Abstract
Background and Purpose
Designer β-keto amphetamines (e.g. cathinones, ‘bath salts’ and ‘research chemicals’) have become popular recreational drugs, but their pharmacology is poorly characterized.
Experimental Approach
We determined the potencies of cathinones to inhibit DA, NA and 5-HT transport into transporter-transfected HEK 293 cells, DA and 5-HT efflux from monoamine-preloaded cells, and monoamine receptor binding affinity.
Key Results
Mephedrone, methylone, ethylone, butylone and naphyrone acted as non-selective monoamine uptake inhibitors, similar to cocaine. Mephedrone, methylone, ethylone and butylone also induced the release of 5-HT, similar to 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and other entactogens. Cathinone, methcathinone and flephedrone, similar to amphetamine and methamphetamine, acted as preferential DA and NA uptake inhibitors and induced the release of DA. Pyrovalerone and 3,4-methylenedioxypyrovalerone (MDPV) were highly potent and selective DA and NA transporter inhibitors but unlike amphetamines did not evoke the release of monoamines. The non-β-keto amphetamines are trace amine-associated receptor 1 ligands, whereas the cathinones are not. All the cathinones showed high blood–brain barrier permeability in an in vitro model; mephedrone and MDPV exhibited particularly high permeability.
Conclusions and Implications
Cathinones have considerable pharmacological differences that form the basis of their suggested classification into three groups. The predominant action of all cathinones on the DA transporter is probably associated with a considerable risk of addiction.
Keywords: designer drug, cathinone, amphetamine, legal high, monoamine transporter, serotonin, dopamine, noradrenaline
Introduction
Stimulant drug abuse remains a major public health issue worldwide. While ‘old stimulants’, including cocaine, methamphetamine and amphetamine, and ‘entactogens’, including 3,4-methylenedioxymethamphetamine (MDMA, ecstasy), 3,4-methylenedioxy-N-ethylamphetamine (MDEA) and 3,4-methlyenedioxyphenyl-N-methyl-2-butanamine (MBDB), continue to be used, novel designer cathinones are emerging. Cathinones differ from amphetamines by the presence of a ketone oxygen group at the β-position (Figure 1). The β-keto-amphetamines are distributed as ‘bath salts’, ‘research chemicals’ and ‘plant food’ via the Internet and have been advertised as ‘legal highs’ with similar psychotropic effects to MDMA or cocaine (Spiller et al., 2011).
Figure 1.
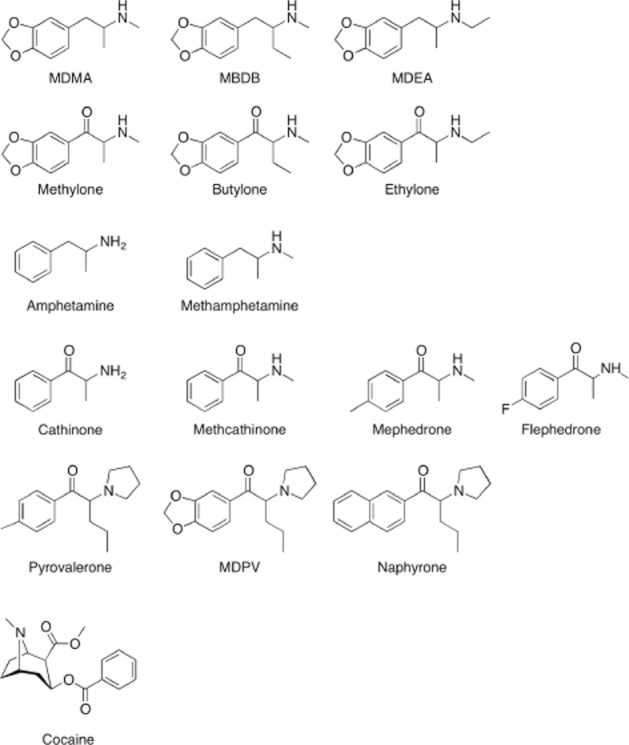
Chemical structures of cathinones, related amphetamines and cocaine.
As β-keto analogues of amphetamines, cathinones may be expected to have amphetamine-like effects because of their structural similarity. Cathinones enhance DA, NA and 5-HT neurotransmission (Hadlock et al., 2011; Kehr et al., 2011; Baumann et al., 2012; Lopez-Arnau et al., 2012; Martinez-Clemente et al., 2012). However, the molecular pharmacology of this novel class of stimulant drugs is poorly documented. In particular, a systematic comparative characterization of the effects of different cathinones on the human DA, NA and 5-HT transporters and comparisons with classic stimulants are lacking.
In the present study, we assessed the in vitro pharmacology of cathinone, methcathinone, mephedrone (4-methylmethcathinone), flephedrone (4-flouromethcathinone), methylone (3,4-methylenedioxymethcathinone, β-keto-MDMA), ethylone (3,4-methylenedioxyethylcathinone, β-keto-MDEA), butylone (β-keto-MBDB), pyrovalerone, 3,4-methylenedioxypyrovalerone (MDPV) and naphyrone (naphthylpyrovalerone). We determined the potencies of these cathinones to inhibit DA, NA and 5-HT transport in vitro. We also tested whether cathinones are releasers of DA or 5-HT and characterized the binding affinities of these drugs for monoamine transporters, dopamine D1–3 receptors, α1 and α2 adrenoceptors, 5-HT1A, 5-HT2A and 5-HT2C receptors, the trace amine-associated receptor 1 (TA1 receptor) and the histamine H1 receptor. Finally, blood-brain–barrier (BBB) permeability was assessed using a human in vitro model. The pharmacological profiles of the novel cathinones were compared with their non-β-keto amphetamine analogues, including MDMA, MDEA, MBDB, amphetamine and methamphetamine as well as with cocaine.
Methods
The drug target nomenclature conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Drugs
The hydrochloride salts of the drugs (purity >98.5%) were supplied by Lipomed (Arlesheim, Switzerland), with the exception of naphyrone, which was synthesized according to Meltzer et al. (2006). Racemic drugs were used except for d-amphetamine and d-methamphetamine.
Radioligand binding
The radioligand binding assays were performed as described previously (Revel et al., 2011; Hysek et al., 2012b). Briefly, membrane preparations from HEK 293 cells (Invitrogen, Zug, Switzerland) that overexpress the respective human transporters (Tatsumi et al., 1997) or receptors (except for rat/mouse TA1 receptor) (Revel et al., 2011) were incubated with the radiolabelled selective ligands at concentrations equal to Kd, and ligands displacement by the compounds was measured. Specific binding of the radioligand to the target receptor was defined as the difference between the total binding and nonspecific binding determined in the presence of selected competitors in excess. The following radioligands and competitors were used: N-methyl-[3H]-nisoxetine and indatraline (NA transporter [NET]), [3H]-citalopram and indatraline (5-HT transporter [SERT]) and [3H]-WIN35,428 and indatraline (DA transporter [DAT]). [3H]-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) and indatraline (5-HT1A receptor), [3H]-ketanserin and spiperone (5-HT2A receptor), [3H]-mesulergine and mianserin (5-HT2C receptor), [3H]-prazosin and risperidone (α1 adrenoceptor), [3H]-rauwolscine and phentolamine (α2 adrenergic receptor), [3H]-SCH 23390 and butaclamol (DA D1 receptor), [3H]-spiperone and spiperone (DA D2 and D3 receptors), [3H]-pyrilamine and clozapine (histamine H1 receptor) and [3H]-RO5166017 and RO5166017 (TA1 receptor). All radioligands were obtained from Perkin-Elmer (Schwerzenbach, Switzerland), with the exception of [3H]-RO5166017, which was synthesized at Roche (Basel, Switzerland).
Monoamine uptake transporter inhibition
The potencies of the drugs to inhibit the SERT, NET and DAT were evaluated in HEK 293 cells that stably expressed human SERT, NET and DAT (Tatsumi et al., 1997) as previously described (Hysek et al., 2012b). The DAT/SERT ratio was calculated as 1/DAT IC50:1/SERT IC50.
Monoamine release
We assessed DAT- and SERT-mediated DA and 5-HT efflux in HEK 293 cells that overexpressed human DAT or SERT respectively. We cultured the cells in 24-well plates (XF24, Seahorse Biosciences, North Billerica, MA) coated with poly-d-lysine to 70–100% confluency. After removing the culture medium, we added 85 μL release buffer (Krebs–HEPES that contained 130 mM NaCl, 1.3 mM KCl, 2.2 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 10 mM HEPES, 10 mM d-glucose, 0.2 mg·mL−1 ascorbic acid and 10 μM pargyline) with 10 nM [3H]-5-HT (SERT cells) or 10 nM [3H]-DA and 1 μM unlabelled DA (DAT cells). We filled the cells with the respective radiolabelled monoamine for 20 min at 37°C. We then removed the buffer and washed twice with warm buffer. We induced [3H]-5-HT and [3H]-DA release by adding 1000 μL release buffer that contained the drugs in different concentrations or controls. We incubated the cells for 15 min at 37°C and shaked at 300 r.p.m. on a rotary shaker. We then stopped the release by removing the buffer and washing the cells twice with ice-cold buffer. The release time was based on the difference between drug-stimulated and spontaneous release (control) over time, which reached its maximum before 15 min. We then added 65 μL lysis buffer and lysed the cells overnight in a refrigerator. We mixed 50 μL of the cell lysate suspension with 2.5 mL UltimaGold and determined radioactivity. The radioactive counts in the cells where no drug was present in the release buffer (control) was defined as 100%, and the percentages of radioactivity that remained in the cells treated with drugs were calculated. Pure uptake transporter inhibitors, including imipramine, citalopram, cocaine and mazindol, have been shown to produce apparent substrate efflux from monoamine-preloaded HEK cells that is explained by inhibition of transporter-mediated re-uptake of the substrate that diffuses out of the cells (Scholze et al., 2000). The DAT inhibitor mazindol and SERT inhibitor citalopram reduced the amount of preloaded DA and 5-HT (mean ± SD) by 15.6 ± 7 and 19.6 ± 8%, respectively, at the maximal concentration of 100 μM (Emax) compared with controls. This nonspecific release was subtracted from total release at the maximal drug concentration of 100 μM to yield Emax values of specific transporter-mediated release. We considered any drug that produced significantly higher maximal DA efflux compared with mazindol to be a DA releaser and a drug that produced significantly higher maximal 5-HT efflux compared with citalopram as a 5-HT releaser. EC50 values were calculated using Prism (GraphPad, San Diego, CA). anova followed by Dunnett's tests were used to compare drug effects with the control condition. Efflux was studied in DAT- and SERT-expressing cells because the action of a drug on the DA and 5-HT system was considered to be relevant for predicting its stimulant-like properties and abuse potential (Rothman and Baumann, 2006).
Cytotoxicity
Cell membrane integrity was verified using the ToxiLight BioAssay Kit (Lonza, Basel, Switzerland) for all of the drugs (10 and 100 μM) after 4 h of incubation at 37°C.
Transendothelial BBB transport
Transendothelial transport was assessed for a selection of compounds using a human in vitro BBB permeability model (Sano et al., 2010; 2012). Conditionally immortalized human brain capillary endothelial cells (TY09) were obtained from the Department of Neurology and Clinical Neuroscience, Yamaguchi University, Japan. TY09 cells express the human blood-to-brain influx and brain-to-blood efflux transporters, form tight cell monolayers and retain BBB-specific properties independent of cell passage number (Sano et al., 2012). The cells were grown in growth medium (EGM-MV BulletKit CC-3125, Lonza, Verviers, Belgium) supplemented with 20% FBS (AMIMED, BioConcept, Allschwil, Switzerland), 100 U·mL−1 penicillin (Sigma, Buchs, Switzerland) and 100 mL·mL−1 streptomycin (Sigma). The cells were seeded on Transwell polycarbonate membrane inserts (Corning, Baar, Switzerland; 0.4 μm pore size, 12 mm insert diameter) precoated with rat tail collagen type 1 solution (Becton Dickinson, Allschwil, Switzerland) at a density of 5 × 104 cells·cm−2 and grown to confluence. Before the initiation of the transport studies, the cell culture medium was replaced with prewarmed transport buffer (HBSS supplemented with 10 mM HEPES and 1 mM Na-Pyruvate, pH 7.4), and 1.5 μM of the test substance was added to the donor compartment of a Transwell filter insert. The extracellular marker Lucifer yellow CH dilithium salt (Sigma) was always combined with the test compound in the same experiment to provide a control for cellular tightness. The initial concentration of Lucifer yellow applied was 10 μM. After 10, 20, 30, 45 and 60 min, 200 μL samples were collected from the acceptor compartment and replaced by buffer. Additionally, a sample of 200 μL was taken from the stock solution and analysed. Lucifer yellow was quantified by fluorescence spectroscopy using a Spectramax M2 spectrophotometer (Molecular Devices, Sunnyvale, CA).
Drug concentrations were determined using HPLC coupled to tandem MS. The analytes were extracted using methanol that contained 0.1 μg·mL−1 MDMA-d5 (Lipomed, Arlesheim, Switzerland). Chromatographic separation was performed on a Shimadzu HPLC system (Shimadzu, Reinach, Switzerland). A Reprosil Fluosil 100 PFP column (50 × 2 mm, 2.2 μm, Dr Maisch, Ammerbuch-Entringen, Germany) was used for the separation of the analytes. Eluent A (0.1% formic acid in water) and eluent B (0.1% formic acid in methanol) were used with the following gradient: 5% B for 0–0.4 min, 5–98% B for 0.4–1.9 min, 98% B for 1.9–2.7 min, and 5% B for 2.7–3 min. The mobile phases were delivered at a constant flow rate of 0.35 mL·min−1. The total run time was 3.0 min. The column oven was set at 40°C. The injection volume was 10 μL. MS detection was performed using a triple quadrupole MS (API3200, Applied Biosystems, Rotkreuz, Switzerland) operated in electrospray ionization positive-ion mode. The assays were linear in the concentration range of 1.2–300 ng·mL−1 for all of the analytes. The selected mass-to-charge (m/z) ratio transitions of the protonated MH+ analyte ions used in selective reaction monitoring mode were the following: MDMA 194 → 163, MDMA-d5 199 → 165, mephedrone 178 → 160, methylone 208 → 160, cocaine 304 → 182, cathinone 150 → 132, methcathinone 164 → 146, amphetamine 136 → 91, methamphetamine 150 → 91, MDPV 276 → 126. The dwell time was set at 20 ms for all of the analytes.
Permeability coefficients were calculated according to Equations 1–3 (Cecchelli et al., 1999):
| (1) |
where X is the cumulative amount of drug transported to the acceptor compartment, and Cd is the concentration of the substance in the donor compartment at each time point. Cd is calculated by subtracting the accumulated transported amount of drug from the initial amount in the donor compartment determined from the stock solution. Cl refers to the total cleared volume at each time point. The permeability-surface area product (PS) is determined by plotting Cl as a function of time. The slope of the curve represents the PS value. The PS values of the cell monolayer plus filter (PStotal) and porous filter (PSfilter) were determined and used for the calculation of the permeability coefficient (Pe) according to the following equations:
| (2) |
| (3) |
where A is the surface area of the filter. The Pe ratios were obtained by normalizing the Pe values of the test compounds (Pe test) with the corresponding Pe values of the extracellular marker Lucifer yellow (Pe Lucifer yellow): Pe ratio = Pe test/Pe Lucifer yellow. Pe ≤ 1 indicates low trancellular permeability as observed with highly hydrophilic compounds, such as sucrose. Pe > 1 and <3 indicates intermediate permeability, and Pe ≥ 3 indicates high permeability (Sano et al., 2012). Estimates of partition coefficient (CLogP) values (Ghose et al., 1998) were calculated using ChemDraw Ultra 11 (CambridgeSoft, Cambridge, MA).
Results
Receptor binding profiles
The monoamine transporter and receptor binding affinities are shown in Table 1. Pyrovalerone and MDPV exhibited very high affinity for the DAT and NET in the low nanomolar range (<10 nM), consistent with their high potency as DAT and NET inhibitors (Table 2). Cathinone and methcathinone showed similar monoamine transporter binding profiles to amphetamine and methamphetamine, with binding affinities for the DAT and NET in the low micromolar range (<10 μM) and no affinity for the SERT (>30 μM). Transporter binding affinities for the DAT and SERT were generally lower than the respective potencies as transporter inhibitors for those compounds that also released DA or 5-HT respectively. Mephedrone, flephedrone and methcathinone were the only cathinones that exhibited relevant (<10 μM) 5-HT2A receptor binding. These compounds and cathinone also bound to α1 adrenoceptors, which was not seen for the other drugs investigated. Cocaine and all of the cathinones showed lower binding affinity for TA1 receptor compared with the non-β-keto analogue amphetamines.
Table 1.
Monoamine transporter and receptor binding affinities
| NET | DAT | SERT | 5-HT1A | 5-HT2A | 5-HT2C | α1A | α2A | D1 | D2 | D3 | H1 | TA1rat | TA1mouse | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDMA | 30.5 ± 8.0 | 6.5 ± 2.5 | 13.3 ± 0.6 | 12.2 ± 0.8 | 7.8 ± 2.4 | >13 | >6 | 15.0 ± 10 | >13.6 | 25.2 ± 12 | >17.7 | >14.4 | 0.37 ± 0.12 | 2.4 ± 1.1 |
| MBDB | 11.9 ± 1.4 | 3.1 ± 0.3 | 5.80 ± 0.7 | 15.5 ± 3.8 | 6.62 ± 1.8 | >13 | >6 | 16.9 ± 4 | >12.5 | NA | >16 | >13 | 1.2 ± 0.1 | 3.6 ± 1.1 |
| MDEA | 6.17 ± 1.4 | 1.35 ± 0.06 | 4.32 ± 0.6 | 18.8 ± 0.78 | >13 | >13 | >6 | 12.2 ± 0.5 | >12.5 | NA | >16 | >13 | 0.32 ± 0.6 | 4.9 ± 1.7 |
| Ethylone | 9.89 ± 0.9 | 1.43 ± 0.4 | 9.04 ± 0.6 | 17.0 ± 2.4 | >13 | >13 | >6 | >25 | >12.5 | NA | >16 | >13 | >12.5 | >10 |
| Mephedrone | >25 | 3.4 ± 0.8 | >30 | >20 | 2.1 ± 0.7 | >13 | 3.48 ± 2.2 | 11.0 ± 5.0 | >13.6 | >30 | >9.2 | >14.4 | 4.3 ± 2.0 | >10 |
| Naphyrone | 0.18 ± 0.02 | 0.04 ± 0.01 | 0.18 ± 0.02 | 6.00 ± 0.21 | 11 ± 2.2 | >13 | >6 | 8.0 ± 2.8 | >12.5 | NA | >16 | 2.28 ± 0.27 | >12.5 | >10 |
| Butylone | 8.13 ± 0.7 | 0.44 ± 0.03 | 14.1 ± 4.1 | >20 | >13 | >13 | >6 | >25 | >12.5 | NA | >16 | >13 | >12.5 | >10 |
| Cocaine | 4.47 ± 2.6 | 0.28 ± 0.07 | 1.1 ± 0.09 | >20 | >13 | >13 | >6 | >20 | >13.6 | >30 | >17.7 | >14.4 | >10 | >10 |
| Methylone | >25 | 2.73 ± 0.2 | >30 | >20 | >13 | >13 | >6 | >20 | >13.6 | >30 | >9.2 | >14.4 | >12.5 | >10 |
| Flephedrone | >25 | 12.2 ± 3.1 | >30 | >20 | 1.4 ± 0.6 | >13 | 1.52 ± 0.05 | >20 | >13.6 | >30 | >17.7 | >14.4 | 5.4 ± 1.7 | >10 |
| Cathinone | 3.50 ± 2.7 | 19.8 ± 1.9 | >30 | >20 | >13 | >13 | 5.40 ± 1.1 | 8.9 ± 2.7 | >13.6 | >30 | >17.7 | >14.4 | 2.2 ± 0.70 | 2.1 ± 0.73 |
| Methcathinone | 1.45 ± 0.7 | 1.28 ± 0.2 | >30 | 12.7 ± 3.5 | 3.0 ± 0.6 | >13 | 3.93 ± 1.3 | 11.9 ± 3.9 | >13.6 | >30 | >9.2 | >14.4 | 4.1 ± 1.2 | >10 |
| Amphetamine | 1.00 ± 0.6 | 5.68 ± 3.8 | >25 | 6.74 ± 1.38 | >13 | >13 | >6 | 2.8 ± 0.8 | >13.6 | >30 | >17.7 | >14.4 | 0.23 ± 0.18 | 0.09 ± 0.06 |
| Methamphetamine | 4.28 ± 2.1 | 1.85 ± 0.9 | 26.7 ± 11 | 8.07 ± 0.75 | >13 | >13 | >6 | 6.1 ± 1.6 | >13.6 | >30 | >17.7 | >14.4 | 0.35 ± 0.12 | 0.55 ± 0.2 |
| Pyrovalerone | 0.06 ± 0.005 | 0.03 ± 0.005 | 4.97 ± 0.3 | 13.4 ± 2.1 | >13 | >13 | >6 | >20 | >13.6 | >30 | >9.2 | 10.7 ± 1.5 | >12.5 | >10 |
| MDPV | 0.08 ± 0.02 | 0.01 ± 0.002 | 2.86 ± 0.1 | 10.29 ± 4.7 | >13 | >13 | >6 | >20 | >13.6 | >30 | >9.2 | >14.4 | 7.2 ± 1.1 | >10 |
Values are Ki given as μM (mean ± SD).
NA, not assessed.
Table 2.
Monoamine transport inhibition
| NET | DAT | SERT | DAT/SERT ratio | Recreational dose* mg | |
|---|---|---|---|---|---|
| IC50 (μM) (95% CI) | IC50 (μM) (95% CI) | IC50 (μM) (95% CI) | Ratio (95% CI) | ||
| MDMA | 0.447 (0.33–0.60) | 17 (12–24) | 1.36 (1.0–2.0) | 0.08 (0.04–0.16) | 100 |
| MBDB | 2.80 (1.9–4.1) | 22 (20–26) | 2.04 (1.4–3.0) | 0.09 (0.05–0.15) | 200 |
| MDEA | 1.02 (0.78–1.3) | 9.3 (8.0–11) | 1.27 (0.93–1.7) | 0.14 (0.01–0.21) | 125 |
| Ethylone | 2.54 (2.0–3.2) | 5.68 (4.9–6.5) | 4.46 (3.8–5.2) | 0.8 (0.6–1.1) | 175 |
| Mephedrone | 0.254 (0.22–0.30) | 3.31 (2.6–4.2) | 4.64 (3.7–5.9) | 1.4 (0.9–2.4) | 150 |
| Naphyrone | 0.25 (0.20–0.32) | 0.47 (0.40–0.55) | 0.96 (0.85–1.09) | 2.0 (1.5–2.7) | 25 |
| Butylone | 2.02 (1.5–2.7) | 2.90 (2.5–3.4) | 6.22 (4.3–9.0) | 2.1 (1.3–3.6) | 150 |
| Cocaine | 0.451 (0.38–0.59) | 0.768 (0.6–1.0) | 2.37 (2.0–2.9) | 3.1 (2–4.8) | 75 |
| Methylone | 0.542 (0.39–0.75) | 4.82 (3.8–6.1) | 15.5 (10–26) | 3.3 (1.5–6.8) | 150 |
| Flephedrone | 0.246 (0.16–0.37) | 6.35 (4.2–9.5) | >10 | 5.8 (0.8–41) | 200 |
| Cathinone | 0.199 (0.15–0.26) | 14.0 (10–20) | >100 | >10 | 50 |
| Methcathinone | 0.085 (0.06–0.17) | 1.12 (0.83–1.5) | >10 | >10 | 59 |
| Amphetamine | 0.094 (0.06–0.14) | 1.30 (0.83–2.0) | >10 | >10 | 30 |
| Methamphetamine | 0.064 (0.04–0.09) | 1.05 (0.74–1.5) | >10 | >10 | 30 |
| Pyrovalerone | 0.043 (0.03–0.06) | 0.035 (0.03–0.04) | 13.0 (10.8–15.8) | >100 | 20 |
| MDPV | 0.044 (0.03–0.07) | 0.031 (0.03–0.04) | 9.30 (6.8–12.8) | >100 | 5 |
Values are means of three to four independent experiments and 95% confidence intervals (CI).
Drugs are ranked according to the DAT/SERT ratio = 1/DAT IC50 : 1/SERT IC50.
Estimated average.
Inhibition of monoamine transporters
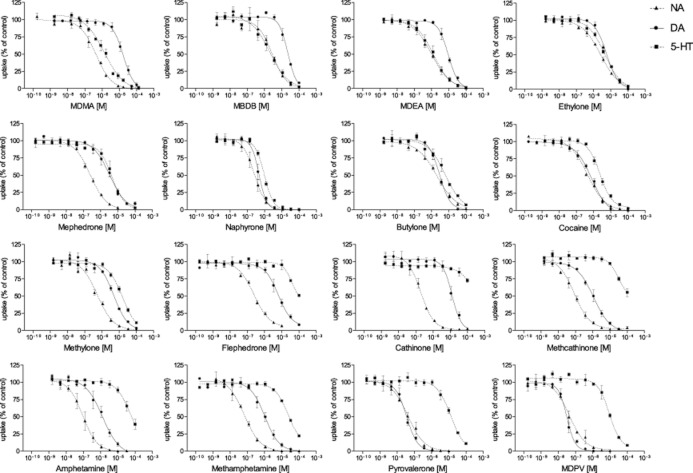
The effects of the cathinones and reference substances on monoamine transporter function are shown in Figure 2. IC50 values for monoamine transport inhibition and DAT/SERT inhibition ratios are shown in Table 2. Significant differences were observed in the absolute and relative potencies of the cathinones to inhibit monoamine transporter function. Pyrovalerone and its derivative MDPV were the most potent DAT inhibitors, significantly more potent than all of the other drugs. The rank order of potency for DAT inhibition was MDPV and pyrovalerone >> naphyrone, cocaine, methamphetamine, amphetamine and methcathinone > butylone, mephedrone, methylone, ethylone, flephedrone and MDEA > cathinone, MDMA and MBDB. The rank order of potency for SERT inhibition was naphyrone, MDEA and MDMA > MBDB, cocaine, ethylone, mephedrone and butylone >> all of the others. The DAT/SERT inhibition ratios ranged from >100 for pyrovalerone and MDPV (mostly DAT inhibition) to 0.08 for MDMA (mostly SERT inhibition). The entactogens MDMA, MBDB and MDEA were the only drugs that blocked the SERT significantly more potently than the DAT (DAT/SERT ratio << 1). Ethylone, mephedrone, naphyrone, butylone and methylone were similar to cocaine, with DAT/SERT selectivity ratios in the range of 1–4. Cathinone and methcathinone were similar to their non-β-keto analogues amphetamine and methamphetamine, with DAT/SERT inhibition ratios >10. The rank order of potency for NET inhibition was pyrovalerone and MDPV > methamphetamine, methcathinone and amphetamine > cathinone, flephedrone, naphyrone and mephedrone > MDMA, cocaine and methylone > MDEA, butylone, ethylone and MBDB. DAT and NET but not SERT inhibition potency (IC50) values were correlated with psychotropic effective doses (Table 2) as reported from experimental studies (Martin et al., 1971) or by recreational users (Derungs et al., 2011) http://www.erowid.org; accessed June 20, 2012). The Spearman rank correlation coefficients were rs = 0.73 and 0.79 respectively (both P < 0.01).
Figure 2.
Monoamine uptake inhibition. Potencies of different drug concentrations to inhibit the accumulation of NA, DA and 5-HT into NA, DA and 5-HT transporter-transfected HEK 293 cells respectively. The data are expressed as the mean ± SEM of three to five independent experiments. The lines show the data fit by nonlinear regression. IC50 values are presented in Table 2.
Monoamine release
Amphetamine, methamphetamine, cathinone, methcathinone, flephedrone, mephedrone and MDMA released DA through the DAT (Figure 3 and Table 3). However, the potency of MDMA to release DA was low (EC50 > 10 μM). The entactogens MDMA, MDEA and MBDB, as well as the cathinones methylone, ethylone, butylone and mephedrone released 5-HT through the SERT. Amphetamine, methamphetamine, methcathinone and flephedrone also released 5-HT, however, only at very high concentrations (EC50 > 33 μM). The pyrovalerone derivatives, including pyrovalerone, naphyrone and MDPV, produced no DA or 5-HT efflux similar to cocaine, indicating that these pyrovalerone derivatives act as very potent transporter inhibitors but not substrate releasers.
Figure 3.
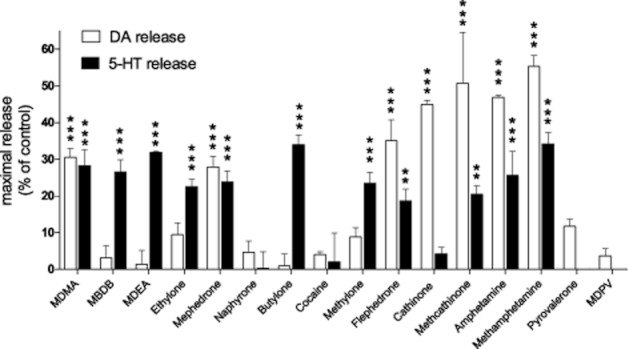
Dopamine and 5-HT release. HEK 293 cells that stably express the DA or 5-HT transporter were preloaded with [3H]-DA or [3H]-5-HT, respectively, washed and incubated with drugs. Transporter-mediated release is expressed as % reduction in monoamine cell content at the maximal drug concentration (100 μM) compared with controls. **P < 0.01, ***P < 0.001, significant effects compared with controls. The EC50 values are shown in Table 3. The data are expressed as the mean ± SEM of three independent experiments.
Table 3.
Monoamine release from monoamine-preloaded cells
| DAT | SERT | |
|---|---|---|
| EC50 (μM) (95% CI) | EC50 (μM) (95% CI) | |
| MDMA | 22 (8.9–53) | 5.63 (3.5–9.2) |
| MBDB | >100 | 2.49 (1.0–6.9) |
| MDEA | >100 | 2.88 (1.6–5.0) |
| Ethylone | >100 | 9.90 (2.4–40) |
| Mephedrone | 3.75 (1.7–8.4) | 5.98 (3.2–11) |
| Naphyrone | >100 | >100 |
| Butylone | >100 | 5.5 (1.8–17) |
| Cocaine | >100 | >100 |
| Methylone | >100 | >10 |
| Flephedrone | 12.5 (5.7–28) | >33 |
| Cathinone | 5.64 (3.0–10) | >100 |
| Methcathinone | 2.36 (1.7–3.3) | >33 |
| Amphetamine | 1.76 (1.1–2.9) | >33 |
| Methamphetamine | 1.56 (0.9–2.8) | >33 |
| Pyrovalerone | >100 | >100 |
| MDPV | >100 | >100 |
Values are means.
Cytotoxicity
None of the drugs showed apparent cytotoxicity at the concentrations used in the functional assays.
Transendothelial transport
All of the positively tested drugs exhibited Pe ratios ≥3, indicating high permeability (Table 4). Pe ratios for mephedrone and MDPV were >10, suggesting very high permeability. Additionally, the apical to basolateral transport of MDPV was significantly greater (P < 0.05) than basolateral to apical transport, consistent with active transport by one of the blood-to-brain influx carriers. Pe ratios could not be calculated for cocaine and cathinone because of low recovery.
Table 4.
Blood-brain barrier permeability
| Pe ratio | |||||
|---|---|---|---|---|---|
| Apical to basolateral | Basolateral to apical | Permeability | aActive transport | bCLogP | |
| MDMA | 6.0 ± 0.56 | 7.4 ± 2.4 | + | No | 1.85 |
| Mephedrone | 14.0 ± 10.4 | 12.2 ± 6.1 | ++ | No | 1.67 |
| Methylone | 6.1 ± 2.8 | 5.3 ± 1.3 | + | No | 1.39 |
| Methcathinone | 5.9 ± 2.8 | 8.5 ± 3.2 | + | No | 1.19 |
| Amphetamine | 6.3 ± 3.7 | 5.2 ± 1.3 | + | No | 1.74 |
| Methamphetamine | 5.4 ± 1.1 | 6.4 ± 3.0 | + | No | 1.74 |
| MDPV | 37.2 ± 11.3 | 12.0 ± 11.2 | ++ | Yes | 3.80 |
Data are expressed as mean ± SD (n = 3–9).
Pe ratios indicate the blood–brain barrier permeability of the drug in relation to the extracellular marker lucifer yellow (Pe = 1).
+, high permeability (Pe ratio >3). ++, very high permeability (Pe ratio >10).
P <0.05 significant difference between apical to basolateral compared with basolateral to apical transport indicating active transport.
CLogP, prediction of partition coefficient (lipophilicity).
Discussion
All of the cathinones were inhibitors of the monoamine transporters, but their selectivity for the SERT, NET and DAT varied considerably. Further, most of the compounds were substrate releasers. Thus, important pharmacological differences were found between different cathinones. We classified the cathinones into three groups based on, firstly, their relative potency to act as SERT, NET and DAT inhibitors and, secondly, their action as substrate releasers: (1) cocaine-MDMA-mixed cathinones (including mephedrone, methylone, ethylone, butylone and naphyrone, which act as relatively nonselective monoamine uptake inhibitors similar to cocaine and, with the exception of naphyrone, also as MDMA-like 5-HT releasers); (2) methamphetamine-like cathinones (including cathinone, methcathinone and flephedrone, which act as preferential catecholamine inhibitors and DA releasers, similar to amphetamine and methamphetamine); and (3) pyrovalerone–cathinones (including pyrovalerone and MDPV, which act as very potent and selective catecholamine uptake blockers but not substrate releasers).
The potency of drugs of abuse to inhibit the NET and DAT or activate the NA and DA system is associated with their psychostimulant effects and enhanced abuse liability (Rothman et al., 2001). Consistently, we found that the doses of the drugs abused by humans correlated with their potency to inhibit catecholamine transport. In contrast, relatively increased activation of the 5-HT system is linked to a reduction in abuse potential (Wee et al., 2005; Rothman and Baumann, 2006; Baumann et al., 2011) and more ‘entactogenic’ MDMA-like subjective drug effects (Liechti et al., 2000a). Thus, the relative in vitro effect on the DAT versus SERT is useful to predict drug characteristics in vivo and compare novel cathinones with known psychostimulants. MDMA is selective for the SERT, with a DAT/SERT inhibition ratio of 0.08 (present study) and DA/5-HT release potency ratio <1 (Baumann et al., 2012), and produces positive mood effects in humans with little psychostimulation (Liechti et al., 2001). Cocaine has a DAT/SERT ratio close to unity, and methamphetamine is more selective for the DAT, with a DAT/SERT inhibition ratio >10 and DA/5-HT release ratio >100 (Baumann et al., 2012) and has mostly psychostimulant effects in humans.
Cocaine-MDMA-mixed cathinones
Mephedrone, methylone, ethylone, butylone and naphyrone exhibited relative DAT versus SERT inhibition potencies in the range of 1–5, similar to cocaine. Uptake inhibition studies using rat synaptosomes found that mephedrone was equally potent at the DAT and SERT (Hadlock et al., 2011). Methylone and butylone were slightly more potent DAT than SERT inhibitors at the human transporter as previously shown for methylone (Cozzi et al., 1999). Equal uptake inhibition potencies for the DAT and SERT were shown for methylone and butylone using rat brain synaptosomes (Nagai et al., 2007; Lopez-Arnau et al., 2012). Ethylone was an equipotent inhibitor of all three transporters, and we are not aware of other published data. Compared with methylone, ethylone and butylone, the respective non-β-keto analogue entactogens MDMA, MDEA and MBDB were 10-fold more selective for the SERT versus DAT, consistent with previous data on methylone and MDMA (Cozzi et al., 1999; Nagai et al., 2007). Together the data indicate that the cocaine–MDMA–mixed cathinones are more dopaminergic with regard to monoamine transporter inhibition than their entactogen analogs.
In terms of monoamine release, the cocaine–MDMA–mixed cathinones were comparable with MDMA. Ethylone and butylone released 5-HT, comparable with their non-β-keto entactogen analogues MBDB and MDEA, but with lower potency. Previous studies found that the monoamine release profiles of mephedrone and methylone resembled those of MDMA, with DAT/SERT and NET/DAT ratios close to unity (Nagai et al., 2007; Baumann et al., 2012). However, mephedrone was a more potent releaser of DA than MDMA in the present study and from striatal suspensions preloaded with DA (Hadlock et al., 2011). An in vivo microdialysis study in rats showed that mephedrone also produced a rapid and pronounced increase in nucleus accumbens DA levels, comparable with amphetamine and unlike MDMA, which only moderately elevates DA levels (Kehr et al., 2011). Both mephedrone and MDMA also produced strong increases in extracellular 5-HT, whereas amphetamine had only a moderate effect on 5-HT levels (Kehr et al., 2011). Other microdialysis studies showed that mephedrone and methylone elevated extracellular DA and 5-HT levels in the rat nucleus accumbens, with relatively higher effects on 5-HT levels (Aarde et al., 2011; Baumann et al., 2012), similar to MDMA and unlike methamphetamine, which preferentially increases DA (Baumann et al., 2012). Thus, mephedrone shares some of the DA-releasing properties of amphetamine and methamphetamine and 5-HT-releasing property of MDMA. Mephedrone also produced relatively weak motor stimulation similar to MDMA, unlike amphetamine that strongly increases locomotor activity in rats (Kehr et al., 2011), and a preference to move along the walls of the test box (Motbey et al., 2012) as previously described for MDMA. Like MDMA, mephedrone also reduced voluntary wheel running in rats, while running was increased by methamphetamine or MDPV (Huang et al., 2012). Similar to mephedrone, methylone was also reported to be a weak motor stimulant compared with methamphetamine (Baumann et al., 2012). Drug discrimination studies in rats also showed that methylone generalized well to MDMA and at lower potency also to amphetamine (Dal Cason et al., 1997). Mephedrone is self-administered by rats (Aarde et al., 2011; Hadlock et al., 2011), has been reported to produce strong craving in humans (Brunt et al., 2011) and when administered intranasally is rated by users to be more addictive than cocaine (Winstock et al., 2011). Furthermore, mephedrone showed very high BBB permeability in our in vitro model, confirming that mephedrone readily enters the brain (Hadlock et al., 2011). Overall, the pharmacological effects of mephedrone and methylone appear to be relatively similar to those of MDMA but share more of the DA system-stimulating properties of amphetamine and methamphetamine and the DAT versus SERT inhibition profile of cocaine. The subjective effects of mephedrone have been reported to be similar to those of cocaine (Winstock et al., 2011) but also MDMA (Carhart-Harris et al., 2011). Importantly, MDMA is mostly used orally, whereas intranasal administration is the most common route of use for mephedrone (Winstock et al., 2011) and cocaine. Users noted that the high obtained with the intranasal use of mephedrone was similar to or better than the high produced by cocaine (Winstock et al., 2011). These observations indicate that the oral use of mephedrone produces overall similar effects to MDMA, whereas intranasal use results in more cocaine-like psychotropic effects. Similar to mephedrone and methylone, ethylone and butylone may be associated with an increased risk of addiction compared to their non-β-keto analogues because of the stronger relative activation of the DA system.
The pyrovalerone derivative naphyrone exhibited a monoamine uptake transporter inhibition profile that was very close to that of cocaine, with equal relative potency at all three transporters. Similar to cocaine, naphyrone was not a monoamine releaser. Naphyrone is distinct from pyrovalerone and its derivative MDPV because of its higher absolute and relative SERT-inhibiting potency. Although the structure would suggest similar pharmacological effects to the other pyrovalerone derivatives, the additional SERT inhibition may indicate more similar effects to cocaine in humans (Derungs et al., 2011).
Methamphetamine-like cathinones
Cathinone and methcathinone exhibited a relative monoamine transporter inhibition profile that was very similar to that of the non-β-keto analogues amphetamine and methamphetamine, with high inhibitory potencies at the DAT and low potencies at the SERT, consistent with previous findings (Cozzi et al., 1999; Fleckenstein et al., 1999). Cathinone and methcathinone were also potent releasers of DA but not 5-HT, similar to amphetamine and methamphetamine. Cathinone and methcathinone have previously been shown to release radiolabelled DA and 5-HT from rat brain preparations with similar DA versus 5-HT selectivity to amphetamine (Kalix, 1990) and methamphetamine (Glennon et al., 1987), but with two- to three-fold lower potency. Methcathinone has been shown to be a substrate for the transporter (Cozzi and Foley, 2003), similar to the classic amphetamines and MDMA (Rothman et al., 2001; Verrico et al., 2007). Cathinone and methcathinone produce amphetamine-like locomotor stimulation in animals (Glennon et al., 1987; Kelly, 2011), and cathinone is self-administered by rats (Gosnell et al., 1996) or rhesus monkeys (Johanson and Schuster, 1981; Woolverton and Johanson, 1984) with reinforcing efficacies comparable to amphetamine and cocaine. Clinically, cathinone and methcathinone have been reported to produce similar toxicity to amphetamine, including hypertension, hyperthermia, euphoria, locomotor activation and hallucinations following higher or repeated doses (Kalix, 1990; Widler et al., 2005). Thus, the pharmacology of cathinone and methcathinone is qualitatively very close to that of amphetamine and methamphetamine.
Flephedrone was a DAT but not a SERT inhibitor, similar to its analogue 4-fluoroamphetamine (Nagai et al., 2007). Flephedrone released DA but not 5-HT (IC50 >33 μM). The DAT/SERT selectivity profile of flephedrone is therefore equal to the methamphetamine-like cathinones. In contrast, flephedrone had higher 5-HT2A receptor binding that was similar to mephedrone and MDMA. We are not aware of any other in vitro or in vivo data on flephedrone. Agitation and psychosis were reported in a patient who insufflated flephedrone and MDPV powder (Thornton et al., 2012).
Pyrovalerone cathinones
Pyrovalerone and its derivative MDPV were very potent DAT inhibitors as previously shown (Meltzer et al., 2006) and at least 10-fold more potent than cocaine and methamphetamine. In contrast to the pyrovalerone derivative naphyrone, MDPV and pyrovalerone are weak inhibitors of the SERT, resulting in high DAT selectivity, with DAT/SERT inhibition ratios >100. MDPV and pyrovalerone were also the most potent NET inhibitors. Despite the high potency to block the DAT, pyrovalerone and MDPV did not produce DA efflux. Thus, pyrovalerone derivative cathinones are pure transporter uptake inhibitors. Consistent with the potent effect on catecholamine carriers, MDPV, compared with mephedrone, produced behavioural effects in animals at lower doses (Aarde et al., 2011) and has been reported to produce mostly sympathomimetic toxicity and psychotic reactions in humans (Spiller et al., 2011). Similar to methamphetamine, MDPV and pyrovalerone did not exhibit affinity for the 5-HT2A receptor and exhibited low affinity for TA1 receptors, consistent with the other cathinones.
Pyrovalerone derivatives have been suggested to easily cross the BBB because of their high lipophilicity (Meltzer et al., 2006; Coppola and Mondola, 2012). All of the cathinones and non-β-keto amphetamine analogues showed good membrane permeation in our in vitro BBB model, but we indeed documented very high transmembrane permeability of MDPV and a potential active transport. Although the high brain uptake may contribute to the higher potency of MDPV compared with the non-pyrovalerone cathinones, the high potency at the NET and DAT is more likely to be responsible for the psychotropic effects at low doses in humans. In fact, we found that the potencies to inhibit the NET and DAT were significantly correlated with the doses reported to produce psychotropic effects in recreational users. Consistently, pyrovalerone and MDPV are at least 10-fold more potent inhibitors of the NET or DAT compared with mephedrone; this was demonstrated in the present study and previously (Meltzer et al., 2006), and are used recreationally at approximately 10-fold lower doses than mephedrone (Derungs et al., 2011), whereas both MDPV and mephedrone exhibited very high BBB penetrance in our study. The potency of the pyrovalerone derivatives at the DAT and NET and high brain penetrance could result in high sympathomimetic toxicity and risk of addiction in humans. MDPV has also been shown to be a potent reinforcer in rats, similar to methamphetamine (Watteron et al., 2011).
Structure–activity relationship and binding to monoamine receptors
β-Keto-amphetamines appear to have similar effects on plasma membrane monoamine transporters compared with their non-β-keto analogues, with slightly higher selectivity for the DAT over the SERT. β-Keto-analogue cathinones also exhibited approximately 10-fold lower affinity for the TA1 receptor compared with their respective non-β-keto amphetamines. TA1 receptors play an important role in the modulation of dopaminergic and 5-hydroxytryptaminergic activity (Lindemann et al., 2008; Revel et al., 2011). Activation of TA1 receptors negatively modulates dopaminergic neurotransmission. Importantly, methamphetamine decreased DAT surface expression via a TA1 receptor-mediated mechanism and thereby reduced the presence of its own pharmacological target (Xie and Miller, 2009). MDMA and amphetamine have been shown to produce enhanced DA and 5-HT release and locomotor activity in TA1 receptor knockout mice compared with wild-type mice (Lindemann et al., 2008; Di Cara et al., 2011). Because methamphetamine and MDMA auto-inhibit their neurochemical and functional effects via TA1 receptors, low affinity for these receptors may result in stronger effects on monoamine systems by cathinones compared with the classic amphetamines. The higher selectivity of the cathinones for the DAT and lack of TA1 receptor binding may result in an increased risk of dependence compared with classic non-β-keto analogue stimulants (Rothman and Baumann, 2006). Because 5-HT release dampens the stimulant effects of amphetamine-type drugs, the lower activity of the cathinones at the SERT would be expected to result in more stimulant-like effects (Baumann et al., 2011) compared with the non-β-keto analogues. The α-ethyl-substituted compounds MBDB and butylone exhibited fivefold lower absolute and relative NET inhibition potencies than their α-methyl-analogues MDMA and methylone, in line with previous studies (Montgomery et al., 2007). The lower affinity for NET has been associated with the low stimulant and euphorigenic properties of MBDB (Montgomery et al., 2007).
Several of the drugs evaluated in the present study exhibited moderate direct affinity for 5-HT receptors and adrenoceptors, with Ki values in the 1–10 μM range. Direct interactions between MDMA and 5-HT2 receptors rather than indirect agonist effects via 5-HT release have been suggested to contribute to MDMA-induced excitation and hallucinogen-like perceptual alterations at higher doses (Liechti et al., 2000b; 2001). MDMA, MBDB, mephedrone, flephedrone and methcathinone bound to 5-HT2A receptors, consistent with previous data on mephedrone, methylone and MDMA (Lopez-Arnau et al., 2012; Martinez-Clemente et al., 2012). Stimulation of 5-HT2A receptors has also been shown to enhance DA release (Gudelsky et al., 1994), potentially increasing abuse liability. Although flephedrone and methcathinone show low potency at the SERT, these drugs may have direct effects on the 5-HT system via 5-HT2A receptor activation at higher doses. Amphetamine and methamphetamine bound to 5-HT1A receptors, potentially resulting in behavioural effects that are opposite to those induced by 5-HT2A receptor stimulation (Nichols, 2004; Gatch et al., 2011). Mephedrone, flephedrone, cathinone and methcathinone exhibited affinity for α1A adrenoceptors, which have been implicated in stimulant-induced vasoconstriction, hyperthermia (Hysek et al., 2012b) and euphoria (Newton et al., 2012). Finally, amphetamine and methamphetamine bound to α2A receptors, which modulate NA release and sympathomimetic toxicity (Hysek et al., 2012a).
It is important to note that we assessed the effects of racemic cathinones, whereas stereoselective and drug-specific interactions have been demonstrated for amphetamines (Lyon et al., 1986; Steele et al., 1987; Acquas et al., 2007). Furthermore, we did not investigate drug interactions with intracellular targets such as monoamine oxidase or the vesicular monoamine transporter.
In summary, considerable differences were found in the pharmacology of the different cathinones. Mephedrone, methylone, ethylone, butylone and naphyrone acted as non-selective monoamine uptake inhibitors, similar to cocaine and, with the exception of naphyrone, also induced the release of 5-HT, similar to MDMA. Cathinone and methcathinone were found to be selective catecholamine uptake inhibitors and releasers, similar to their non-β-keto analogues amphetamine and methamphetamine. Pyrovalerone and MDPV were shown to be highly potent and selective catecholamine transporter inhibitors but not substrate releasers.
Acknowledgments
We thank V Metzler and A van der Klooster for technical assistance and Prof A Pfaltz for providing support for the synthesis of naphyrone. This work was supported by the Swiss National Science Foundation (no. 323230_126231 and 3232B_144996) and the Translational Medicine Hub Innovation Fund of F Hoffmann-La Roche and the University of Basel.
Glossary
- BBB
blood–brain barrier
- DA
dopamine
- DAT
dopamine transporter
- MBDB
3,4-methlyenedioxyphenyl-N-methyl-2-butanamine
- MDEA
3,4-methylenedioxy-N-ethylamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- MDPV
3,4-methylenedioxypyrovalerone
- NET
NA transporter
- Pe
permeability coefficient
- SERT
5-HT transporter
- TA receptor
trace amine-associated receptor
Conflict of interest
None.
References
- Aarde SA, Wright MJ, Buczynski MW, Angrish D, Parsons LH, Houseknecht KL, et al. Behavioral and termoregulatory effects of novel cathinone derivative drugs 4-MMC and MDPV. Neuropsychopharmacology. 2011;36:S441. [Google Scholar]
- Acquas E, Pisanu A, Spiga S, Plumitallo A, Zernig G, Di Chiara G. Differential effects of intravenous R,S-(+/−)-3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and its S(+)- and R(−)-enantiomers on dopamine transmission and extracellular signal regulated kinase phosphorylation (pERK) in the rat nucleus accumbens shell and core. J Neurochem. 2007;102:121–132. doi: 10.1111/j.1471-4159.2007.04451.x. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2011;25:1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend. 2011;118:19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Cecchelli R, Dehouck B, Descamps L, Fenart L, Buee-Scherrer VV, Duhem C, et al. In vitro model for evaluating drug transport across the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:165–178. doi: 10.1016/s0169-409x(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Coppola M, Mondola R. 3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett. 2012;208:12–15. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Foley KF. Methcathinone is a substrate for the serotonin uptake transporter. Pharmacol Toxicol. 2003;93:219–225. doi: 10.1046/j.1600-0773.2003.pto930504.x. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- Derungs A, Schietzel S, Meyer MR, Maurer HH, Krahenbuhl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone) Clin Toxicol (Phila) 2011;49:691–693. doi: 10.3109/15563650.2011.592838. [DOI] [PubMed] [Google Scholar]
- Di Cara B, Maggio R, Aloisi G, Rivet JM, Lundius EG, Yoshitake T, et al. Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA) J Neurosci. 2011;31:16928–16940. doi: 10.1523/JNEUROSCI.2502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein AE, Haughey HM, Metzger RR, Kokoshka JM, Riddle EL, Hanson JE, et al. Differential effects of psychostimulants and related agents on dopaminergic and serotonergic transporter function. Eur J Pharmacol. 1999;382:45–49. doi: 10.1016/s0014-2999(99)00588-9. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–289. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose AK, Viswanadhan VN, Wendoloski JJ. Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragment methods: an analysis of ALogP and CLogP methods. J Phys Chem A. 1998;102:3762–3772. [Google Scholar]
- Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Yracheta JM, Bell SM, Lane KE. Intravenous self-administration of cathinone by rats. Behav Pharmacol. 1996;7:526–531. [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK, Nash JF. Potentiation of 3,4-methylenedioxymethamphetamine-induced dopamine release and serotonin neurotoxicity by 5-HT2 receptor agonists. Eur J Pharmacol. 1994;264:325–330. doi: 10.1016/0014-2999(94)90669-6. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Brugger R, Simmler LD, Bruggisser M, Donzelli M, Grouzmann E, et al. Effects of the a2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Pharmacol Exp Ther. 2012a;340:286–294. doi: 10.1124/jpet.111.188425. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Rickli A, Simmler LD, Donzelli M, Grouzmann E, et al. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Br J Pharmacol. 2012b;166:2277–2288. doi: 10.1111/j.1476-5381.2012.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola V, Vischer N, Donzelli M, Krähenbühl S, et al. Duloxetine inhibits effects of MDMA (‘ecstasy’) in vitro and in humans in a randomized placebo-controlled laboratory study. Plos ONE. 2012c;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A comparison of the behavioral effects of l- and dl-cathinone and d-amphetamine. J Pharmacol Exp Ther. 1981;219:355–362. [PubMed] [Google Scholar]
- Kalix P. Pharmacological properties of the stimulant khat. Pharmacol Ther. 1990;48:397–416. doi: 10.1016/0163-7258(90)90057-9. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000a;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (‘Ecstasy’) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000b;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 2001;154:161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon RA, Glennon RA, Titeler M. 3,4-Methylenedioxymethamphetamine (MDMA): stereoselective interactions at brain 5-HT1 and 5-HT2 receptors. Psychopharmacology (Berl) 1986;88:525–526. doi: 10.1007/BF00178519. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharmacol. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery T, Buon C, Eibauer S, Guiry PJ, Keenan AK, McBean GJ. Comparative potencies of 3,4-methylenedioxymethamphetamine (MDMA) analogues as inhibitors of [3H]noradrenaline and [3H]5-HT transport in mammalian cell lines. Br J Pharmacol. 2007;152:1121–1130. doi: 10.1038/sj.bjp.0707473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict Biol. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, Brown G, Kosten TR, Mahoney JJ, Haile CN. Noradrenergic a1 receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. Plos ONE. 2012;7:e30854. doi: 10.1371/journal.pone.0030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A. 2011;108:8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann N Y Acad Sci. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sano Y, Shimizu F, Abe M, Maeda T, Kashiwamura Y, Ohtsuki S, et al. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood-brain barrier function. J Cell Physiol. 2010;225:519–528. doi: 10.1002/jcp.22232. [DOI] [PubMed] [Google Scholar]
- Sano Y, Kashiwamura Y, Abe M, Dieu L, Huwyler J, Shimizu F, et al. A stable human brain microvascular endothelial cell line retaining its barrier-specific nature, independent of the passage number. Clin Exp Neuroimmunol. 2012 (in press) [Google Scholar]
- Scholze P, Zwach J, Kattinger A, Pifl C, Singer EA, Sitte HH. Transporter-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J Pharmacol Exp Ther. 2000;293:870–878. [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of ‘bath salts’ and ‘legal highs’ (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol. 1987;36:2297–2303. doi: 10.1016/0006-2952(87)90594-6. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8:310–313. doi: 10.1007/s13181-012-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK. MDMA (ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology. 2007;189:489–503. doi: 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]
- Watteron LR, Kufahl PR, Nemirovsky NE, Sewalia K, Olive MF. Potent reinforcing effects of the synthetic cathinone methylenedioxypyrovalerone (MDPV) in rats. Neuropsychopharmacology. 2011;36:S440. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Widler P, Mathys K, Brenneisen R, Kalix P, Fisch HU. Pharmacodynamics and pharmacokinetics of khat: a controlled study. Clin Pharmacol Ther. 1994;55:556–562. doi: 10.1038/clpt.1994.69. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J Exp Anal Behav. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Miller GM. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharmacol Exp Ther. 2009;330:316–325. doi: 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]