2 induces oviductal contraction via endothelin receptor subtype A in rats. J Endocrinol. 2007;193:383–391. doi: 10.1677/JOE-07-0089. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinami T, Ishikawa M, Inoue A, Yanagisawa M, Masaki T, Yoshida MC, et al. Chromosomal assignments of the human endothelin family genes: the endothelin-1 gene (EDN1) to 6p23-p24, the endothelin-2 gene (EDN2) to 1p34, and the endothelin-3 gene (EDN3) to 20q13.2-q13.3. Am J Hum Genet. 1991;48:990–996. [PMC free article] [PubMed] [Google Scholar]
- Arvidsson K, Nemoto T, Mitsui Y, Ohashi S, Nakanishi H. The solution structure of human endothelin-2 a 1H-NMR and CD study. Eur J Biochem. 1998;257:380–388. doi: 10.1046/j.1432-1327.1998.2570380.x. [DOI] [PubMed] [Google Scholar]
- Bacon CR, Davenport AP. Endothelin receptors in human coronary artery and aorta. Br J Pharmacol. 1996;117:986–992. doi: 10.1111/j.1476-5381.1996.tb15292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, et al. Expression of endothelin 1 and endothelin a receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–727. [PubMed] [Google Scholar]
- Bagnato A, Loizidou M, Pflug BR, Curwen J, Growcott J. Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol. 2011;163:220–233. doi: 10.1111/j.1476-5381.2011.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barst RJ, Langleben D, Badesch D, Frost A, Lawrence EC, Shapiro S, et al. Treatment of pulmonary arterial hypertension with the selective endothelin-a receptor antagonist sitaxsentan. J Am Coll Cardiol. 2006;47:2049–2056. doi: 10.1016/j.jacc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2003;25:317–340. [PubMed] [Google Scholar]
- Belaidi E, Joyeux-Faure M, Ribuot C, Launois SH, Levy P, Godin-Ribuot D. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J Am Coll Cardiol. 2009;53:1309–1317. doi: 10.1016/j.jacc.2008.12.050. [DOI] [PubMed] [Google Scholar]
- Bertelsen GA, Rebello S, Gulati A. Characteristics of endothelin receptors in the cerebral cortex and spinal cord of aged rats. Neurobiol Aging. 1992;13:513–519. doi: 10.1016/0197-4580(92)90080-h. [DOI] [PubMed] [Google Scholar]
- Bhalla A, Haque S, Taylor I, Winslet M, Loizidou M. Endothelin receptor antagonism and cancer. Eur J Clin Invest. 2009;39(Suppl. 2):74–77. doi: 10.1111/j.1365-2362.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- Bloch KD, Eddy RL, Shows TB, Quertermous T. cDNA cloning and chromosomal assignment of the gene encoding endothelin 3. J Biol Chem. 1989;264:18156–18161. [PubMed] [Google Scholar]
- Bloch KD, Hong CC, Eddy RL, Shows TB, Quertermous T. cDNA cloning and chromosomal assignment of the endothelin 2 gene: vasoactive intestinal contractor peptide is rat endothelin 2. Genomics. 1991;10:236–242. doi: 10.1016/0888-7543(91)90505-9. [DOI] [PubMed] [Google Scholar]
- Bohm F, Pernow J, Lindstrom J, Ahlborg G. ETA receptors mediate vasoconstriction, whereas ETB receptors clear endothelin-1 in the splanchnic and renal circulation of healthy men. Clin Sci (Lond) 2003;104:143–151. doi: 10.1042/CS20020192. [DOI] [PubMed] [Google Scholar]
- Borgeson DD, Grantham JA, Williamson EE, Luchner A, Redfield MM, Opgenorth TJ, et al. Chronic oral endothelin type a receptor antagonism in experimental heart failure. Hypertension. 1998;31:766–770. doi: 10.1161/01.hyp.31.3.766. [DOI] [PubMed] [Google Scholar]
- Bridges PJ, Jo M, Al-Alem L, Na G, Su W, Gong MC, et al. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction. Reprod Fertil Dev. 2010;22:780–787. doi: 10.1071/RD09194. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Sharma P, Stevens PA. Association between diastolic blood pressure and variants of the endothelin-1 and endothelin-2 genes. J Cardiovasc Pharmacol. 2000;35:S41–S43. doi: 10.1097/00005344-200000002-00010. [DOI] [PubMed] [Google Scholar]
- Cameron IT, Plumpton C, Champeney R, Van Papendorp C, Ashby MJ, Davenport AP. Identification of endothelin-1, endothelin-2 and endothelin-3 in human endometrium. J Reprod Fertil. 1993;98:251–255. doi: 10.1530/jrf.0.0980251. [DOI] [PubMed] [Google Scholar]
- Chang I, Bramall A, Baynash AG, Mcinnes RR, Stewart DJ, Yanagisawa M. 2009. Genetic study of ET-2 in mice. Eleventh International Conference on Endothelin, 15.
- Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- Chao HH, Hong HJ, Liu JC, Lin JW, Chen YL, Chiu WT, et al. Leptin stimulates endothelin-1 expression via extracellular signal-regulated kinase by epidermal growth factor receptor transactivation in rat aortic smooth muscle cells. Eur J Pharmacol. 2007;573:49–54. doi: 10.1016/j.ejphar.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lydon JP, Conneely OM, O'Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Clapéron A, Rose C, Gane P, Collec E, Bertrand O, Ouimet T. The Kell protein of the common K2 phenotype is a catalytically active metalloprotease, whereas the rare Kell K1 antigen is inactive. J Biol Chem. 2005;280:21272–21283. doi: 10.1074/jbc.M500100200. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Corder R, Douthwaite JA, Lees DM, Khan NQ, Viseu dos Santos AC, Wood EG, et al. Endothelin-1 synthesis reduced by red wine. Nature. 2001;414:863–864. doi: 10.1038/414863a. [DOI] [PubMed] [Google Scholar]
- Cozza EN, Chiou S, Gomez-Sanchez CE. Endothelin-1 potentiation of angiotensin II stimulation of aldosterone production. Am J Physiol. 1992;262:R85–R89. doi: 10.1152/ajpregu.1992.262.1.R85. [DOI] [PubMed] [Google Scholar]
- D'Orléans-Juste P, Telemaque S, Claing A. Different pharmacological profiles of big-endothelin-3 and big-endothelin-1 in vivo and in vitro. Br J Pharmacol. 1991;104:440–444. doi: 10.1111/j.1476-5381.1991.tb12448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orléans-Juste P, Houde M, Rae GA, Bkaily G, Carrier E, Simard E. Endothelin-1 (1-31): from chymase-dependent synthesis to cardiovascular pathologies. Vascul Pharmacol. 2008;49:51–62. doi: 10.1016/j.vph.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Davenport AP. International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Maguire JJ. Endothelin. Handb Exp Pharmacol. 2006;176:295–329. doi: 10.1007/3-540-32967-6_9. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Kuc RE, Maguire JJ, Harland SP. ETA receptors predominate in the human vasculature and mediate constriction. J Cardiovasc Pharmacol. 1995;26(Suppl. 3):S265–S267. [PubMed] [Google Scholar]
- Davenport AP, Kuc RE, Plumpton C, Mockridge JW, Barker PJ, Huskisson NS. Endothelin-converting enzyme in human tissues. J Mol Histol. 1998;30:359–374. [Google Scholar]
- Devesly P, Phillips PE, Johns A, Rubanyi G, Parker-Botelho LH. Receptor kinetics differ for endothelin-1 and endothelin-2 binding to Swiss 3T3 fibroblasts. Biochem Biophys Res Commun. 1990;172:126–134. doi: 10.1016/s0006-291x(05)80182-2. [DOI] [PubMed] [Google Scholar]
- Dickstein K, De Voogd HJ, Miric MP, Willenbrock R, Mitrovic V, Pacher R, et al. Effect of single doses of slv306, an inhibitor of both neutral endopeptidase and endothelin-converting enzyme, on pulmonary pressures in congestive heart failure. Am J Cardiol. 2004;94:237–239. doi: 10.1016/j.amjcard.2004.03.074. [DOI] [PubMed] [Google Scholar]
- Douglas SA, Hiley CR. Endothelium-dependent vascular activities of endothelin-like peptides in the isolated superior mesenteric arterial bed of the rat. Br J Pharmacol. 1990;101:81–88. doi: 10.1111/j.1476-5381.1990.tb12093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle JR, Fecker LF, Orfanos CE, Geilen CC. Endothelin-1 decreases basic apoptotic rates in human melanoma cell lines. J Invest Dermatol. 2002;119:549–555. doi: 10.1046/j.1523-1747.2002.01848.x. [DOI] [PubMed] [Google Scholar]
- Elferink JG, de Koster BM. The effect of endothelin-2 (ET-2) on migration and changes in cytosolic free calcium of neutrophils. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:130–135. doi: 10.1007/BF00168749. [DOI] [PubMed] [Google Scholar]
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Fecteau MH, Honoré JC, Plante M, Labonté J, Rae GA, Pedro DJ. Endothelin-1 (1-31) is an intermediate in the production of endothelin-1 after big endothelin-1 administration in vivo. Hypertension. 2005;46:87–92. doi: 10.1161/01.HYP.0000170460.24604.23. [DOI] [PubMed] [Google Scholar]
- Flores JA. Gene expression of endothelin-1 in the porcine ovary: follicular development. Biol Reprod. 2000;63:1377–13782. doi: 10.1093/biolreprod/63.5.1377. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Compton AM, Bennett T. Regional hemodynamic effects of endothelin-2 and sarafotoxin-S6b in conscious rats. Am J Physiol. 1990;258:R912–R917. doi: 10.1152/ajpregu.1990.258.4.R912. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, Bennett T. Inhibition by phosphoramidon of the regional haemodynamic effects of proendothelin-2 and -3 in conscious rats. Br J Pharmacol. 1992;107:584–590. doi: 10.1111/j.1476-5381.1992.tb12787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaid A, Gibson SJ, Herrero MT, Gentleman S, Legon S, Yanagisawa M, et al. Topographical localisation of endothelin mRNA and peptide immunoreactivity in neurones of the human brain. Histochemistry. 1991;95:303–314. doi: 10.1007/BF00266781. [DOI] [PubMed] [Google Scholar]
- Grant K, Loizidou M, Taylor I. Endothelin-1: a multifunctional molecule in cancer. Br J Cancer. 2003;88:163–166. doi: 10.1038/sj.bjc.6700750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton JP, Rae GA, Bkaily G, D'Orléans-Juste P. ET(B) receptor blockade potentiates the pressor response to big endothelin-1 but not big endothelin-2 in the anesthetized rabbit. Hypertension. 2000;35:726–731. doi: 10.1161/01.hyp.35.3.726. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Balkwill FR. Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation – a potential mechanism. Eur J Immunol. 2001;31:480–489. doi: 10.1002/1521-4141(200102)31:2<480::aid-immu480>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Naylo RS, Balkwill FR. Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Mol Cancer Ther. 2002a;1:1273–1281. [PubMed] [Google Scholar]
- Grimshaw MJ, Wilson JL, Balkwill FR. Endothelin-2 is a macrophage chemoattractant: implications for macrophage distribution in tumors. Eur J Immunol. 2002b;32:2393–2400. doi: 10.1002/1521-4141(200209)32:9<2393::AID-IMMU2393>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Grimshaw MJ, Hagemann T, Ayhan A, Gillett CE, Binder C, Balkwill FR. A role for endothelin-2 and its receptors in breast tumor cell invasion. Cancer Res. 2004;64:2461–2468. doi: 10.1158/0008-5472.can-03-1069. [DOI] [PubMed] [Google Scholar]
- Guimaraes CL, Calixto JB, Rae GA. Potent constrictor actions of endothelin-1, endothelin-2, and endothelin-3 in rat isolated portal vein. Hypertension. 1992;19(Suppl. 2):S79–S86. doi: 10.1161/01.hyp.19.2_suppl.ii79. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- Haq A, Kayali M, Hammami MM, Jaroudi K, al-Sedairy ST. Immunoreactive endothelin-1, endothelin-2 and big endothelin-1 in follicular fluids of women undergoing ovulation induction for in-vitro fertilization. Hum Reprod. 1996;11:269–273. doi: 10.1093/humrep/11.2.269. [DOI] [PubMed] [Google Scholar]
- Harland SP, Kuc RE, Pickard JD, Davenport AP. Characterization of endothelin receptors in human brain cortex, gliomas, and meningiomas. J Cardiovasc Pharmacol. 1995;26(Suppl. 3):S408–S411. [PubMed] [Google Scholar]
- Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coro- nary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248:C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Hocher B, Liefeldt L, Thone-Reineke C, Orzechowski HD, Distler A, Bauer C, et al. Characterization of the renal phenotype of transgenic rats expressing the human endothelin-2 gene. Hypertension. 1996;28:196–201. doi: 10.1161/01.hyp.28.2.196. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, et al. Targeted and natural (piebald-lethal) mutations of the endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Howard PG, Plumpton C, Davenport AP. Anatomical localization and pharmacological activity of mature endothelins and their precursors in human vascular tissue. J Hypertens. 1992;10:1379–1386. doi: 10.1097/00004872-199211000-00010. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuva Y, Miyauchi T, Goto K, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Nat Acad Sci USA. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Tsujioka K, Tomoi M, Saida K, Mitsui Y. Differential activities of two distinct endothelin family peptides on ileum and coronary artery. FEBS Lett. 1989;247:337–340. doi: 10.1016/0014-5793(89)81365-1. [DOI] [PubMed] [Google Scholar]
- Iwashima A, Kobayashi M, Saida K, Kagamu H, Ohashi S, Arakawa M, et al. Contraction and intracellular calcium-ion elevation of cultured human aortic smooth muscle cells by endothelin-1, vasoactive intestinal contractor (VIC) and the derivatives. In Vitro Cell Dev Biol Anim. 1997;33:751–756. doi: 10.1007/s11626-997-0153-8. [DOI] [PubMed] [Google Scholar]
- Jankovic SM, Jankovic SV, Lukic G, Radonjic V, Cupara S, Stefanovic S. Contractile effects of endothelins on isolated ampullar segment of human oviduct in luteal phase of menstrual cycle. Pharmacol Res. 2009;59:69–73. doi: 10.1016/j.phrs.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Johnstrom P, Fryer TD, Richards HK, Harris NG, Barret O, Clark JC, et al. Positron emission tomography using 18F-labelled endothelin-1 reveals prevention of binding to cardiac receptors owing to tissue-specific clearance by ET B receptors in vivo. Br J Pharmacol. 2005;144:115–122. doi: 10.1038/sj.bjp.0706064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma Y, Miyauchi T, Kobayashi T, Yuki K, Maeda S, Sakai S, et al. Myocardial expression of endothelin-2 is altered reciprocally to that of endothelin-1 during ischemia of cardiomyocytes in vitro and during heart failure in vivo. Life Sci. 1999;65:1671–1683. doi: 10.1016/s0024-3205(99)00416-6. [DOI] [PubMed] [Google Scholar]
- Kalra PR, Moon JC, Coats AJ. Do results of the enable (endothelin antagonist bosentan for lowering cardiac events in heart failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol. 2002;85:195–197. doi: 10.1016/s0167-5273(02)00182-1. [DOI] [PubMed] [Google Scholar]
- Karet FE, Davenport AP. Localization of endothelin peptides in human kidney. Kidney Int. 1996;49:382–387. doi: 10.1038/ki.1996.56. [DOI] [PubMed] [Google Scholar]
- Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150:3392–3400. doi: 10.1210/en.2008-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipper E, Levit A, Mastich Y, Berisha B, Schams D, Meidan R. Induction of endothelin-2 expression by luteinizing hormone and hypoxia: possible role in bovine corpus luteum formation. Endocrinology. 2010;151:1914–1922. doi: 10.1210/en.2009-0767. [DOI] [PubMed] [Google Scholar]
- Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, et al. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147:1770–1779. doi: 10.1210/en.2005-1228. [DOI] [PubMed] [Google Scholar]
- Kojima K, Nihei Z. Expression of endothelin-1 immunoreactivity in breast cancer. Surg Oncol. 1995;4:309–315. doi: 10.1016/s0960-7404(10)80043-x. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Kurihara Y, Yazaki Y. Lessons from gene deletion in the endothelin system. Handb Exp Pharmacol. 2001;152:141–154. [Google Scholar]
- Lambert GL, Barker S, Corder R. Comparison of the regulation of endothelin-2 and endothelin-converting enzyme-1 b [correction of beta] by forskolin and TNF-alpha in ACHN cells. J Cardiovasc Pharmacol. 1998;31(Suppl. 1):S49–S51. doi: 10.1097/00005344-199800001-00016. [DOI] [PubMed] [Google Scholar]
- Lambert GL, Barker S, Lees DM, Corder R. Endothelin-2 synthesis is stimulated by the type-1 tumour necrosis factor receptor and cAMP: comparison with endothelin-converting enzyme-1 expression. J Mol Endocrinol. 2000;24:273–283. doi: 10.1677/jme.0.0240273. [DOI] [PubMed] [Google Scholar]
- Landan G, Bdolah A, Wollberg Z, Kochva E, Graur D. The evolutionary history of the sarafotoxin/endothelin/endothelin-like superfamily. J Cardiovasc Pharmacol. 1991;17(Suppl. 7):S517–S519. doi: 10.1097/00005344-199100177-00148. [DOI] [PubMed] [Google Scholar]
- Lee ME, Bloch KD, Clifford JA, Quertermous T. Functional analysis of the endothelin-1 gene promoter. Evidence for an endothelial cell-specific cis-acting sequence. J Biol Chem. 1990;265:10446–10450. [PubMed] [Google Scholar]
- Lee S, Lin M, Mele A, Farmar J, Russo D, et al. Proteolytic processing of big endothelin-3 by the Kell blood group protein. Blood. 1999;94:1440–1450. [PubMed] [Google Scholar]
- Liefeldt L, Rylski B, Walcher F, Manhart J, Kron S, Rosenke YW, et al. Effects of transgenic endothelin-2 overexpression on diabetic cardiomyopathy in rats. Eur J Clin Invest. 2010;40:203–210. doi: 10.1111/j.1365-2362.2009.02251.x. [DOI] [PubMed] [Google Scholar]
- Lin WW, Lee CY. Intestinal relaxation by endothelin isopeptides: involvement of Ca(2+)-activated K+ channels. Eur J Pharmacol. 1992;219:355–360. doi: 10.1016/0014-2999(92)90475-j. [DOI] [PubMed] [Google Scholar]
- Lydon JP, Demayo FP, Funk CR, Mani S, Hughes AR, Montgomery CA, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- McCartney SA, Ballinger AB, Vojnovic I, Farthing MJ, Warner TD. Endothelin in human inflammatory bowel disease: comparison to rat trinitrobenzenesulphonic acid-induced colitis. Life Sci. 2002;71:1893–1904. doi: 10.1016/s0024-3205(02)01923-9. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol. 1995;115:191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JJ, Kuc RE, Davenport AP. Vasoconstrictor activity of novel endothelin peptide, ET-1(1-31), in human mammary and coronary arteries in vitro. Br J Pharmacol. 2001;134:1360–1366. doi: 10.1038/sj.bjp.0704384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Plumpton C, Barker PJ, Huskisson NS, Davenport AP. Localization of immunoreactive endothelin and proendothelin in the human lung. Pulm Pharmacol. 1992;5:175–182. doi: 10.1016/0952-0600(92)90038-i. [DOI] [PubMed] [Google Scholar]
- Marone G, DE Crescenzo G, Adt M, Patella V, Arbustini E, Genovese A. Immunological characterization and functional importance of human heart mast cells. Immunopharmacology. 1995;31:1–18. doi: 10.1016/0162-3109(95)00037-3. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ishikawa Y, Kozakai T, Uchide T, Komatsu Y, Saida K. Vasoactive intestinal contractor/endothelin-2 gene expression in the murine central nervous system. Biochem Biophys Res Commun. 2003;300:661–668. doi: 10.1016/s0006-291x(02)02872-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Suzuki N, Onda H, Fujino M. Abundance of endothelin-3 in rat intestine, pituitary gland and brain. Biochem Biophys Res Commun. 1989;164:74–80. doi: 10.1016/0006-291x(89)91684-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Suzuki N, Kitada C, Fujino M. Endothelin family peptides in human plasma and urine: their molecular forms and concentrations. Peptides. 1994;15:505–510. doi: 10.1016/0196-9781(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Mattera GG, Eglezos A, Renzetti AR, Mizrahi J. Comparison of the cardiovascular and neural activity of endothelin-1, -2, -3 and respective proendothelins: effects of phosphoramidon and thiorphan. Br J Pharmacol. 1993;110:331–337. doi: 10.1111/j.1476-5381.1993.tb13813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meidan R, Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol Metab. 2007;18:379–385. doi: 10.1016/j.tem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Quertermous T, Hong CC, Bloch KD. Regional and maturation-associated expression of endothelin 2 in rat gastrointestinal tract. J Histochem Cytochem. 1995;43:203–209. doi: 10.1177/43.2.7822776. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Davenport AP. Cerebellar neurons and glia respond differentially to endothelins and sarafotoxin s6b. Brain Res. 1992;581:299–306. doi: 10.1016/0006-8993(92)90721-k. [DOI] [PubMed] [Google Scholar]
- Na G, Bridges PJ, Koo Y, Ko C. Role of hypoxia in the regulation of periovulatory edn2 expression in the mouse 1. Can J Physiol Pharmacol. 2008;2:310–319. doi: 10.1139/y08-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Ogimoto A, Okayama H, Ohtsuka T, Shigematsu Y, Hamada M, et al. A985G polymorphism of the endothelin-2 gene and atrial fibrillation in patients with hypertrophic cardiomyopathy. Circ J. 2007;71:1932–1936. doi: 10.1253/circj.71.1932. [DOI] [PubMed] [Google Scholar]
- Nakamuta M, Ohashi M, Tabata S, Tanabe Y, Goto K, Naruse M, et al. High plasma concentrations of endothelin-like immunoreactivities in patients with hepatocellular carcinoma. Am J Gastroenterol. 1993;88:248–252. [PubMed] [Google Scholar]
- Negus RP, Turner L, Burke F, Balkwill FR. Hypoxia down-regulates mcp-1 expression: implications for macrophage distribution in tumors. J Leukoc Biol. 1998;63:758–765. doi: 10.1002/jlb.63.6.758. [DOI] [PubMed] [Google Scholar]
- O'Reilly G, Charnock-Jones DS, Davenport AP, Cameron IT, Smith SK. Presence of messenger ribonucleic acid for endothelin-1, endothelin-2, and endothelin-3 in human endometrium and a change in the ratio of ETA and ETB receptor subtype across the menstrual cycle. J Clin Endocrinol Metab. 1992;75:1545–1549. doi: 10.1210/jcem.75.6.1464662. [DOI] [PubMed] [Google Scholar]
- O'Reilly G, Charnock-Jones DS, Cameron IT, Smith SK, Davenport AP. Endothelin-2 mRNA splice variants detected by RT-PCR in cultured human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 1993a;22(Suppl. 8):S18–S21. doi: 10.1097/00005344-199322008-00007. [DOI] [PubMed] [Google Scholar]
- O'Reilly G, Charnock-Jones DS, Morrison JJ, Cameron IT, Davenport AP, Smith SK. Alternatively spliced mRNAs for human endothelin-2 and their tissue distribution. Biochem Biophys Res Commun. 1993b;193:834–840. doi: 10.1006/bbrc.1993.1701. [DOI] [PubMed] [Google Scholar]
- Ohkubo S, Ogi K, Hosoya M, Matsumoto H, Suzuki N, Kimura C, et al. Specific expression of human endothelin-2 (ET-2) gene in a renal adenocarcinoma cell line. Molecular cloning of cDNA encoding the precursor of ET-2 and its characterization. FEBS Lett. 1990;274:136–140. doi: 10.1016/0014-5793(90)81348-r. [DOI] [PubMed] [Google Scholar]
- Oliver FJ, de la Rubia G, Feener EP, Lee ME, Loeken MR, Shiba T, et al. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem. 1991;266:23251–23256. [PubMed] [Google Scholar]
- Packer M, Mcmurray J, Massie B, Caspi A, Charlon V, Cohensolal A, et al. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, et al. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20:2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- Paulino EC, Steil AA, Jancar S. Effect of endothelins on human neutrophil activation by immune complexes. Int Immunopharmacol. 2006;6:1119–1125. doi: 10.1016/j.intimp.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Plumpton C, Champeney R, Ashby MJ, Kuc RE, Davenport AP. Characterization of endothelin isoforms in human heart: endothelin-2 demonstrated. J Cardiovasc Pharmacol. 1993;22(Suppl. 8):S26–S28. doi: 10.1097/00005344-199322008-00009. [DOI] [PubMed] [Google Scholar]
- Plumpton C, Ashby MJ, Kuc RE, O'Reilly G, Davenport AP. Expression of endothelin peptides and mRNA in the human heart. Clin Sci (Lond) 1996;90:37–46. doi: 10.1042/cs0900037. [DOI] [PubMed] [Google Scholar]
- Poli E, Barboso G, Casoli C, Starcich R, Bertaccini G. Positive inotropic effect of endothelin-2 on human atrium preparations in vitro. Cardioscience. 1991a;2:99–104. [PubMed] [Google Scholar]
- Poli E, Casoli C, Spaggiari I, Starcich R, Bertaccini G. Contractile effect of endothelin-2 on the isolated human saphenous vein. Arch Int Pharmacodyn Ther. 1991b;313:108–119. [PubMed] [Google Scholar]
- Rodriguez-Pascual F, Redondo-Horcajo M, Magan-Marchal N, Lagares D, Martinez-Ruiz A, Kleinert H, et al. Glyceraldehyde-3-phosphate dehydrogenase regulates endothelin-1 expression by a novel, redox-sensitive mechanism involving mRNA stability. Mol Cell Biol. 2008;28:7139–7155. doi: 10.1128/MCB.01145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubert P, Gillard V, Plas P, Chabrier PE, Braquet P. Binding characteristics of endothelin isoforms (et-1, ET-2, and et-3) in vascular smooth muscle cells. J Cardiovasc Pharmacol. 1991;17(Suppl. 7):S104–S108. doi: 10.1097/00005344-199100177-00027. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- Saida K, Mitsui Y, Ishida N. A novel peptide, vasoactive intestinal contractor, of a new (endothelin) peptide family. Molecular cloning, expression, and biological activity. J Biol Chem. 1989;264:14613–14616. [PubMed] [Google Scholar]
- Saida K, Hashimoto M, Mitsui Y, Ishida N, Uchide T. The prepro vasoactive intestinal contractor (VIC)/endothelin-2 gene (EDN2): structure, evolution, production, and embryonic expression. Genomics. 2000;64:51–61. doi: 10.1006/geno.1999.6083. [DOI] [PubMed] [Google Scholar]
- Saida K, Kometani N, Uchide T, Mitsui Y. Sequence analysis and expression of the mouse full-length vasoactive intestinal contractor/endothelin-2 gene (EDN2): comparison with the endothelin-1 gene (EDN1) Clin Sci (Lond) 2002;103(Suppl. 48):S84–S89. doi: 10.1042/CS103S084S. [DOI] [PubMed] [Google Scholar]
- Sakai S, Miyauchi T, Kobayashi M, Yamagushi I, Goto K, Sugishita Y. Inhibition of myocardial endothelin pathway improves long-term survival in heart failure. Nature. 1996;384:353–355. doi: 10.1038/384353a0. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Randriantsoa A. Differences between endothelin receptors mediating contraction of guinea-pig aorta and pig coronary artery. Eur J Pharmacol. 1993;249:199–206. doi: 10.1016/0014-2999(93)90433-i. [DOI] [PubMed] [Google Scholar]
- Seed A, Kuc RE, Maguire JJ, Hillier C, Johnston F, Essers H, et al. 2012. The dual endothelin converting enzyme/neutral endopeptidase inhibitor SLV-306 (daglutril), inhibits systemic conversion of big endothelin-1 in humans. Life Sci, doi: 10.1016/j.lfs.2012.03.022. [DOI] [PubMed]
- Sharma P, Hingorani A, Jia H, Hopper R, Brown MJ. Quantitative association between a newly identified molecular variant in the endothelin-2 gene and human essential hypertension. J Hypertens. 1999;17:1281–1287. doi: 10.1097/00004872-199917090-00007. [DOI] [PubMed] [Google Scholar]
- Shimada K, Takahashi M, Tanzawa K. Cloning and functional expression of endothelin-converting enzyme from rat endothelial cells. J Biol Chem. 1994;269:18275–18278. [PubMed] [Google Scholar]
- Shirasuna K, Watanabe S, Oki N, Wijayagunawardane MP, Matsui M, Ohtani M, et al. A cooperative action of endothelin-1 with prostaglandin f(2alpha) on luteal function in the cow. Domest Anim Endocrinol. 2006;31:186–196. doi: 10.1016/j.domaniend.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Stow LR, Jacobs ME, Wingo CS, Cain BD. Endothelin-1 gene regulation. FASEB J. 2011;25:16–28. doi: 10.1096/fj.10-161612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizchi R. Slv-306. Solvay. Curr Opin Investig Drugs. 2003;4:329–332. [PubMed] [Google Scholar]
- Takai S, Shiota N, Jin D, Miyazaki M. Chymase processes big-endothelin-2 to endothelin-2-(1-31) that induces contractile responses in the isolated monkey trachea. Eur J Pharmacol. 1998;358:229–233. doi: 10.1016/s0014-2999(98)00622-0. [DOI] [PubMed] [Google Scholar]
- Takizawa S, Uchide T, Adur J, Kozakai T, Kotake-Nara E, Quan J, et al. Differential expression of endothelin-2 along the mouse intestinal tract. J Mol Endocrinol. 2005;35:201–209. doi: 10.1677/jme.1.01787. [DOI] [PubMed] [Google Scholar]
- Tanese K, Fukuma M, Ishiko A, Sakamoto M. Endothelin-2 is upregulated in basal cell carcinoma under control of hedgehog signaling pathway. Biochem Biophys Res Commun. 2010;391:486–491. doi: 10.1016/j.bbrc.2009.11.085. [DOI] [PubMed] [Google Scholar]
- Telemaque S, D'Orléans-Juste P. Presence of a phosphoramidon-sensitive endothelin-converting enzyme which converts big-endothelin-1, but not big-endothelin-3, in the rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:505–507. doi: 10.1007/BF00172593. [DOI] [PubMed] [Google Scholar]
- Tokito F, Suzuki N, Hosoya M, Matsumoto H, Ohkubo S, Fujino M. Epidermal growth factor (EGF) decreased endothelin-2 (ET-2) production in human renal adenocarcinoma cells. FEBS Lett. 1991;295:17–21. doi: 10.1016/0014-5793(91)81374-h. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Murphy LJ. Molecular pharmacology of endothelin converting enzymes. Biochem Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Turner L, Scotton C, Negus R, Balkwill F. Hypoxia inhibits macrophage migration. Eur J Immunol. 1999;29:2280–2287. doi: 10.1002/(SICI)1521-4141(199907)29:07<2280::AID-IMMU2280>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Uchide T, Masuda H, Mitsui Y, Saida K. Gene expression of vasoactive intestinal contractor/endothelin-2 in ovary, uterus and embryo: comprehensive gene expression profiles of the endothelin ligand-receptor system revealed by semi-quantitative reverse transcription-polymerase chain reaction analysis in adult mouse tissues and during late embryonic development. J Mol Endocrinol. 1999;22:161–171. doi: 10.1677/jme.0.0220161. [DOI] [PubMed] [Google Scholar]
- Uchide T, Adur J, Fukamachi T, Saida K. Quantitative analysis of endothelin-1 and vasoactive intestinal contractor/endothelin-2 gene expression in rats by real-time reverse transcriptase polymerase chain reaction. J Cardiovasc Pharmacol. 2000;36:S5–S8. doi: 10.1097/00005344-200036051-00004. [DOI] [PubMed] [Google Scholar]
- Vallender TW, Lahn BT. Localized methylation in the key regulator gene endothelin-1 is associated with cell type-specific transcriptional silencing. FEBS Lett. 2006;580:4560–4566. doi: 10.1016/j.febslet.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Walles B, Gröschel-Stewart U, Kannisto P, Owman C, Sjöberg NO, Unsicker K. Immunocytochemical demonstration of contractile cells in the human ovarian follicle. Experientia. 1990;46:682–683. doi: 10.1007/BF01939933. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Proteolytic activity of mycoplasma salivarium and mycoplasma orale 1. Med Microbiol Immunol. 1975;161:127–132. doi: 10.1007/BF02121754. [DOI] [PubMed] [Google Scholar]
- Wright C, Cody W, Dunbar JJ, Doherty A, Hingorani G, Rapundalo S. Characterization of endothelins as chemoattractants for human neutrophils. Life Sci. 1994;55:1633–1641. doi: 10.1016/0024-3205(94)00330-0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Yanagisawa M, Kapur RP, Richardson JA, Williams SC, Clouthier DE, et al. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yap EY, Battistini B, McKay KO. Contraction to big endothelin-1, big endothelin-2 and big endothelin-3, and endothelin-converting enzyme inhibition in human isolated bronchi. Br J Pharmacol. 2000;129:170–176. doi: 10.1038/sj.bjp.0703006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar S, Tsukamoto H, Kalra VK. Ethanol induced expression of ET-1 and ET-BR in liver sinusoidal endothelial cells and human endothelial cells involves hypoxiainducible factor-1alpha and microrNA-199. J Immunol. 2009;183:5232–5243. doi: 10.4049/jimmunol.0901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H, Zhang Y, Penfold S, Rollins BJ. Mcp-1-mediated chemotaxis requires activation of signal transduction pathways. J Leukoc Biol. 1997;61:529–532. doi: 10.1002/jlb.61.4.529. [DOI] [PubMed] [Google Scholar]
- Yorimitsu K, Shinmi O, Nishiyama M, Moroi K, Sugita Y, Saito T, et al. Effect of phosphoramidon on big endothelin-2 conversion into endothelin-2 in human renal adenocarcinoma (ACHN) cells. Analysis of endothelin-2 biosynthetic pathway. FEBS Lett. 1992;314:395–398. doi: 10.1016/0014-5793(92)81513-l. [DOI] [PubMed] [Google Scholar]
- Yorimitsu K, Moroi K, Inagaki N, Saito T, Masuda Y, Masaki T, et al. Cloning and sequencing of a human endothelin converting enzyme in renal adenocarcinoma (ACHN) cells producing endothelin-2. Biochem Biophys Res Commun. 1995;208:721–727. doi: 10.1006/bbrc.1995.1397. [DOI] [PubMed] [Google Scholar]
- Yoshinaga M, Chijiiwa Y, Misawa T, Harada N, Nawata H. EndothelinB receptor on guinea pig small intestinal smooth muscle cells. Am J Physiol. 1992;262:G308–G311. doi: 10.1152/ajpgi.1992.262.2.G308. [DOI] [PubMed] [Google Scholar]


