Abstract
Background and Purpose
Prokineticin 2 (PK2) has recently been shown to acutely reduce food intake in rodents. We aimed to determine the CNS sites and receptors that mediate the anorectic effects of peripherally administered PK2 and its chronic effects on glucose and energy homeostasis.
Experimental Approach
We investigated neuronal activation following i.p. administration of PK2 using c-Fos-like immunoreactivity (CFL-IR). The anorectic effect of PK2 was examined in mice with targeted deletion of either prokineticin receptor 1 (PKR1) or prokineticin receptor 2 (PKR2), and in wild-type mice following administration of the PKR1 antagonist, PC1. The effect of IP PK2 administration on glucose homeostasis was investigated. Finally, the effect of long-term administration of PK2 on glucose and energy homeostasis in diet-induced obese (DIO) mice was determined.
Key Results
I.p. PK2 administration significantly increased CFL-IR in the dorsal motor vagal nucleus of the brainstem. The anorectic effect of PK2 was maintained in mice lacking the PKR2 but abolished in mice lacking PKR1 and in wild-type mice pre-treated with PC1. DIO mice treated chronically with PK2 had no changes in glucose levels but significantly reduced food intake and body weight compared to controls.
Conclusions and Implications
Together, our data suggest that the anorectic effects of peripherally administered PK2 are mediated via the brainstem and this effect requires PKR1 but not PKR2 signalling. Chronic administration of PK2 reduces food intake and body weight in a mouse model of human obesity, suggesting that PKR1-selective agonists have potential to be novel therapeutics for the treatment of obesity.
Keywords: obesity, prokineticin 2, PK2, mice, hypothalamus, brainstem, food intake, body weight
Introduction
The control of body weight is a complex physiological process. There is a critical need to identify novel regulatory pathways in energy homeostasis, which are suitable for pharmacological manipulation in order to treat patients with obesity.
Prokineticin 2 (PK2) is an 81-amino-acid peptide that binds to and activates two G-protein-coupled receptors, the PK receptor 1 (PKR1) and the PK receptor 2 (PKR2) (Lin et al., 2002; Masuda et al., 2002; Soga et al., 2002). PK2 is highly expressed in several regions of the CNS, including the hypothalamus, with lower levels of expression in peripheral tissues (Wechselberger et al., 1999; Li et al., 2001; Cheng et al., 2006). PKR1 is expressed in discrete CNS regions such as the dorsal motor nucleus (DMN) of brainstem, hypothalamic regions such as the arcuate nucleus (ARC), dorsomedial hypothalamus (DMH) and zona incerta, and focal areas of the olfactory system and hippocampus. PKR2 has a wide pattern of expression throughout the CNS, but is not expressed in the DMN. PK2 has been implicated in numerous physiological processes; for example, it potently stimulates contraction of gastrointestinal smooth muscle and is involved in neurogenesis and development of the CNS (Li et al., 2001; Prosser et al., 2007a). PK2 mRNA expression is most concentrated in the suprachiasmatic nucleus of the hypothalamus, the site of the master circadian clock, and PK2 is thought to have a role in regulation of circadian output (Cheng et al., 2002; 2005; Li et al., 2006; Prosser et al., 2007b). Levels of hypothalamic PK2 expression are low when rodents feed (during the dark phase). Furthermore, a mouse model with targeted deletion of PK2 and humans with homozygous inactivating mutations in PK2 or the PK2 receptor develop obesity (Li et al., 2006; Sarfati et al., 2010). This evidence suggests that PK2 may be a novel regulator of food intake in humans. Consistent with this hypothesis, we recently demonstrated that i.c.v, administration of PK2 to rats potently reduces food intake, and that its anorectic effect may be mediated by the hypothalamic melanocortin system (Gardiner et al., 2010). Additionally, we have shown that acute peripheral administration of PK2 to lean and diet-induced obese (DIO) mice significantly reduces food intake and body weight (Gardiner et al., 2010). This suggests that the PKR1 and/or 2 may be a potential target for the development of novel anti-obesity therapies. It is therefore important to determine the mechanism of action of peripherally administered PK2 on food intake, its effects on glucose homeostasis and determine the efficacy of chronic administration of PK2 in obesity. We have investigated the region of the CNS and receptor, which mediates the anorectic effects of acute peripherally administered PK2. We have also investigated the effect of acute administration of PK2 on glucose homeostasis. Finally, we determined the effect of chronic peripheral administration of PK2 on food intake, body weight and glucose homeostasis in a mouse model of human obesity. These data have important implications for the therapeutic potential of PK2-mimetics as anti-obesity agents.
Methods
Animals
Studies 1, 5 and 6
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). Animal procedures undertaken were approved by the British Home Office Animals (Scientific Procedures) Act 1986 (Project License 70/6402). Adult male C57Bl/6 mice weighing 20–25 g (Harlan, Bicester, UK) were singly housed in individually ventilated cages (width 24.5 cm, length 41.5 cm and depth 18.5 cm) at 21–23°C with a 12 h light/dark cycle. Animals had ad libitum access to food (RM1 diet; SDS, Witham, UK) and water, unless specified in procedure protocol. For the chronic study, adult male C57Bl/6J mice (Harlan, the Netherlands) were maintained as above but had ad libitum access to a 60 kcal% fat diet (D12492, Research Diets Inc., New Brunswick, NJ) supplemented with chocolate Delicato Balls (Delicato, Segeltorp, Sweden) for 13 weeks prior to and during the study.
Study 2
PKR2 knockout mice were generated as previously described using the C57BL/6-Tyrc-Brd mouse strain (Prosser et al., 2007b). Female animals were used since only 1/10 male animals were homozygotes. All female PKR2 homozygotes had unopened vaginal orifices, indicating sexual immaturity.
Studies 3 and 4
All experiments were performed under protocols approved by the Animal Care and Use Committee of the Italian Ministry of Health according to European Commission directives. Pkr1-null mutant mice were generated by Lexicon Genetics (The Woodlands, TX) as already described (Negri et al., 2006). Germ-line chimeras were crossed with C57BL/6J females to generate heterozygotes and then inter-crossed, giving rise to overtly healthy mutant offspring in the expected Mendelian ratio. Progeny were genotyped with PCR. Pkr1-null mice were viable and largely indistinguishable from wild-type littermates. Adult male PKR1(+/+) and PKR1(–/–) mice, weighing 20–23 g, were housed in temperature-controlled rooms (22–25°C) with access to water and food ad libitum.
Experimental design and measurements
Study 1. Effect of peripheral PK2 administration on c-fos-like immunoreactivity in the brainstem and hypothalamus
Male C57Bl/6 mice were administered either 540 nmol kg−1 PK2 (Peprotech, London, UK) or saline i.p. in the early light phase (n = 6 per group). This dose of PK2 was chosen as it has been shown to significantly reduce food intake in mice (Gardiner et al., 2010). Ninety minutes later, mice were killed by i.p. injection of pentobarbitone. C-fos-like immunoreactivity was determined using immunocytochemistry for c-fos on brain sections as previously described (Batterham et al., 2002). Total numbers of c-fos-positive cells were counted bilaterally in matched sections from hypothalamic nuclei and the brainstem.
Study 2. Effect of peripheral PK2 administration on food intake in PKR2 knockout mice
Ad libitum fed wild-type (n = 13) and PKR2 knockout female mice (n = 6) were administered either saline, 20 nmol kg−1 PK2 or 60 nmol kg−1 PK2 i.p. at the beginning of the dark phase. A crossover study design was used. These doses were chosen because they have previously been shown to significantly, but not maximally, reduce acute food intake (Gardiner et al., 2010). Following injection, a known amount of food was returned to the cages; and food was re-weighed at 1, 2, 4, 6 and 24 h post injection.
Study 3. Effect of peripheral PK2 administration on food intake in PKR1 knockout mice
Ad libitum fed male wild-type and male PKR1(–/–) mice (n = 6 per group) were administered saline, 20 nmol kg−1 PK2 or 60 nmol kg−1 PK2 i.p. at the beginning of the dark phase. These doses were chosen because they have previously been shown to significantly, but not maximally, reduce acute food intake (Gardiner et al., 2010). Following injection, a known amount of food was returned to the cages; and food was re-weighed at 1, 2, 4, 6 and 24 h post injection.
Study 4. Effect of peripheral PK2 administration on food intake in wild-type mice with or without co-administration of a PKR1 antagonist
Effects of PK2 administration (60 nmol kg−1 i.p.) were studied in mice at the beginning of the dark phase, in the presence of a previously described PKR1 antagonist, PC1 (Balboni et al., 2008; Giannini et al., 2009). The dose of PC1 (220 nmol kg−1 s.c.) was chosen since it is significantly reduces pain and inflammation (Giannini et al., 2009). Ad libitum fed male wild-type (n = 6–7 per group) were administered i.p. saline, PK2 alone, PC1 alone, or PK2 with PC1, at the beginning of the dark phase. Following injection, a known amount of food was returned to the cages; and food was re-weighed at 1, 2 and 12 h post injection.
Study 5. Effect of peripheral PK2 administration on glucose homeostasis
In order to assess the effects of PK2 on glucose tolerance, ad libitum fed male C57Bl/6 mice received an i.p. injection of 2 g kg−1 d-glucose. Animals were then immediately injected with either saline or 540 nmol kg−1 PK2 (n = 12 per group) in the early light phase. To determine the effects of PK2 on insulin tolerance, male C57Bl/6 mice were fasted for 24 h then given an i.p. injection of 1.5 units kg−1 Humulin (Eli Lilly, Basingstoke, UK), followed by either saline or 540 nmol kg−1 PK2 (n = 12 per group) in the early light phase.
In both studies, tail vein blood was collected at 0, 15, 30, 60, 90, 120 and 150 min after injection. Plasma glucose was measured using the Acensia Contour blood glucose monitoring system (Bayer HealthCare, Newbury, UK).
Study 6. Effect of chronic peripheral PK2 administration on food intake and body weight in DIO mice
DIO mice weighing 40–55 g were implanted s.c. with Alzet mini-osmotic pumps (Model 2002, ALZET, Cupertino, CA) as previously described (Thompson et al., #b1001; 700/id). The pumps contained either 0.9% saline (n = 11), PK2 (60 nmol kg−1 h−1 or 4.4 mg/168 μL) (n = 10) or PK2 (120 nmol kg−1 h−1 or 8.8 mg/168 μL) (n = 10) with sufficient peptide for continuous infusion for 14 days. These doses of PK2 were chosen as they have previously been shown to significantly reduce food intake when the hourly dose was administered as a single i.p. injection to mice (Gardiner et al., 2010). A further two groups (n = 11) were implanted with a pump containing saline and were pair-fed to the PK2-treated mice.
Food intake and body weight
Food intake and body weight were monitored daily for 13 days.
Behavioural analysis
In order to determine if chronic peripheral PK2 administration had any effect on behaviour, behavioural analysis was conducted on day 8 post surgery as previously described (Fray et al., 1980).
Plasma collection for hormone analysis
Mice were fasted overnight on day 13 of the study, and fasting plasma glucose was measured on the morning of day 14 by tail vein sampling and blood glucose meter (Ascensia, Bayer). Mice were then killed by asphyxiation with CO2. Plasma was collected by cardiac puncture for analysis of insulin and leptin.
Plasma assays
Plasma insulin was measured using a mouse plasma insulin elisa kit (Crystal Chem Inc., Downers Grove, IL). Plasma leptin was measured using elisa (Crystal Chem Inc.).
Data analysis and statistical procedures
Values are presented as mean ± SEM, except c-fos and behavioural analysis data, which are presented as median and inter-quartile range. For c-fos ICC data, the Mann–Whitney U-test was used. Data from the PKR2 knockout study and behavioural studies were compared using Kruskal–Wallis one-way anova on ranks. In the chronic study, differences in cumulative food intake and body weight through time were compared across experimental groups using generalized estimating equation curve analysis (Stata 9.1, Statacorp, College Station, TX). Plasma assays were analysed using a one-way anova, followed by post hoc Dunnett's test. Plasma glucose levels were compared using an unpaired Student's t-test. P-values < 0.05 were considered significant.
Results
Study 1: Peripheral PK2 administration increases c-fos-like immunoreactivity in the dorsal motor nucleus of the brainstem
I.p. administration of 540nmol kg−1 PK2 to C57Bl/6 mice significantly increased c-fos-like immunoreactivity in the dorsal motor vagal nucleus (DMV) of the brainstem compared with saline-injected controls at 90 minutes post-injection [c-fos counts in DMV median (interquartile range): saline 0 (0–1), PK2 18 (9.5–34), n = 6 per group] (Figure 1A,B). No significant differences in c-fos expression were observed in the other areas of the brainstem or hypothalamus (Figure 1C).
Figure 1.
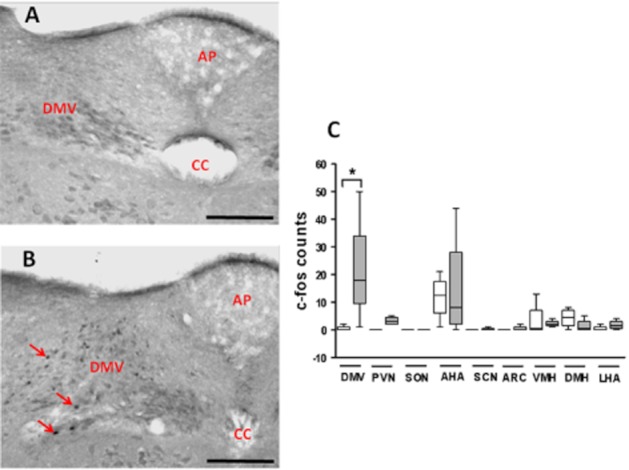
Effect of saline or PK2 on c-fos-like immunoreactivity in the brain of mice. (A, B) Representative light-field microphotographs showing the effect of i.p. saline (A) or 540 nmol kg−1 PK2 (B) administration on c-fos-like immunoreactivity in the brain of mice. The arrows indicate cfos-like immunoreactivity in the DMV nucleus of the brainstem in PK2-treated animals. AP = area postrema, CC = central canal. A and B: 200× magnification. Scale bars are equivalent to 100 μm, n = 6 per group. (C) Median and interquartile ranges of c-fos-like immunoreactivity in DMV, PVN, SON, AHA, suprachiasmatic nucleus (SCN), ARC, ventromedial nucleus (VMH), dorsomedial nucleus (DMH) and lateral hypothalamic area (LHA). The saline and 540 nmol kg−1 PK2 groups are denoted by the left and right bars in each pair respectively.
Study 2: Peripherally administered PK2 retains its anorectic effects in PKR2 knockout mice
I.p. administration of 20 and 60 nmol kg−1 PK2 significantly reduced 0–1 h food intake in both wild-type and PKR2 knockout mice (Figure 2). There were no significant changes in food intake for PK2-injected animals compared with saline controls at any other time point in wild-type or PKR2 knockout mice (Supplementary Table S1).
Figure 2.
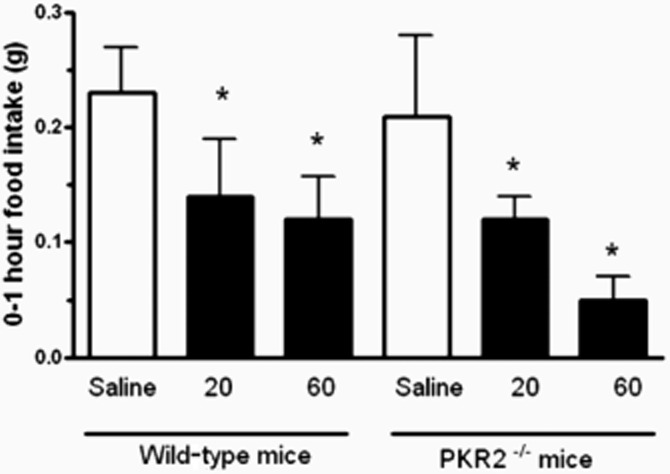
Food intake in wild-type and PKR2 knockout mice following saline or PK2 injection. 0–1 h food intake in wild-type and PKR2 knockout mice following i.p. injection of either saline, 20 nmol kg−1 PK2 or 60 nmol kg−1 PK2. *P < 0.05 versus saline, n = 6–13 per group.
Study 3: Peripherally administered PK2 does not alter food intake in PKR1 knockout mice
I.p. administration of 20 and 60 nmol kg−1 PK2 significantly reduced 0–1 h food intake in wild-type, but not in PKR1(–/–) mice (Figure 3). There were no significant changes in food intake for PK2-injected animals compared with saline controls at any other time point (Supplementary Table S2).
Figure 3.
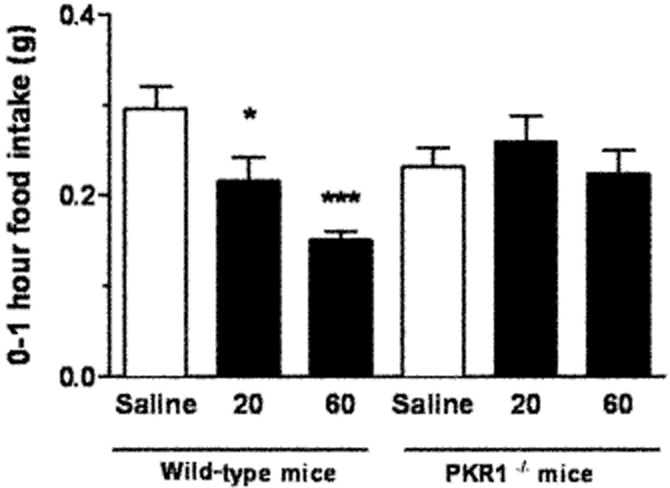
Food intake in wild-type and PKR1 knockout mice following saline or PK2 injection. 0–1 h food intake in wild-type and PKR1 knockout mice following i.p. injection of either saline, 20 nmol kg−1 PK2 or 60 nmol kg−1 PK2. *P < 0.05 versus saline, n = 6 per group.
Study 4. Peripheral PK2 administration does not alter food intake in wild type mice with co-administration of a PKR1 antagonist
S.c. injection of the PKR1-preferring antagonist, PC1 (220 nmol kg−1), had no effect on spontaneous feeding in male mice (Figure 4). As previously observed, i.p. administration of 60 nmol kg−1 PK2 significantly reduced 0–1 h food intake in male mice; however, this dose of PK2 had no effect on 0–1 h food intake in the presence of PC1 (Figure 4).
Figure 4.
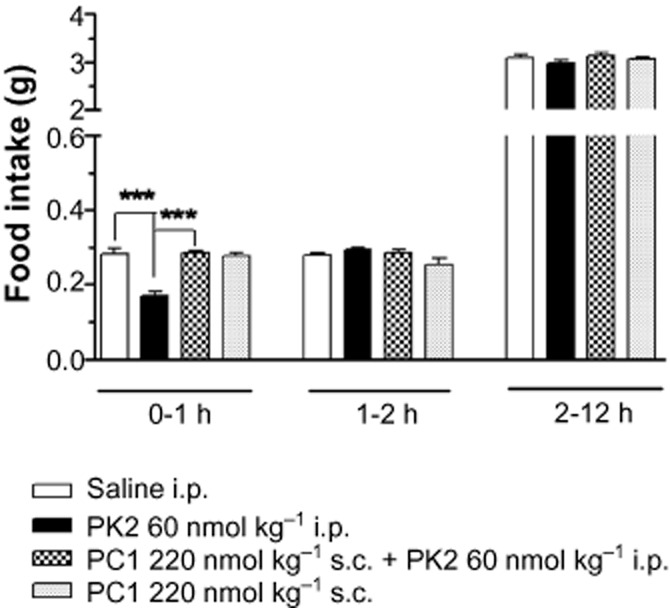
Food intake in wild-type mice following saline or PK2 injection in the presence of a PKR1 antagonist, PC1. Food intake at 0–1, 1–2 and 2–12 h in wild-type mice following administration of saline, PK2 alone, PC1 alone or PK2 with PC1. PK2 was administered i.p. at the dose, 60 nmol kg−1. PC1 was administered s.c. at the dose, 220 nmol kg−1. ***P < 0.001; n = 6–7 per group.
Study 5: Peripheral PK2 administration has no effect on glucose homeostasis
Administration of 540 nmol kg−1 PK2 had no effect on plasma glucose following i.p. glucose administration (Figure 5A). Similarly, following i.p. insulin administration, there were no differences in plasma glucose between animals administered saline or 540 nmol kg−1 PK2 at any time point studied (Figure 5B).
Figure 5.
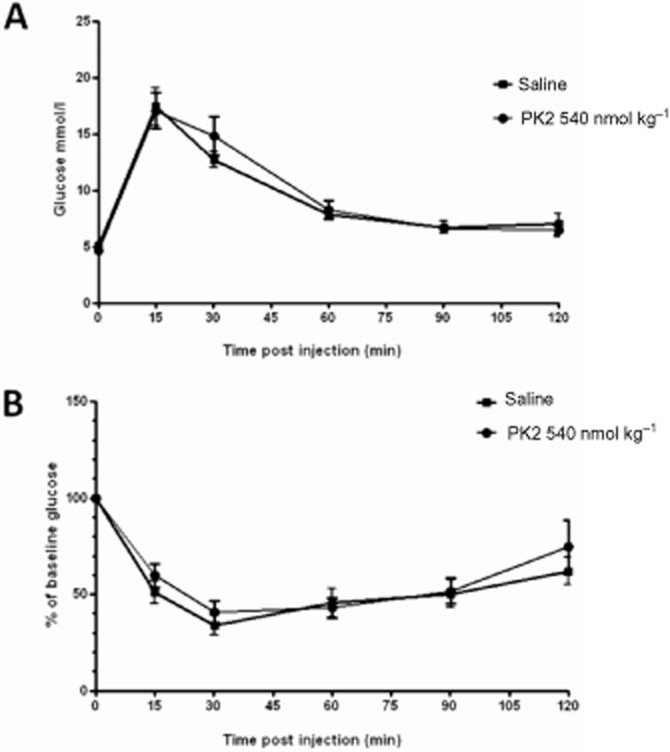
Effect of saline or PK2 on glucose homeostasis in mice. Plasma glucose levels in mice injected with either 2 g kg−1 d-glucose (A) or 1.5 units kg−1 Humulin (B) followed by saline or 540 nmol kg−1 PK2 treatment. Plasma glucose was measured at 0, 15, 30, 60, 90, 120 and 150 min after injection. n = 12 per group.
Study 6: Chronic peripheral administration of PK2 reduces food intake and body weight in diet-induced obese mice
Food intake and body weight
Administration of 60 nmol kg−1 h−1 PK2 significantly reduced cumulative food intake between days 1 and 3 post surgery compared with saline controls, and administration of 120 nmol kg−1 h−1 PK2 significantly reduced cumulative food intake between days 1 and 9 compared with saline controls (Figure 6A). PK2 administered at 60 nmol kg−1 h−1 and 120 nmol kg−1 h−1 significantly reduced body weight gain between day 1 and day 13 post surgery compared with saline controls. Mice pair-fed to each dose of PK2 lost a similar amount of weight to those administered PK2 (Figure 6B).
Figure 6.
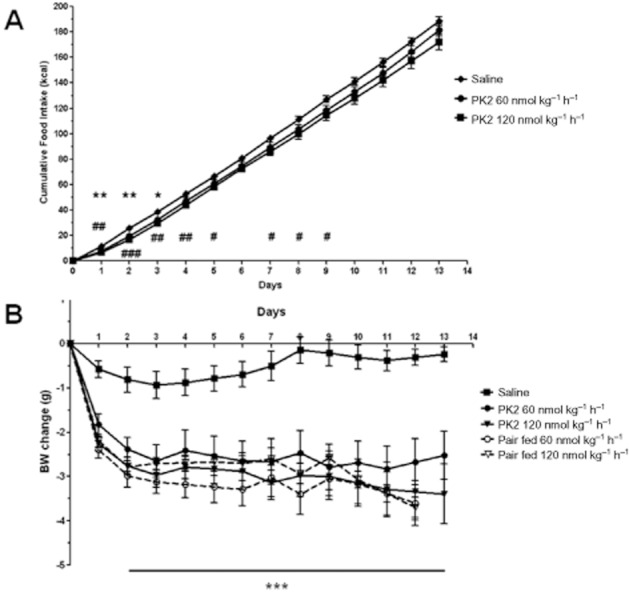
Effect of chronic administration of saline or PK2 on food intake and body weight in diet-induced obese mice. Cumulative food intake (A) and body weight change (B) in diet-induced obese mice implanted with a mini-osmotic pump containing either saline, PK2 (60 nmol kg−1 h−1) or PK2 (120 nmol kg−1 h−1). Figure 4: *P < 0.05, **P < 0.01 60 nmol kg−1 h−1 PK2 versus saline; #P < 0.05, ##P < 0.01, ###P < 0.001 120 nmol kg−1 h−1 PK2 versus saline, n = 10–11 per group. Figure 4: ***P < 0.001 all groups versus saline.
Behavioural analysis
Administration of 120 nmol kg−1 h−1 PK2 significantly reduced sleeping behaviour compared with saline controls [sleeping episodes median (interquartile range): saline 15 (15–20.5), PK2 13 (9–15), P < 0.05, n = 10–11 per group]. No other differences in behaviour were observed between groups (data not shown).
Plasma analysis
At the termination of the study, there was no significant difference in fasting plasma glucose, insulin or leptin in PK2-treated mice when compared with saline (Table 1). There was a significant reduction in plasma glucose and insulin in the two pair-fed groups compared with saline controls.
Table 1.
The effect of a 13 day infusion of saline or PK2 on fasting plasma glucose, insulin and leptin in mice
| Saline | PK2 60 nmol kg−1 h−1 | Pair fed to PK2 60 nmol kg−1 h−1 | PK2 120 nmol kg−1 h−1 | Pair fed to PK2 120 nmol kg−1 h−1 | |
|---|---|---|---|---|---|
| Glucose (mmol L−1) | 8.34 ± 0.44 | 7.74 ± 0.26 | 7.77 ± 0.33 | 7.03 ± 0.52 | 7.57 ± 0.26 |
| Insulin (ng mL−1) | 2.46 ± 0.31 | 1.87 ± 0.18 | 1.60 ± 0.20* | 1.77 ± 0.26 | 1.41 ± 0.16** |
| Leptin (ng mL−1) | 26.68 ± 0.82 | 24.69 ± 0.81 | 25.36 ± 0.83 | 24.93 ± 0.87 | 26.12 ± 0.62 |
Three groups of ad libitum fed mice (n = 10–11 per group) received a 13 day infusion of saline or 60 or 120 nmol kg−1 h−1 PK2. Two further groups of mice also received saline but were pair fed to the PK2-treated groups. Fasting plasma glucose, insulin and leptin were measured on day 14. *P < 0.05, **P < 0.01 versus saline.
Discussion
We previously demonstrated that PK2 potently inhibits food intake in rodents, which suggests that it may be a novel regulator of food intake (Gardiner et al., 2010). In the current study, we observed that peripheral administration of PK2 significantly reduced food intake in mice. PK2 binds to and activates two closely related G-protein-coupled receptors, the PKR1 and PKR2 (Lin et al., 2002; Masuda et al., 2002; Soga et al., 2002). PK2 has similar efficacy at the PKR1 and the PKR2 (Soga et al., 2002). It was therefore important to investigate which receptor subtype mediates the anorectic effects of PK2. We observed that peripherally administered PK2 (20 or 60 nmol kg−1) inhibited food intake in mice lacking a functional PKR2 gene but had no effect on food intake in mice lacking a functional PKR1 gene. Furthermore, we have demonstrated that the anorectic effects of PK2 are abolished by co-administration of a PK1R antagonist. Our data therefore suggest that peripherally administered PK2 inhibits food intake through a PKR1- rather than PKR2-dependent mechanism. We cannot exclude that higher doses of PK2 than the doses studied (20 and 60 nmol kg−1) would inhibit food intake in a PKR2-dependent manner; however, our data suggest that PKR1 is the receptor that mediates the anorectic effects of peripherally administered PK2.
We previously demonstrated that i.c.v. administration of PK2 increased c-fos immunoreactivity within the hypothalamic arcuate nucleus (ARC) supraoptic nucleus (SON), ARC, paraventricular nucleus (PVN) and anterior hypothalamic area (AHA) (Gardiner et al., 2010); and direct injection of PK2 into these same nuclei significantly reduced food intake. By contrast, peripheral administration of PK2 does not increase c-fos immunoreactivity in any of these hypothalamic regions. Therefore, both central and peripheral PK2 administration inhibit food intake; however, our data suggest that hypothalamic activation is associated with central but not peripheral PK2 administration.
PKR1 is known to be expressed in the DMV nucleus of the brainstem (Cheng et al., 2006). Furthermore, we observed elevated c-fos immunoreactivity (a marker of neuronal activation) within the DMV following peripheral PK2 administration. This suggests that peripherally administered PK2 may directly act through the DMV to inhibit food intake. However, we cannot exclude that the anorectic effects of peripheral PK2 are mediated through a peripheral, intermediary signal.
A recent report has suggested that administration of PK2 impaired glucose clearance in a glucose tolerance test (Zhou et al., 2009). It was therefore important to investigate the effect of acute and chronic PK2 administration on glucose homeostasis, particularly if PK2 is to be used as a therapeutic target for the treatment of obesity. Our data suggest that acute or chronic PK2 administration does not have an adverse effect on glucose homeostasis. The differences in the effects of PK2 on glucose homeostasis in our studies and the previous report may have been due to the different doses of PK2 administered, or differences in animal housing conditions.
In order to determine the therapeutic potential of PK2 as an anti-obesity agent, we determined the effects of chronic administration of PK2 in DIO mice, which provides a mouse model of human obesity. Continuous administration of PK2 to DIO mice significantly reduced food intake and body weight compared with saline controls. There was no attenuation of the effect of PK2 on body weight, suggesting that tachyphylaxis did not occur. Animals pair-fed to both PK2 treatment groups lost a similar amount of weight to animals administered PK2, suggesting that the reduction in body weight was primarily mediated by a decrease in food intake and not an increase in energy expenditure.
Negri et al. (2004) previously studied the effects of the reptilian homologue of PK2, Bv8, on food intake in rats. They observed that Bv8 inhibited food intake for up to 12 h following administration. It would be interesting to determine if differential pharmacokinetics between PK2 and Bv8 explain their contrasting durations of anorectic action.
In summary, we have demonstrated that peripherally administered PK2 acts via the brainstem to reduce food intake in mice. This effect is not dependent upon signalling through the PKR2. Importantly, PK2 did not adversely affect glucose homeostasis. Chronic continuous infusion of PK2 significantly reduced body weight and food intake in a mouse model of human obesity. Our data suggest that PKR1-selective agonists have potential to be novel therapeutics for the treatment of obesity.
Acknowledgments
The section is funded by grants from the MRC, BBSRC, NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, an FP7- HEALTH- 2009- 241592 EurOCHIP grant and is supported by the NIHR Imperial Biomedical Research Centre Funding Scheme. SSH is funded by a Welcome Trust Clinical Research Fellowship. CNJ is funded by an NIHR Clinical Lectureship, AMS/Wellcome Starter Grant, and SFE Early Career Grant. WSD is funded by an NIHR Career Development Fellowship. Study 4 was partly funded by an academic grant from Astra Zeneca.
Glossary
- AHA
anterior hypothalamic area
- ARC
arcuate nucleus
- CFL-IR
c-fos-like immunoreactivity
- DIO
diet-induced obesity
- DMH
dorsomedial nucleus
- DMV
dorsal motor vagal nucleus of the brainstem in
- PVN
paraventricular nucleus
- LHA
lateral hypothalamic area
- PK2
prokineticin 2
- PKR1
prokineticin receptor 1
- PKR2
prokineticin receptor 2
- SON
supraoptic nucleus
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1 Effect of i.p. administration of saline or PK2 to wild-type or PKR2 knockout mice.
Table S2 Effect of i.p. administration of saline or PK2 to wild-type or PKR1 knockout mice.
References
- Balboni G, Lazzari I, Trapella C, Negri L, Lattanzi R, Giannini E, et al. Triazine compounds as antagonists at Bv8-prokineticin receptors. J Med Chem. 2008;51:7635–7639. doi: 10.1021/jm800854e. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bittman EL, Hattar S, Zhou QY. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci. 2005;6:17. doi: 10.1186/1471-2202-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Leslie FM, Zhou QY. Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol. 2006;498:796–809. doi: 10.1002/cne.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray PJ, Sahakian BJ, Robbins TW, Koob GF, Iversen SD. An observational method for quantifying the behavioural effects of dopamine agonists: contrasting effects of d-amphetamine and apomorphine. Psychopharmacology (Berl) 1980;69:253–259. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- Gardiner JV, Bataveljic A, Patel NA, Bewick GA, Roy D, Campbell D, et al. Prokineticin 2 is a hypothalamic neuropeptide that potently inhibits food intake. Diabetes. 2010;59:397–406. doi: 10.2337/db09-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini E, Lattanzi R, Nicotra A, Campese AF, Grazioli P, Screpanti I, et al. The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proc Natl Acad Sci U S A. 2009;106:14646–14651. doi: 10.1073/pnas.0903720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, et al. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou H. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, De Felice M, Colucci A, Melchiorri P. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. Br J Pharmacol. 2004;142:141–151. doi: 10.1038/sj.bjp.0705686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Colucci A, Mignogna G, Barra D, et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26:6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Caldwell MA. Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci. 2007a;26:3339–3344. doi: 10.1111/j.1460-9568.2007.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc Natl Acad Sci U S A. 2007b;104:648–653. doi: 10.1073/pnas.0606884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfati J, Dodé C, Young J. Kallmann syndrome caused by mutations in the PROK2 and PROKR2 genes: pathophysiology and genotype-phenotype correlations. Front Horm Res. 2010;39:121–132. doi: 10.1159/000312698. [DOI] [PubMed] [Google Scholar]
- Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, et al. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, et al. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab. 2006;291:E1074–E1082. doi: 10.1152/ajpendo.00040.2006. [DOI] [PubMed] [Google Scholar]
- Wechselberger C, Puglisi R, Engel E, Lepperdinger G, Boitani C, Kreil G. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett. 1999;462:177–181. doi: 10.1016/s0014-5793(99)01473-8. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li JD, Huang Q. 2009. Prokineticin receptor antagonists and uses thereof. PCT/US2009/068304(WO 2010/077976 A2), 1-88.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


