Abstract
Glucokinase activators (GKAs) represent one of the leading hopes for the next generation of type 2 diabetes (T2D) therapeutics, showing efficacy in reducing blood glucose and HbA1c levels in animal models of T2D and short-term human trials. While the hypoglycaemic risks of GCK activation in pancreatic beta-cells have long been appreciated, the hepatic effects of GKAs have generally been perceived to be without significant side effect. In this issue of the British Journal of Pharmacology, De Ceuninck et al. report that acute and chronic GKA treatment of normoglycaemic and hyperglycaemic rodent models results in significant accumulation of triglycerides in the liver. This suggests GKA-mediated activation of hepatic glucose uptake and suppression of endogenous glucose production may come at a significant cost; namely, the development of hepatic steatosis. This raises important questions regarding the safety of GKAs and emphasizes that both plasma and hepatic lipid profiles should be carefully monitored in on-going and future studies of these molecules.
Keywords: glucokinase, glucokinase regulatory protein, glucokinase activator, hepatic steatosis, type 2 diabetes, hepatopharmacology, triglycerides
Type 2 diabetes (T2D) is a major global health concern, with diabetes-related costs representing more than 10% of global health care expenditures in 2011 and recent estimates suggesting more than 310 million individuals are affected worldwide (International Diabetes Federation, 2011). While this is primarily attributable to lifestyle changes including decreased physical activity and increased obesity, this rapid rise in prevalence emphasizes the inefficacy of current therapeutic strategies. Accordingly, there is a substantial need for the development of new treatments that can sustainably and safely lower glycaemia.
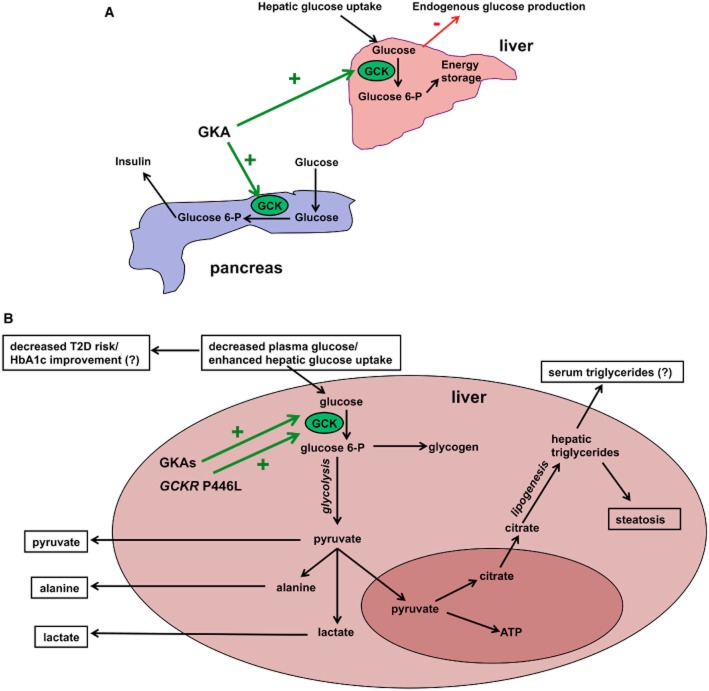
The critical role of glucokinase (GCK) in human glucose homeostasis, most convincingly demonstrated by the disruption of glycaemic control in individuals with both gain- and loss-of-function mutations within GCK, initially highlighted modulation of GCK as a possible therapeutic target (Osbak et al., 2009). GCK functions as the glucose sensor in the liver and pancreatic beta-cell, two of the primary sites of dysregulation in T2D pathogenesis, and activation of GCK in both cell types would in theory be of substantial benefit in the hyperglycaemic context of T2D (Figure 1A).
Figure 1.
Metabolic effects of GCK activation. (A) Schematic representation of the beneficial effects of GKAs on the pancreas and liver. GKAs lead to increased glucose-stimulated insulin secretion in the beta-cell and increase the glucose-6-phoshate : glucose ratio in the liver, resulting in enhanced hepatic glucose uptake and suppressed endogenous glucose production. (B) Potential shared hepatic pathways for seven phenotypic traits and metabolites significantly associated with both GKAs and the GCKR P446L variant. Activation of GCK by GKAs results in increased hepatic glucose uptake (Grimsby et al., 2003; De Ceuninck et al., 2012; Nissim et al., 2012), which likely underlies the association of P446L with plasma glucose concentrations (Dupuis et al., 2010). Decreased plasma glucose levels contribute to reduction of T2D risk, as seen with GCKR P446L (Dupuis et al., 2010), and/or decreased HbA1c, as observed with GKAs in rodent models and in short-term, but not long-term, human trials (Meininger et al., 2011; De Ceuninck et al., 2012). GKAs increase flux through glycolysis and hepatic lactate, pyruvate, and alanine output (Nissim et al., 2012). GCKR P446L is associated with plasma levels of all three glycolytic products (Suhre et al., 2011; Kettunen et al., 2012). However, enhanced glycolytic flux also stimulates de novo lipogenesis; both GKAs and P446L have been shown to increase hepatic steatosis (Speliotes et al., 2011; De Ceuninck et al., 2012), and P446L is associated with plasma triglycerides (Saxena et al., 2007). Emerging evidence suggests GKAs may also affect plasma triglycerides (Meininger et al., 2011; De Ceuninck et al., 2012). Significant published associations with both GCKR P446L and GKAs are shown in the black boxes, whilst contradictory findings for GKAs are indicated by a ‘?’.
Nearly a decade ago, the first publication describing a small-molecule GCK activator (GKA), RO-28–1675 from Hoffman-La Roche, seemed to realize both advantages (Grimsby et al., 2003). Favourable effects on the beta-cell were suggested by concurrent reduction of blood glucose levels with increased plasma insulin levels following a single oral dose in multiple rodent models of normoglycaemia and T2D. Liver-mediated effects were demonstrated by increased liver glucose uptake and suppressed endogenous glucose production during a hyperglycaemic pancreatic clamp in male Sprague–Dawley and Zucker Diabetic Fatty (ZDF) rats. Since this initial report, broad interest from the pharmaceutical industry and academia has resulted in efforts to generate diverse GKAs with differing kinetic and pharmacokinetic properties, the description of preclinical effects of several GKAs and the filing of more than 100 patents. This diversity complicates generalization, as a number of structural classes of GKAs exist with differing effects and potencies. However, GKAs all enhance GCK activity in vitro by increasing the affinity of GCK for glucose, bind to a pocket distal to the active site known as the allosteric activator site and generally share a common structural core. Notably, in vivo effects in animal models generally correlate well with in vitro compound efficacies. Chronic or acute treatments in such models have been predominantly promising, particularly in the lowering of blood glucose, and several GKAs have progressed to human clinical trials.
However, the potential risks of GCK activation have also been well documented. Historically, the primary concern has focused on the beta-cell, where GCK activation may lead to episodes of hypoglycaemia due to insulin secretion even at low glucose concentrations as observed in individuals with GCK activating mutations (Osbak et al., 2009). Additionally, chronic activation may lead to beta-cell stress and ultimate beta-cell failure, a side effect seen in long-term treatment with sulphonylureas. While there is little evidence to date demonstrating GKAs contribute to beta-cell stress, episodes of hypoglycaemia have been reported in animal models and in human studies of individuals with T2D treated with GKAs, particularly at higher doses (Bonadonna et al., 2010; Meininger et al., 2011).
These concerns have led to increased focus on the effects of GCK in the liver, and some efforts to develop or prioritize liver-specific GKAs. A metabolic analysis of acute GCK activation in the liver by the GKA Piragliatin demonstrated that GKAs activate hepatic glucose uptake, glycolysis and downstream energetic pathways in rats (Nissim et al., 2012). However, concern has been raised about the effects of hepatic GCK activation. Increased glucose uptake and glycolytic flux could result in increased hepatic lipid biosynthesis, as noted in rodent models overexpressing Gck. For example, transient adenoviral overexpression of Gck in normal Wistar rats reduces blood glucose levels but significantly increases triglyceride levels (O'Doherty et al., 1999), while transgenic overexpression of Gck in mouse liver causes a progressive, age-dependent development of glucose intolerance and significantly increases fasting plasma glucose, insulin and triglyceride concentrations at 12 months of age (Ferre et al., 2003). However, abnormal lipid profiles are not generally a feature of GCK activating mutations (Osbak et al., 2009), and while the available data from chronic GKA studies in rodents have been sparse and inconsistent, the general consensus has been that GKAs do not affect lipid profiles. Alarmingly, a recent report on the GKA MK-0941 contradicts this notion, as plasma triglyceride concentrations were significantly increased at one or more doses in two separate clinical trials (Meininger et al., 2011). This suggested a significant unmet need to comprehensively characterize the effects of GKAs on lipid profiles, both in the liver and in plasma, under acute and chronic treatment conditions.
The study by De Ceuninck et al. (2012) in this issue of the British Journal of Pharmacology convincingly demonstrates that hepatic triglyceride content is increased by both short-term and long-term GKA treatment in db/db mice, normoglycaemic Wistar rats and ZDF rats. Importantly, up to nine GKAs were tested representing three distinct structural classes and a range of in vitro and in vivo efficacies, including an inactive control molecule with high structural similarity to one of the active GKAs. While GKAs showed the expected positive effects on induction of glycogen synthesis in rat hepatocytes, insulin secretion from INS-1E cells and dose-dependent improvement of glycaemic response on oral administration in db/db mice, significant increases in hepatic triglyceride content were detectable after only 4 days of treatment and were significantly increased after treatment regimes lasting 2, 4, 6 or 8 weeks. Significant increases in hepatic triglyceride content were also observed after 4 week GKA treatment of ZDF and normoglycaemic Wistar rats. Importantly, across each study, there was a strong correlation between the degree of triglyceride accumulation and the ability of GKAs to lower HbA1c.
This study represents an important advance in understanding the effects of acute and chronic GKA treatment on hepatic lipid profiles. It is therefore imperative to investigate how these findings will translate to the effects of GKAs in humans. While GCK activating mutations do not seem to replicate the lipid phenotypes seen by GKA treatment, GCK is regulated post-transcriptionally in the liver by the glucokinase regulatory protein (GKRP; gene name: GCKR). A non-synonymous variant in GKRP, changing proline at position 446 to leucine, has been shown to increase the activity of GCK (Beer et al., 2009; Rees et al., 2012). Genetic studies have demonstrated that carriers of the leucine allele have significantly increased plasma triglyceride levels and incidence of hepatic steatosis but significantly reduced fasting plasma glucose levels and risk of developing T2D (Saxena et al., 2007; Dupuis et al., 2010; Speliotes et al., 2011). These genetic and functional studies are beginning to elucidate the consequences of hepatic GCK activation in humans and paint a picture strikingly consistent with the emerging hepatic effects of GKAs from rodent models (Figure 1B) (De Ceuninck et al., 2012; Nissim et al., 2012).
Available data convincingly indicate hepatic metabolic flux is enhanced under conditions of GCK activation, with significant intra- and extra-hepatic consequences, but the precise downstream consequences and the relative importance of the pathways highlighted in Figure 1 remain unanswered questions. For example, in spite of the associations of the P446L variant with increased hepatic steatosis and plasma triglyceride levels, P446L is not associated with coronary artery disease, myocardial infarction, or stroke, and this variant is protective for T2D. There is also evidence in the current report from De Ceuninck et al. (2012) that, although the effects of GKAs on hepatic triglyceride content are invariably consistent, resultant effects on plasma triglycerides are dependent on the animal model employed. Future studies of both the P446L variant and GKAs may provide additional insight into the systemic metabolic consequences of GCK activation in the liver. Furthermore, the combination of these two research directions could be equally informative; it could be of substantial interest to investigate potential differential effects of GKA treatment by P446L genotype, as two in three individuals of Western European descent (and an even higher proportion of individuals of East Asian descent) carry one or two copies of the GCK-activating leucine allele.
In summary, optimism for GKAs has been somewhat diminished by recent findings, with the time-dependent loss of HbA1c-lowering efficacy reported in clinical trials of MK-0941 of particular concern (Meininger et al., 2011). The potential side effects of GKA treatment also are coming under increased scrutiny; evidence is accumulating that activation of GCK in the liver may carry significant risks. While it has been suggested that careful management of dosing, or the use of GKAs with less marked effects on the glucose affinity of GCK, may reduce adverse events associated with GKAs, the findings of De Ceuninck et al. (2012) suggest there is a direct relationship between treatment efficacy and adverse side effects. Accordingly, it may not be a trivial matter to optimize GKAs to provide maximal glycaemic benefit while minimizing complications. However, the advances in our understanding of the consequences of GCK activation may help focus such efforts and future clinical studies.
Acknowledgments
The authors apologize to colleagues whose work they were unable to cite due to space limitations. ALG is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science (095101/Z/10Z).
Glossary
- GCK
glucokinase
- GKA
glucokinase activator
- GKRP
glucokinase regulatory protein
- T2D
type 2 diabetes
- ZDF
Zucker diabetic fatty
Conflict of interest
None.
References
- Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PR, Orho-Melander M, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18:4081–4088. doi: 10.1093/hmg/ddp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna RC, Heise T, Arbet-Engels C, Kapitza C, Avogaro A, Grimsby J, et al. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab. 2010;95:5028–5036. doi: 10.1210/jc.2010-1041. [DOI] [PubMed] [Google Scholar]
- De Ceuninck F, Kargar C, Ilic C, Caliez A, Rolin JO, Umbdenstock T, et al. Small molecule glucokinase activators disturb lipid homeostasis and induce fatty liver in rodents: a warning for therapeutic applications in humans. Brit J Pharmacol. 2012;168:339–353. doi: 10.1111/j.1476-5381.2012.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia. 2003;46:1662–1668. doi: 10.1007/s00125-003-1244-z. [DOI] [PubMed] [Google Scholar]
- Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, International Diabetes Federation (Ed), 5th Edn. Brussels: International Diabetes Federation; 2011. [Google Scholar]
- Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikainen LP, et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. 2012;44:269–276. doi: 10.1038/ng.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meininger GE, Scott R, Alba M, Shentu Y, Luo E, Amin H, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2560–2566. doi: 10.2337/dc11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim I, Horyn O, Daikhin Y, Wehrli SL, Yudkoff M, Matschinsky FM. Effects of a glucokinase activator on hepatic intermediary metabolism: study with (13)C-isotopomer-based metabolomics. Biochem J. 2012;444:537–551. doi: 10.1042/BJ20120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty RM, Lehman DL, Telemaque-Potts S, Newgard CB. Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes. 1999;48:2022–2027. doi: 10.2337/diabetes.48.10.2022. [DOI] [PubMed] [Google Scholar]
- Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009;30:1512–1526. doi: 10.1002/humu.21110. [DOI] [PubMed] [Google Scholar]
- Rees MG, Wincovitch S, Schultz J, Waterstradt R, Beer NL, Baltrusch S, et al. Cellular characterisation of the GCKR P446L variant associated with type 2 diabetes risk. Diabetologia. 2012;55:114–122. doi: 10.1007/s00125-011-2348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]