Abstract
Background and Purpose
3-Iodothyronamine (T1AM), an endogenous derivative of thyroid hormones, is regarded as a rapid modulator of behaviour and metabolism. To determine whether brain thyroid hormone levels contribute to these effects, we investigated the effect of central administration of T1AM on learning and pain threshold of mice either untreated or pretreated with clorgyline (2.5 mg·kg−1, i.p.), an inhibitor of amine oxidative metabolism.
Experimental Approach
T1AM (0.13, 0.4, 1.32 and 4 μg·kg−1) or vehicle was injected i.c.v. into male mice, and after 30 min their effects on memory acquisition capacity, pain threshold and curiosity were evaluated by the following tests: passive avoidance, licking latency on the hot plate and movements on the hole-board platform. Plasma glycaemia was measured using a glucorefractometer. Brain levels of triiodothyroxine (T3), thyroxine (T4) and T1AM were measured by HPLC coupled to tandem MS. ERK1/2 activation and c-fos expression in different brain regions were evaluated by Western blot analysis.
Results
T1AM improved learning capacity, decreased pain threshold to hot stimuli, enhanced curiosity and raised plasma glycaemia in a dose-dependent way, without modifying T3 and T4 brain concentrations. T1AM effects on learning and pain were abolished or significantly affected by clorgyline, suggesting a role for some metabolite(s), or that T1AM interacts at the rapid desensitizing target(s). T1AM activated ERK in different brain areas at lower doses than those effective on behaviour.
Conclusions And Implications
T1AM is a novel memory enhancer. This feature might have important implications for the treatment of endocrine and neurodegenerative-induced memory disorders.
Keywords: 3-iodothyronamine, thyroid hormones, passive avoidance, object recognition task, pain threshold
Introduction
3-Iodothyronamine (T1AM) is an endogenous primary amine probably produced through thyroid hormone metabolism (Scanlan, 2009; Piehl et al., 2011). T1AM circulates in healthy rodents and humans (Saba et al., 2010; Galli et al., 2012) but it concentrates in tissues, particularly in the liver and brain (Saba et al., 2010). Recently, several studies have explored the consequences of administering T1AM directly into the brain. T1AM injected i.c.v. rapidly induces metabolic effects, including a reduction of body temperature (Doyle et al., 2007), modification of food intake and hyperglycaemia (Manni et al., 2012). T1AM effects were not linearly related to the dosage and depended on the animal species and administration route (Dhillo et al., 2008; Klieverik et al., 2009). We have recently reported that 1.32 μg·kg−1 T1AM injected i.c.v. into fasted mice produced hypophagia and hyperglycaemia, while it reduced plasma fT3 and fasting-induced c-fos activation (Manni et al., 2012). Both hypophagia and fT3 reduction were not linearly related to T1AM doses and they were prevented by in vivo treatment with clorgyline, an inhibitor of oxidative deamination, the major pathway of T1AM catabolism (Saba et al., 2010), suggesting the involvement of rapid desensitizing targets and/or interference with neuromediators producing opposing effects on behaviour. In fact, electrophysiological experiments suggest that T1AM affects the response to catecholamines and other neurotransmitters, acting as a specific inhibitor of dopamine and noradrenaline re-uptake and of monoamine transport into synaptic vesicles (Snead et al., 2007). Therefore, T1AM might be regarded as a neuromodulator.
In the present paper, we investigated whether T1AM is able to produce specific neurological effects such as the modification of memory acquisition and pain threshold in mice, and whether these effects are modified under conditions of MAO inhibition by clorgyline and whether they were associated with changes in triiodothyroxine (T3) and thyroxine (T4) brain levels.
With this aim, we evaluated the behaviour of mice injected i.c.v. with T1AM (0.13, 0.4, 1.32 and 4 μg·kg−1) in the passive avoidance test and in the novel object recognition task, as well as the effects of T1AM on pain threshold, exploratory activity and plasma glycaemia. In parallel experiments, the activation of typical signalling proteins involved in memory acquisition and pain perception, including pERK, pAkT, c-fos and pCREB, were measured in specific brain areas. Brain levels of T3 and T4 and of T1AM were also determined following injection of T1AM at the lowest effective dose.
Methods
Animals
One hundred and twenty male mice (CD1 strain; 20–30 g) from the Morini breeding farm (San Polo d'Enza, Italy) were used. The cages were placed in the experimental room 24 h before the tests for adaptation. Animals were kept at 23 ± 1°C with a 12 h light–dark cycle (light on at 07:00 h) and were fed a standard laboratory diet with water ad libitum. Experiments and animal use procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). The experimental protocols were approved by the Animal Care Committee of the Department of Pharmacology, University of Florence, in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS No. 123) and the European Community Council Directive of 24 November 1986 (86/609/EEC). The authors further attest that all efforts were made to minimize the number of animals used and their suffering. All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
I.c.v. injection technique
I.c.v. administration was performed under light ether anaesthesia according to the method described by Haley and McCormick (1957), with minor modifications. The depth of anaesthesia was checked by monitoring the respiratory rate (which was reduced within 2 min) and testing for lack of a pain response to gentle pressure on the hind paws. The head of the anaesthetized mouse was grasped firmly and the needle of a 10 μL microsyringe was inserted perpendicularly 2 mm through the skull into the brain. Ten microlitres of solution was then slowly injected (in 20 s) into a lateral ventricle. The injection site was 1 mm to the left of the midpoint, on a line drawn through to the anterior base of the ears. Immediately after needle removal, the animal remained quiet for approximately 1 min and then resumed its normal activity. To ascertain that solutions were administered exactly into the cerebral ventricle, some mice were injected with 10 μL of 1:10 India ink and their brains were examined macroscopically after sectioning. The accuracy of the injection technique was confirmed to 95%.
Behavioural tests
The passive avoidance paradigm
This test was performed according to the step-through method described by Jarvik and Kopp (1967). The experimental apparatus consisted of a two-compartment acrylic box with a lighted compartment connected to a darkened one by a guillotine door. The dark chamber was constructed with a pitfall floor. When entering this chamber in the training session, the mice receive a non-painful punishment consisting of a fall (from 40 cm) into a cold water bath (10°C). The test was then repeated 1 and 24 h after the training session. Before the trials, the mice were removed from the cage and injected i.c.v. with 10 μL of vehicle (0.5% DMSO) or T1AM (0.13, 0.40, 1.32 and 4 μg·kg−1; n = 20 for each treatment). Additional mice were pretreated with an i.p. injection of clorgyline (2.5 mg·kg−1) and then received either vehicle or T1AM i.c.v. T1AM was kindly provided by Dr Thomas Scanlan (Portland, OR, USA) and was dissolved in vehicle. Thirty minutes after i.c.v. injections, the mice were placed on an illuminated platform (60 W, 840 lux) and allowed to enter the dark compartment (training session). Because mice prefer dark to light, they usually entered the dark compartment within 5 s. Mice not entering the dark compartment within 60 s during the training session were excluded from the experiment. The extent of punishment memory was expressed as the difference in seconds between training and retention latencies. The latter was measured 1 and 24 h after the training session. In the 1 h and 24 h tests, each animal was placed on the platform and the latency to enter the dark compartment was measured up to a maximum of 300 s.
The novel object recognition task
Experiments were carried out as described by Ennaceur and Delacour (1988), with minor modifications. The mice were subjected to the procedure separately and care was taken to remove any olfactory/taste cues by cleaning the arena and test objects with alcohol between trials. Each mouse completed one session with three successive trials. In trial 1, habituation phase, 24 h before the test day the mouse was placed at the centre of the empty ‘open-field’ arena, located in a sound-attenuated room under dimmed lighting for 2 min. On the following day, mice (n = 20) were injected with T1AM (1.32 μg·kg−1, a dose selected on the basis of our previous experiments) 30 min before the second trial (familiarization). During this trial (trial 2), the sample phase, two objects (such as plastic cubes or cylinders) were placed in opposite corners of the box and the mouse was allowed to explore them. After 24 h, each mouse was again placed in the test arena for 5 min (trial 3) in the presence of one of the familiar objects and of a novel object, and the time spent exploring both objects was again recorded. Object position and presentation order were randomized to prevent bias from order or place preference. Each trial lasted 5 min, with a 3 min inter-trial interval (ITI). During the ITI, the mouse was removed from the testing box and placed in a holding cage. Exploration was defined as the state in which the mouse's nose was kept within 2 cm of the object. Locomotor activity in the arena was measured as the times of grid crossing.
The hole-board test
The hole-board test was performed according to Galeotti et al. (2006). The experimental setting consisted of a 40 cm square plane with 16 flush mounted cylindrical holes (3 cm diameter) distributed four by four in an equidistant, grid-like manner. Mice were placed in the centre of the board one by one and allowed to move about freely for a period of 10 min. Two electric eyes, crossing the plane from midpoint to midpoint of the opposite sides, thus dividing the plane into four equal quadrants, automatically signalled mouse movements (locomotor activity). Miniature photoelectric cells, in each of the 16 holes, recorded hole exploration. Animals were injected i.c.v. with vehicle or T1AM at dosages of 0.4, 1.32 or 4 μg·kg−1 (n = 20 in each group) and tested 30 min after i.c.v. injections.
The hot plate
After the mice had been introduced to a hot plate device (51.5 ± 1°C), the latency of a flinching or jumping response was measured. The cut-off time was set at 45 s to minimize skin damage. Animals were injected i.c.v. with vehicle or T1AM at dosages of 0.13, 0.4, 1.32 and 4 μg·kg−1 (n = 20 in each group). Additional mice were pretreated with clorgyline (2.5 mg·kg−1, i.p.) before receiving either vehicle or T1AM i.c.v. Measurements were performed 30 min after i.c.v. injections.
T1AM-activated signalling in brain
Preparation of mouse brain tissue
Male mice (mean weight, 23 ± 3 g) were removed from the cage and injected i.c.v. under light anaesthesia with 10 μL of vehicle or T1AM (0.044, 0.13 and 1.32, μg·kg−1). Mice (n = 5 for each treatment) were then killed by decapitation, within 30 min of i.c.v. injections. The skull was opened with scissors and the brain surface was cooled down by exposing it to liquid nitrogen vapours for 1–2 s. The brain was excised and the forebrain was divided into two parts: frontal cortex and diencephalon. The hippocampus and amygdala were then quickly removed. The hypothalamus was separated from thalamus, and all tissues were flash-frozen in liquid nitrogen and stored at −80°C until use.
Brain samples were homogenized in homogenization buffer containing (in mM): 50 Tris–HCl (pH 7.5), 150 NaCl, 1 EDTA, 5 sodium pyrophosphate, 10 β-glycerophosphate, 1 Na3VO4, 0.2 PMSF, 25 μg·mL−1 leupeptin, 10 μg·mL−1 aprotinin and 0.1% SDS. Homogenates were then centrifuged at 1000× g for 10 min at 4°C to remove cell debris, and supernatants (brain lysates) were used for Western blot analysis.
Western blot analysis
Brain lysates (20 μg) were separated on 10% SDS-PAGE and transferred into nitrocellulose membranes (120 min at 100 V) using standard procedures. Membranes were blocked in PBST (PBS containing 0.1% Tween) containing 5% non-fat dry milk for 60 min. Following washing, blots were incubated overnight at 4°C with specific antibody against ERK1/2 phosphorylated on Thr202/Tyr204 (pERK1/2, Cell Signaling Technology), c-fos (Santa Cruz Biotechnology, Santa Cruz, CA, USA), pAkt or pCREB (Cell Signalling Technology, Danvers, MA, USA). All primary antibodies were diluted in PTBS containing 3% albumin. After being washed with PBST, the nitrocellulose membrane was incubated with polyclonal goat anti-rabbit HRP-conjugated secondary antisera (1:2000, diluted in PTBS containing 5% non-fat dry milk) and left for 1 h at room temperature. Blots were then extensively washed and developed using an enhanced chemiluminescence detection system (Pierce Scientific, Rockford, IL, USA). Exposition and developing time were standardized for all blots. Densitometric analysis of scanned images was performed on a Macintosh iMac computer using the public domain NIH Image program. α-Tubulin (anti-α-tubulin 05–829, Millipore Corporation, Billerica, MA, USA) was used as loading control. Protein concentration was quantified using Bradford's method (protein assay kit, Bio-Rad Laboratories, Segrate, Milan, Italy).
Assay of T1AM and thyroid hormone brain concentration
Mice (n = 6) were killed by decapitation 30 min after T1AM (1.32 μg·kg−1) or vehicle injection. The brain was isolated and quickly frozen at −80°C. T1AM, T4 and T3 were extracted and their levels were measured by HPLC coupled to tandem MS, as described previously (Saba et al., 2010).
Measurement of plasma glycaemia
Mice were injected i.c.v. with vehicle or T1AM at dosages of 0.13, 0.4 and 1.32 μg·kg−1 (n = 5 in each group). After 30 min, the tail vein was punctured and drops of blood were collected to measure glycaemia by a glucorefractometer.
Statistical analysis
Data are expressed as mean ± SEM of independent experiments. Statistical analysis was performed by one-way ANOVA, followed by Student–Newman–Keuls multiple comparison post hoc test. When the experimental setting included only two groups, unpaired t-test was used. The threshold of statistical significance was set at P < 0.05. Data analysis was performed by GraphPad Prism 5.0 statistical program (GraphPad Software, San Diego, CA, USA).
Results
T1AM increased retention and consolidation time in the passive avoidance paradigm
As described in the Methods section, retention sessions of the passive avoidance test were performed 1 and 24 h after the T1AM injection. Following administration of 0.4 and 0.13 μg·kg−1 T1AM, the latency to enter the dark compartment was increased either in the 1 or the 24 h retention session, but the difference versus the control group did not reach the threshold of statistical significance. However, a significantly higher latency to enter the dark compartment was observed after 1.32 and 4 μg·kg−1 T1AM, both at 1 and 24 h (P < 0.001 and P < 0.05 vs. vehicle-treated mice respectively) (Figure 1A).
Figure 1.
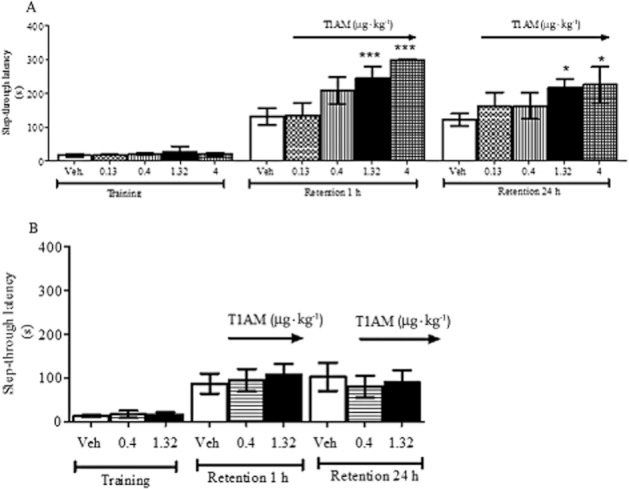
(A) Mice were injected i.c.v. with T1AM (0.13, 0.4, 1.32 or 4 μg·kg−1) or with vehicle (Veh) and subjected to the passive avoidance test as described in the Methods section. Results are expressed as mean ± SEM; n = 20 in each group; *P < 0.05; ***P < 0.001 versus vehicle. (B) Mice pretreated with clorgyline (2.5 mg·kg−1, i.p.), received either vehicle (Veh) or T1AM (0.4 or 1.32 μg·kg−1) i.c.v and were then subjected to the passive avoidance test as described in the Methods section. Results are expressed as mean ± SEM; n = 10 in each group.
T1AM improved memory acquisition: the object recognition task
As 1.32 μg·kg−1 was the lowest effective dose in the passive avoidance test, we next verified whether the pro-cognitive effect of this dose was confirmed in another test, the novel object recognition task, which is based on the natural tendency of rodents to explore unfamiliar objects (Ennaceur and Delacour, 1988).
As shown in Figure 2, mice treated with vehicle spent the same amount of time exploring the familiar and the novel object, while mice injected i.c.v. with 1.3 μg·kg−1 T1AM showed significantly (P < 0.001) enhanced exploratory preference for the novel object in the 24 h retention test (Figure 2A). At this dose, T1AM did not increase locomotor activity (Figure 2B).
Figure 2.
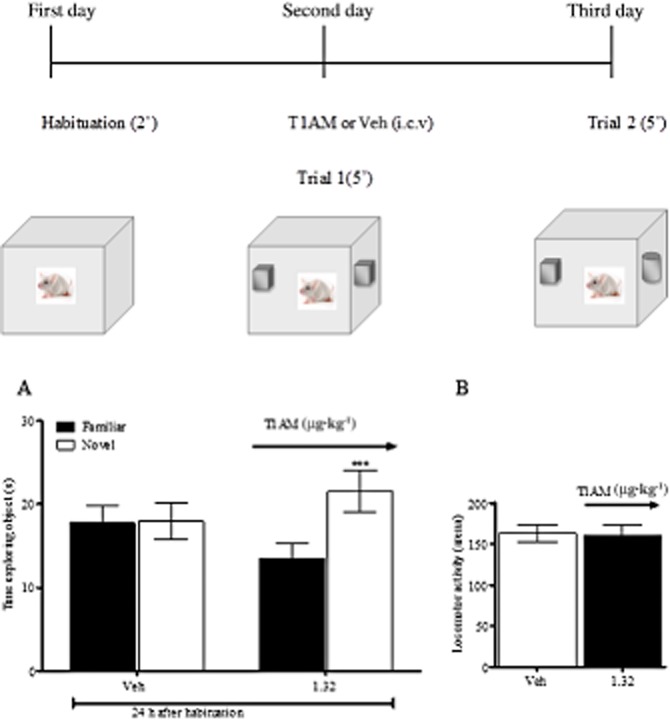
The object recognition task was performed on fed mice, which were injected on day 2 with 1.32 μg·kg−1 T1AM or vehicle (n = 20 per group). The retention session was performed 24 h later (A). Animal movements in the arena (locomotor activity) were not significantly different between the two groups (B). Results are expressed as mean ± SEM; ***P < 0.001 versus vehicle.
T1AM increased exploratory activity: the hole-board test
Memory acquisition is facilitated by increased curiosity. Our results demonstrated that mice injected i.c.v. with T1AM (1.32 and 4 μg·kg−1) showed significantly increased exploratory activity (P < 0.05 vs. vehicle-injected mice), while this effect was not detected in mice injected with 0.4 μg·kg−1 T1AM (Figure 3).
Figure 3.

Mice were injected i.c.v. with T1AM (0.4, 1.32 and 4 μg·kg−1) or vehicle and put on the hole-board platform. Movements and exploratory activities were monitored as described in the Methods section. Results are expressed as mean ± SEM; n = 20 in each group; ***P < 0.001 and **P < 0.01 versus vehicle.
T1AM increased pain sensitivity
As the response to the passive avoidance and object recognition tests may be affected by an analgesic action, we next checked whether T1AM had any effect on the pain threshold by investigating the latency to licking of mice injected i.c.v. with T1AM and then located on the hot plate. As shown in Figure 4, at doses that proved to be effective in the passive avoidance test (1.32 and 4 μg·kg−1), T1AM significantly reduced the threshold of pain perception to hot insults (P < 0.01 and P < 0.05 vs. vehicle respectively). Interestingly, the hyperalgesic effect of T1AM was maintained at 0.4 μg·kg−1 but it was lost at 0.132 μg·kg−1 (Figure 4).
Figure 4.

(A) Mice were placed on the hot plate 30 min after i.c.v. injection of T1AM (0.13, 04, 1.32 and 4 μg·kg−1) or vehicle. The latency to respond to the insult was evaluated as described in the Methods section. Results are expressed as mean ± SEM; n = 20 in each group; *P < 0.05 and **P < 0.01 versus vehicle. (B) Mice were pretreated with clorgyline (2.5 mg·kg−1, i.p.), received either vehicle (Veh) or T1AM (0.13, 0.4 or 1.32 or 4 μg·kg−1) i.c.v and after 30 min were placed on the hot plate. The latency to respond to the insult was evaluated as described in the Methods section. Results are expressed as mean ± SEM; n = 10 in each group; ∧P < 0.05 versus vehicle without clorgyline (A) and *P < 0.05 versus vehicle (with clorgyline).
Effect of clorgyline pretreatment on the response to T1AM
Oxidative deamination by amine oxidases appears to be the chief metabolic pathway for T1AM (Wood et al., 2009; Saba et al., 2010), while other possible modifications are represented by deiodination (Piehl et al., 2008) and sulphorylation (Pietsch et al., 2007). Therefore, it seemed interesting to investigate whether the pro-learning and hyperalgesic effects of T1AM were affected by the MAO inhibitor clorgyline, which was administered i.p. at a dose of 2.5 mg·kg−1 (preliminary experiments showed that i.p. injection of vehicle had no effect either in the vehicle group or in the T1AM-treated group).
In the passive avoidance paradigm, the effect of T1AM was lost after pretreatment with clorgyline (Figure 1B). With regard to pain threshold experiments (Figure 4B), in the presence of clorgyline T1AM still produced a hyperalgesic action, but the dose–response relationship was modified, because a significant effect was detected only with 0.134 μg·kg−1 T1AM (P < 0.05 vs. vehicle), but not with higher concentrations. Notably, clorgyline treatment per se appeared to increase pain sensitivity, because latencies were in general lower (P < 0.05) than those observed in the previous set of experiments (Figure 4A)
T1AM raised plasma glycaemia
Plasma glycaemia is sometimes regarded as a fingerprint of T1AM effects. This was confirmed in the present investigation because i.c.v. T1AM injection increased blood glucose concentration at doses ranging from 0.13 to 1.32 μg·kg−1 (Figure 5). So, hyperglycaemia occurred even at doses that did not produce any behavioural effect. Quantitative analysis showed that the EC50 values for the effects on memory, curiosity and pain threshold ranged from 0.67 to 1.06 μg·kg−1. The potency of T1AM in raising plasma glycaemia appears to be even higher, as even the lowest dose used (0.132 μg·kg−1) produced a maximum response.
Figure 5.

Blood samples were collected from the tail vein of fed mice injected i.c.v. with vehicle or with T1AM at dosages of 0.13, 0.4, 1.32 μg·kg−1 (n = 5 in each group). Glycaemia was measured by a glucorefractometer 30 min after i.c.v. injections. Results are expressed as mean ± SEM;*P < 0.05 versus vehicle.
T1AM induced ERK1/2 phosphorylation in different cerebral areas
Memory acquisition and storage are typically associated with increased ERK1/2 phosphorylation and increased expression of transcription factors c-fos and pCREB. After treatment with 1.32 μg·kg−1 T1AM, pERK could be detected, but its levels were not significantly different from those found in vehicle-injected mice (data not shown). However, after treatment with T1AM at doses of 0.132 and 0.04 μg·kg−1, that is, 10-fold and 33-fold lower than the lowest dose proved to be effective on memory acquisition and retention, pERK turned out to be significantly higher than that observed in the vehicle group (Figure 6).
Figure 6.

Thirty minutes after i.c.v injection of T1AM (0.04, 0.13 μg·kg−1) or vehicle, mice (n = 3 per group) were killed and brain regions separated as described in the Methods section. Immunodetection for pERK was carried out on protein lysates from each region separated on SDS-PAGE gels. Results are the mean ± SEM of the densitometry of three different gels. (A) Results of a typical experiment. (B) Cumulative results. *P < 0.05 versus vehicle.
Similar to pERK, pCREB, pAKT and c-fos were not significantly modified in any brain area by 1.32 μg·kg−1 T1AM (data not shown).
Brain levels of thyroid hormones and T1AM
To exclude the possibility that the behavioural effects of T1AM were affected by fluctuations of the thyroid hormones, we measured T3, T4 and T1AM concentrations in the brains of mice injected with 1.32 μg·kg−1 T1AM. Brain T3 and T4 levels, although quite variable, were consistent with previous reports in rodents (Escobar-Morreale et al., 1996; Pinna et al., 2002) and were not significantly modified after T1AM administration. The latter produced a 34-fold increase in brain T1AM concentration. Taking into account brain weight, it was calculated that less than 10% of the injected T1AM was recovered in the brain after 30 min (Table 1). Although our method allows the detection of T1AM catabolites such as thyronamine, 3-iodothyroacetic acid and thyroacetic acid (Saba et al., 2010), none of these compounds was observed, suggesting that their brain concentrations were below the limits of detection of the assay.
Table 1.
Thyroid hormone and T1AM levels measured in the brains of mice injected i.c.v. with T1AM (1.32 μgkg−1)
| Treatment | T1AM recovered (pmol·g−1) | T3 (ng·g−1) | T4 (ng·g−1) |
|---|---|---|---|
| Vehicle (n = 6) | 0.39 ± 0.102 | 2.28 ± 0.8 | 3.32 ± 1.6 |
| T1AM (231 pmol·g−1 of brain; n = 6) * | 13.1 ± 0.10 | 2.31 ± 0.12 | 2.60 ± 0.35 |
Calculated considering the mean weight of brains as 0.432 ± 0.05 g.
Discussion
We report for the first time that T1AM given i.c.v. behaves as a memory enhancer in mice. This effect was achieved by stimulating curiosity without modifying locomotor activity or producing any analgesic effect. At the dosages active on memory, T1AM also turned out to be hyperalgesic. Interestingly, T1AM was similarly potent at stimulating memory and curiosity, suggesting that increased exploratory activity is part of the memory-enhancing effect, whereas it appeared to be more potent at producing hyperalgesic and hyperglycaemic effects. The latter therefore represents a sort of ‘fingerprint’ of T1AM pharmacological properties.
In cell cultures and in isolated hearts, T1AM showed a short half-life largely due to cellular uptake and oxidative deamination to 3-iodothyroacetic acid (Saba et al., 2010). This observation was confirmed in our experimental setting. By 30 min after its i.c.v. injection, the brain T1AM concentration had decreased from a nominal value of 231 pmol·g−1 to 13 pmol·g−1, consistent with its degradation and/or transport into the systemic circulation (Manni et al., 2012). Therefore, we wondered whether T1AM metabolite(s) might contribute to its effects. To ascertain the role of oxidative deamination, we repeated the passive avoidance and the hot plate tests in animals pretreated with clorgyline, which has been reported to increase T1AM concentration and to abolish the production of deaminated derivatives, particularly of 3-iodothyroacetic acid (Saba et al., 2010; Manni et al., 2012). Under these conditions, in the passive avoidance test, the response to T1AM was markedly reduced and did not reach the threshold of statistical significance. In the pain threshold experiments, a significant response was still observed, but the dose–response relationship was modified, as the dose of 0.13 μg·kg−1, which was ineffective in the absence of clorgyline, turned out to be hyperalgesic, while this effect disappeared at higher doses. These results suggest either that T1AM acts on rapid desensitizing targets, or that some of its effects are mediated by deaminated derivatives, such as 3-iodothyroacetic acid. Although the latter could not be detected in brain tissue, it should be pointed out that the assay procedure was optimized to detect T1AM, and had a low sensitivity for acid derivatives such as 3-iodothyroacetic acid (Saba et al., 2010).
The interpretation of our findings is further complicated by the observation that clorgyline per se induced a significant reduction in the pain threshold. This might imply that endogenous T1AM and/or its derivatives play a physiological role in pain sensitivity but might also be explained by the hypothesis that the response to T1AM is antagonized by different aminergic systems. Notably, the effect of T1AM on feeding was also modulated by clorgyline (Manni et al., 2012), while behavioural effects of MAO inhibition have been reported in different experimental models (Whitaker-Azmitia et al., 1994). These issues deserve further investigation, and in particular, it would be interesting to evaluate the effects of i.c.v. injections of 3-iodothyroacetic acid.
T1AM has been reported to interact with trace amine-associated receptor 1 (TA1 receptor), which is expressed together with other members of the TA receptor family in different brain regions (Zucchi et al., 2006). Interestingly, TAAR genes are located in a region that is associated with psychiatric disorders in linkage studies (Revel et al., 2011). However, the role of TA receptors could not be directly demonstrated in the present study because specific TA receptor antagonists are not available. Different receptors might also be involved, including α2 adrenoceptors, which have been implicated in the pancreatic response to T1AM (Regard et al., 2007).
ERK1/2 is a member of the family of MAPKs. The corresponding signalling cascade has been reported to activate cAMP-responsive element binding protein (CREB) and other transcription factors, inducing the synthesis of proteins that are required for the stabilization of new memories (Kida et al., 2002; Pittenger et al., 2002) and the regulation of long-term synaptic plasticity (Roberson and Sweatt, 1999). It is well known that pERK2 and pAkt are cross-talking signals involved in memory (Chen et al., 2008) and that pERK2 and pAkt exert opposite effects on the expression and activity of several transcription factors essential for the synthesis of new proteins necessary for memory stabilization, including CREB and c-fos (Peng et al., 2011). However, in this context, we were unable to detect modifications of pERK, pCREB or c-fos levels in any of the brain regions analysed following injection of 1.32 μg·kg−1 T1AM, while we detected significantly higher pERK levels after exposure to lower T1AM doses, namely 0.04 and 0.13 μg·kg−1. This finding suggests that ERK1/2 signalling might be quickly and selectively activated by low T1AM doses, whereas rapid desensitization occurs at higher doses. Memory acquisition requires activation of receptor cascades and synthesis of new proteins involved in memory retention, and ERK phosphorylation appears to be a very early event, which precedes gross behavioural effects, but its specific causal role remains to be determined. The observation that T1AM activates pERK in brain regions such as the amygdala and hippocampus might also be related to the hyperalgesic response (Schicho et al., 2005; Ji et al., 2009; Liu et al., 2012), which was elicited by low T1AM doses. The signalling cascade leading to the hyperalgesic effect is unknown, but T1AM might inhibit the release of analgesic mediators (Hu et al., 1957) on the basis of its putative effect on α2 adrenoceptors (Regard et al., 2007). In any case, the hyperalgic and pro-learning effects cannot be accounted for by changes in thyroid hormone concentration (Guasti et al., 2007), which were not detected in brain tissue after T1AM injection.
It has already been reported that i.c.v. injection of T1AM modulates insulin and/or glucagon secretion and produces a rise in plasma glycaemia (Manni et al., 2012). In the present work, we observed that hyperglycaemia occurs even at the dosage of 0.13 μg·kg−1, which is 10 times lower than that previously used (Manni et al., 2012). There is no evidence that moderate hyperglycaemia may produce behavioural effects similar to those reported in the present investigation. However, this issue deserves further investigation. In particular, it would be interesting to assess the behavioural effects of T1AM on the diabetic/hypothyroid mouse.
To the best of our knowledge, this is the first report indicating that the central effects of T1AM include the regulation of complex behavioural functions involved in learning and pain perception. These actions were associated with an increase in local T1AM concentration of about one order of magnitude, suggesting a novel potential physiological role of endogenous T1AM and/or its deaminated derivative(s). Thyroid hormones are essential for the development of mammalian brain and maintenance of optimal cognitive ability in different periods of life (Bauer et al., 2008). In adulthood, thyroid dysfunction leads to neurological and behavioural abnormalities, including memory impairment. Adult-onset hypothyroidism is also associated with clinically relevant cognitive dysfunctions such as psychotic behaviour, hallucinations, confusion and learning defects (Rivas and Naranjo, 2007). Central hypothyroidism has been reported in patients with Alzheimer's disease (Sampaolo et al., 2005) and the analysis of different experimental models suggests that the effects on cognition rely on hippocampal modifications. In the present work, we demonstrated that T1AM, an endogenous compound related to thyroid hormones, stimulates the acquisition of memory in the mouse and that this effect does not involve significant modifications of brain thyroid hormone levels. It should be noted, thyroid hormone levels found in the brains of our mice were similar to those demonstrated by Escobar-Morreale et al. (1996) and Pinna et al. (2002). Due to these novel effects, our results suggest that pharmacological administration of T1AM might be useful in neurodegenerative and endocrine disorders associated with memory deficits.
Acknowledgments
This work was supported by a grant from the Italian Council for University and Research.
Glossary
- CREB
cyclic AMP-responsive element binding
- PBST
PBS plus Tween
- T1AM
3-iodothyronamine
- T3
triiodothyroxine
- T4
thyroxine
- TA1 receptor
trace amine-associated receptor 1
Conflict of interest
The authors state the absence of any conflict of interest.
References
- Bauer M, Goetz T, Glenn T, Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol. 2008;20:1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen P, Tan H, Ma D, Dou F, Feng J, et al. Regulation of the NMDA receptor-mediated synaptic response by acetylcholinesterase inhibitors and its impairment in an animal model of Alzheimer's disease. Neurobiol Aging. 2008;29:1795–1804. doi: 10.1016/j.neurobiolaging.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillo WS, Bewick GA, White NE, Gardiner JV, Thompson EL, Bataveljic A, et al. The thyroid hormone derivative 3-iodothyronamine increases food intake in rodents. Diabetes Obes Metab. 2008;11:251–260. doi: 10.1111/j.1463-1326.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- Doyle KP, Suchland KL, Ciesielski TM, Lessov NS, Grandy DK, Scanlan TS, et al. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke. 2007;38:2569–2576. doi: 10.1161/STROKEAHA.106.480277. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Escobar del Rey F, Obregon MJ, Morreale de Escobar G. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rats. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Bartolini A, Ghelardini C. Blockade of intracellular calcium release induces an antidepressant-like effect in the mouse forced swimming test. Neuropharmacology. 2006;50:309–316. doi: 10.1016/j.neuropharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Galli E, Marchini M, Saba A, Berti S, Tonacchera M, Vitti P, et al. Detection of 3-iodothyronamine in human patients: a preliminary study. J Clin Endocrinol Metab. 2012;97:E69–E74. doi: 10.1210/jc.2011-1115. [DOI] [PubMed] [Google Scholar]
- Guasti L, Marino F, Cosentino M, Cimpanelli M, Rasini E, Piantanida E, et al. Pain perception, blood pressure levels, and peripheral benzodiazepine receptors in patients followed for differentiated thyroid carcinoma: a longitudinal study in hypothyroidism and during hormone treatment. Clin J Pain. 2007;23:518–523. doi: 10.1097/AJP.0b013e3180735e5e. [DOI] [PubMed] [Google Scholar]
- Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957;12:12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW., 4th Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal-regulated kinase-Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci. 2007;27:13181–13191. doi: 10.1523/JNEUROSCI.0269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik ME, Kopp R. An improved one-trial passive avoidance learning situation. Psychol Rep. 1967;21:221–224. doi: 10.2466/pr0.1967.21.1.221. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Peña de Ortiz S, Kogan JH, Chevere I, Masushige S, et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–355. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klieverik LP, Foppen E, Ackermans MT, Serlie MJ, Sauerwein HP, Scanlan TS, et al. Central effects of thyronamines on glucose metabolism in rats. J Endocrinol. 2009;201:377–386. doi: 10.1677/JOE-09-0043. [DOI] [PubMed] [Google Scholar]
- Liu L, Ji F, Liang J, He H, Fu Y, Cao M. Inhibition by dexmedetomidine of the activation of spinal dorsal horn glias and the intracellular ERK signaling pathway induced by nerve injury. Brain Res. 2012;1427:1–9. doi: 10.1016/j.brainres.2011.08.019. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, , Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni ME, De Siena G, Saba A, Marchini M, Bigagli E, Cinci L, et al. 3-Iodothyronamine: a modulator of the hypothalamus-pancreas-thyroid axes in mouse. Br J Pharmacol. 2012;166:650–658. doi: 10.1111/j.1476-5381.2011.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Zhang Y, Ren B, Zhang J, Wang H. Effect of ketamine administration on memory consolidation, p-CREB and c-fos expression in the hippocampal slices of minor rats. Mol Biol Rep. 2011;38:2401–2407. doi: 10.1007/s11033-010-0374-x. [DOI] [PubMed] [Google Scholar]
- Piehl S, Heberer T, Balizs G, Scanlan TS, Smits R, Koksch B, et al. Thyronamines are isozyme-specific substrates of deiodinases. Endocrinology. 2008;149:3037–3045. doi: 10.1210/en.2007-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl S, Hoefig CS, Scanlan TS, Köhrle J. Thyronamines – past, present, and future. Endocr Rev. 2011;32:64–80. doi: 10.1210/er.2009-0040. [DOI] [PubMed] [Google Scholar]
- Pietsch CA, Scanlan TS, Anderson RJ. Thyronamines are substrates for human liver sulfotransferases. Endocrinology. 2007;148:1921–1927. doi: 10.1210/en.2006-1172. [DOI] [PubMed] [Google Scholar]
- Pinna G, Brödel O, Visser T, Jeitner A, Grau H, Eravci M, et al. Concentrations of seven iodothyronine metabolites in brain regions and the liver of the adult rat. Endocrinology. 2002;143:1789–1800. doi: 10.1210/endo.143.5.8770. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, et al. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, et al. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA. 2011;108:8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas M, Naranjo JR. Thyroid hormones, learning and memory. Genes Brain Behav. 2007;6(Suppl 1):40–44. doi: 10.1111/j.1601-183X.2007.00321.x. Review. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. A biochemical blueprint for long-term memory. Learn Mem. 1999;6:381–388. Review. [PMC free article] [PubMed] [Google Scholar]
- Saba A, Chiellini G, Frascarelli S, Marchini M, Ghelardoni S, Raffaelli A, et al. Tissue distribution and cardiac metabolism of 3-iodothyronamine. Endocrinology. 2010;151:5063–5073. doi: 10.1210/en.2010-0491. [DOI] [PubMed] [Google Scholar]
- Sampaolo S, Campos-Barros A, Mazziotti G, Carlomagno S, Sannino V, Amato G, et al. Increased cerebrospinal fluid levels of 3,3′,5′-triiodothyronine in patients with Alzheimer's disease. J Clin Endocrinol Metab. 2005;90:198–202. doi: 10.1210/jc.2004-1083. [DOI] [PubMed] [Google Scholar]
- Scanlan TS. Minireview: 3-iodothyronamine (T1AM): a new player on the thyroid endocrine team? Endocrinology. 2009;150:1108–1111. doi: 10.1210/en.2008-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicho R, Liebmann I, Lippe IT. Extracellular signal-regulated kinase-1 and -2 are activated by gastric luminal injury in dorsal root ganglion neurons via N-methyl-D-aspartate receptors. Neuroscience. 2005;134:505–514. doi: 10.1016/j.neuroscience.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Snead AN, Santos MS, Seal RP, Miyakawa M, Edwards RH, Scanlan TS. Thyronamines inhibit plasma membrane and vesicular monoamine transport. ACS Chem Biol. 2007;2:390–398. doi: 10.1021/cb700057b. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Zhang X, Clarke C. Effects of gestational exposure to monoamine oxidase inhibitors in rats: preliminary behavioral and neurochemical studies. Neuropsychopharmacology. 1994;11:125–132. doi: 10.1038/npp.1994.42. [DOI] [PubMed] [Google Scholar]
- Wood WJ, Geraci T, Nilsen A, DeBarber AE, Scanlan TS. Iodothyronamines are oxidatively deaminated to iodothyroacetic acids in vivo. Chembiochem. 2009;10:361–365. doi: 10.1002/cbic.200800607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]


