Abstract
Objectives
To assess if cohort segregation policies are effective in preventing cross-infection in cystic fibrosis (CF) clinics.
Design
A prospective cohort study.
Setting
A large adult CF centre in Northwest England.
Participants
All CF patients cared for at the Liverpool adult CF centre 2003–2009.
Methods
Regular sputum sampling with genotyping of pseudomonas aeruginosa (Psa) isolates led to a policy of inpatient and outpatient segregation by microbiological group.
Main outcome measures
Prevalence and cross-infection/super-infection rates of a transmissible Psa strain, i.e. the Liverpool epidemic strain (LES) in adult CF patients at the Liverpool adult CF centre from 2003 to 2009.
Results
There was a decline in the proportion of patients with LES (71–53%) and an increase in those with unique strains (23–31%) and without Psa infection (6–17%) from 2003 to 2009. There were two cases of LES super-infection and one case of new chronic Psa infection (with a unique strain). There were no cases of transmissible strain infection in patients previously uninfected by Psa.
Conclusions
Our segregation policy has halted the spread of the commonest highly transmissible strain in the UK (LES) in our clinic, without endangering patients who were not previously infected with Psa. It confirms that if genotypic surveillance is used, it is unnecessary to segregate patients infected with unique strains from those without Psa infection.
Introduction
Although cystic fibrosis (CF) is the most common potentially lethal inherited disease in the Western World, due to improved management the life-expectancy of CF patients has greatly improved in the last two decades, and in the UK the median survival is 41 years.1 However, the majority of patients still die from end-stage lung disease, due to chronic bacterial infection with pseudomonas aeruginosa (Psa).2 Despite the success of aggressive therapy against acute infection/colonization with this organism, most CF patients become chronically infected by early adulthood, and once established, Psa is impossible to eradicate and can cause a rapid decline in clinical parameters, doubling their mortality.3–5
Initially, it was believed that each individual harboured their own unique Psa strain which was incapable of cross-infecting another individual,6 with or without CF. However, in 1996 Cheng et al.7 demonstrated the widespread presence of an antibiotic resistant Psa at the children's CF centre in Liverpool, UK. Psa genotyping revealed the children to be infected with the same clone, and it was postulated that this particular variant may have the capability to spread to other individuals. Subsequently, we have shown that it can super-infect those with their own unique strains,8 in many cases replacing them, and can also spread to non-CF patients9 and across species.10 This variant, which has now been shown to be widespread throughout UK CF centres11 and has also been reported in Canada,12 is labelled the Liverpool epidemic strain (LES). Reports of other transmissible strains have now been made, not only in the UK13–17 but also elsewhere.18–23
The Liverpool adult centre receives patients from the paediatric centre which reported the first outbreak of LES in 1996, and many of these children have grown up and transferred to the adult sector. A post hoc analysis in 200024 of sputum cultures from our CF patients identified LES in 79% of those chronically infected with Psa, demonstrating the widespread infection of patients inherited from the original reporting paediatric centre.
To control this epidemic, in 2003 we implemented a strict cohort-based segregation policy for all our CF patients alongside our paediatric centre, monitored by regular Psa genotyping which includes markers for the most common known UK transmissible Psa strains. All patients submit microbiological samples at every clinic visit/inpatient stay, and Psa isolates from those without known LES infection undergo genotyping every three months. Psa isolates from patients infected with LES undergo a genotypic check on a yearly basis. In 2009, 1098 genotype tests were carried out on Psa-infected patients. Using this system, we are aware of the Psa genotypes infecting our CF patients at all times.
Based on this, those infected with LES are segregated together into a separate outpatient clinic and a 12-bedded purpose-built inpatient facility, all with separate rooms. Patients infected with Burkholderia species or any known other transmissible Psa strains are isolated from all others, both as outpatients and inpatients. Other patients (including those infected without known transmissible strains of Psa and those without Psa infection) are not segregated from each other, since unique Psa strains by definition cannot spread between patients: this group are supervised in the same outpatient clinics and admitted to individual rooms on the same ward for inpatient treatment. Since patients known to be infected with transmissible strains attend outpatient clinics, radiology, lung function, and pathology and physiotherapy departments at different times and on different days to the remainder, we have ensured their complete segregation within the hospital environment.
Patients chronically infected with MRSA are kept within-group, and managed according to the standard cross-infection prevention protocols for this organism active in the hospital at the time. All our patients with CF are given strict instructions counselling them against mixing with other CF individuals, and those admitted to hospital agree a contract which includes this:
We have continued this prospective surveillance for Psa strains among our patients from 2003 to date, and we now report the results of our segregation policy at our centre for 7 years, up to 2009.
Methods
Lower airway microbiological samples are obtained for culture at every clinic visit and frequently (at least weekly) during inpatient treatment stays by a qualified physiotherapist. Wherever possible, sputum samples are preferred to cough swabs. The samples are sent in sealed sterile containers to the routine microbiology laboratory where they are processed in the standard fashion, within 24 hours, including subculture for Psa. Representative Psa isolates from each sample are stored for future potential Psa genotyping at −80°C. Using our surveillance protocol (outlined above) isolates are batched for genotypic analysis and the clinical database updated accordingly.
Psa genotyping methods have evolved with time, and these have been incorporated into our laboratory protocol:
2003–2005: Psa genotyping was carried out using the rapid amplification of polymorphic DNA (RAPD) technique of sputum Psa isolates from all patients.25 Ambiguous results were checked using pulse field gel electrophoresis.
2005–2008: The method was modified during polymerase chain reaction (PCR) by using the primers PA-SS (Pseudomonas-specific bands at 956 bp) and PS21 (LES-specific bands at 364 bp) to identify LES-positive isolates.24 LES-negative isolates by PS21/PA-SS underwent further typing using RAPD for comparison with other common epidemic strains (Manchester and Midlands1).
2008–2009: In March 2008, by combining the specific primers and modifying the product mix we identified LES, Manchester and Midlands1 strains from one test (combined multiplex PCR).26 Primer F9 added to the PCR mix increased the specificity of the test to identify LES.
Chronic colonization with Psa is defined as isolation of the organism in three or more successive samples taken at least four weeks apart over a six-month period. Patients with Psa were classified as LES or other common known epidemic strain positive if any respiratory sample confirmed their presence on genotypic analysis, with the remainder defined as sporadic strains.
Longitudinal follow-up of CF patients
Data on all existing patients and new patients joining the Liverpool adult CF centre from 2003 to 2009 were collected from hospital records and our departmental database.
Results
Clinic demographics
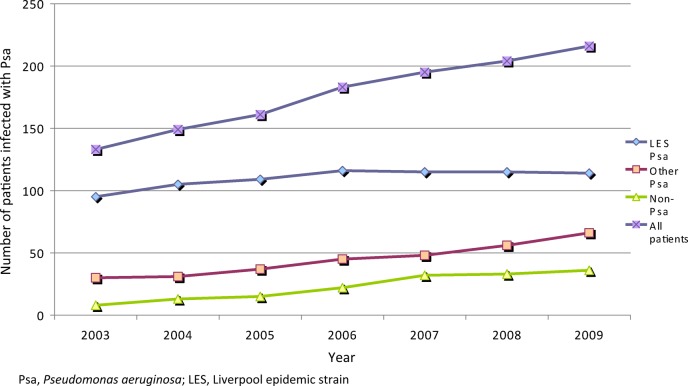
Over time, the adult clinic population has grown from 148 in 2003 to 244 in 2009. The proportion of patients chronically infected with Psa has diminished year on year, from 84% in 2003 to 74% in 2009 (see Figure 1). Of these, 71% were chronically infected with LES in 2003 and this proportion fell steadily to 53% in 2009 (P < 0.001). The proportion of patients transferring into the clinic from the paediatric sector also altered, with progressively fewer patients chronically infected by Psa, and of these, progressively fewer chronically infected by LES (see Table 1). During the study period, 56 patients have left the clinic (40 died [30 LES], 12 transferred elsewhere [6 LES], and 4 lost to follow-up [all sporadic strains]). Patients who underwent lung transplantation remained with their original cohort, since upper airway Psa infection with their original strain is presumed, whether or not the lower airway becomes re-infected with Psa.
Figure 1.
Prevalence of chronic Psa infection at the Liverpool CF clinic (2003–2009).
Table 1.
Incidence and prevalence of LES, other Psa strains and no Psa infection at Liverpool adult cystic fibrosis unit (2003–2009)
| 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|
| LES | 95 (71%) | 105 (70%) | 109 (68%) | 116 (63%) | 115 (58%) | 115 (56%) | 114 (53%) |
| Other Psa | 30 (23%) | 31 (21%) | 37 (23%) | 45 (25%) | 48 (25%) | 56 (27%) | 66 (31%) |
| No Psa | 8 (6%) | 13 (9%) | 15 (9%) | 22 (12%) | 32 (17%) | 33 (17%) | 36 (17%) |
| Super-infection | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Conversion (Non-Psa to Psa) | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| LES new transfers | 11 (57%) | 11 (48%) | 10 (42%) | 10 (33%) | 7 (25%) | 6 (20%) | 3 (13%) |
| Other Psa new transfers(%) | 6 (32%) | 7 (30%) | 11 (46%) | 13 (44%) | 8 (30%) | 14 (48%) | 11 (50%) |
| Non-Psa new transfers(%) | 2 (11%) | 5 (22%) | 3 (12%) | 7 (23%) | 12 (45%) | 9 (32%) | 8 (37%) |
Psa, Pseudomonas aeruginosa; LES, Liverpool epidemic strain
Super-infection
Over the seven-year period, two patients previously chronically infected with unique Psa strains have acquired LES (super-infection; see Table 2). In both cases, these patients had undergone social contact with LES- infected patients outside the hospital environment, despite appropriate counselling against this. There was no evidence of any nosocomial contact.
Table 2.
Details of patients super-infected with LES attending the Liverpool adult CF clinic (2003–2009)
| Date of joining service | Sample type | Date of conversion to LES | Sample type | Known social contacts with other LES patients | Details | |
|---|---|---|---|---|---|---|
| 1 | September 1992 | Sputum | September 2004 | Sputum | Yes | Socialized with 2 LES patients |
| 2 | August 2005 | Cough swab | July 2007 | Sputum | Yes | Knew 2 LES patients from childhood, frequent socialization |
LES, Liverpool epidemic strain
Pseudomonas conversion
There has only been one case of new chronic Psa infection in the uninfected cohort, despite attempted eradication. This occurred in 2009 and genotyping revealed a unique Psa strain. A further six patients developed acute infections with Psa (unique strains) and these were successfully eradicated using recommended protocols. There were no cases of patients without Psa becoming infected with either a known transmissible or an existing unique strain over the time period.
Other epidemic strains
Two patients are infected with the Midlands1 transmissible strain, one since the start of the screening process and another on presentation to our unit in 2005. Both these patients are managed separately from the rest of the clinic population and remain uninfected with any other epidemic Psa strain.
Discussion
Traditional thinking suggested that CF patients with chronic lung infections harboured their own unique organisms and could not transmit these to similar individuals.6 However, this was shown to be incorrect with the outbreak of the Burkholderia cepacia complex (Bcc) epidemic in CF patients attending summer camps in the USA in the late 1980s.27 Not only could these organisms spread to Bcc naïve patients,28,29 but we also showed that different Bcc strains could spread to patients already infected to their detriment.30 Subsequent stringent segregation of infected patients by the CF healthcare community halted this epidemic,31 and now few CF patients harbour these organisms.
However, at that time whether Psa might possess a similar ability was controversial since strains are indistinguishable by phenotypic methods alone. Although an increase in the incidence and prevalence of multiresistant Psa was noted in a Danish CF centre in the 1980s,32 and following phenotypic cohort segregation there was a fall in the annual incidence of new infections, other control measures (early eradication therapy for Psa infection and elective intravenous treatment for those with chronic infection) were also employed. These, coupled with a lack of genotypic identification of the resistant Psa isolates, meant the link between cohort segregation and improving clinical outcomes could not be made.
Transmissible strains may be more antibiotic resistant and have been shown to confer a worse prognosis with increased treatment requirements,13,21 inpatient hospital stays, worsening lung function and nutritional state,14 and will ultimately cause excess mortality.33
Prevention of infection of CF patients with these strains is therefore paramount: since they do not survive long in the environment and nosocomial reservoirs have not been found, patient-to-patient contact is the likely source of their acquisition, such that patient segregation becomes the most important infection control measure.
Unfortunately, CF care is complex and requires the coordinated efforts of a multidisciplinary team: while the grouping of patients together at dedicated centres is associated with improved outcomes, it also means that they are potentially exposed to pathogens, in particular transmissible Psa strains.34
However, the degree of contact necessary between CF individuals to allow transmission of organisms is unknown: although we have shown that LES-infected patients produce an aerosol of viable infected droplets that can be detected for several hours in the environment, and this clone also has an enhanced ability to survive on hard surfaces compared with other strains,35 the amount of exposure necessary for host acute infection/colonization with Psa in general remains unclear. Contact density must be an important factor in predicting cross-infection: in adult patients, the limited contact possible within the environment of an outpatient visit is unlikely to be sufficient, and where cross-infection has been documented, this has followed an inpatient stay.8,13
Nevertheless, complete avoidance of patient-to-patient contact is the gold standard to prevent the passage of organisms from one individual to another: under these circumstances, for inpatient care, all CF patients would be accommodated in separate areas on different wards, and for outpatient care in separate clinics at separate times of the week.
As regards outpatient care, it is impossible to review all patients entirely separately and some units use the approach whereby each patient remains in a single clinic room and is visited in turn by members of the CF multidisciplinary team (MDT),36 thereby ensuring that patient-to-patient contact within the clinic should not occur. However, such a strategy is time consuming, limits the number of patients that can be seen in any one session and even if patients adhere strictly to appointment times, since they are invited to attend the hospital at the same time, some mixing cannot be prevented. Furthermore, it is irrational to stringently segregate outpatients but not inpatients, where the risk of cross-infection is much greater.
Similarly as regards inpatient care, due to limited healthcare resources such segregation is difficult for most units, and many adopt the policy of admitting patients to the same facility, but in different rooms and with agreed rules of conduct while on the ward. However, children and young adults are gregarious and some mixing is inevitable, especially at those social times in the evening and weekends which are difficult to police, and it has been shown that this strategy ultimately results in cross-infection.37
Many adult CF clinics therefore use cohort segregation as the most practicable way of limiting cross-infection. However, Psa strains cannot be separated on phenotypic or antibiogram patterns38 such that policies which rely on these criteria will inevitably allow cross-infection to occur. These include ones that segregate solely on multiresistance or the separation of Psa-positive from Psa-negative patient groups. In the latter, those with sporadic strains cannot by definition cross-infect, and therefore their separation from those who are Psa negative is illogical, but they can in turn become super-infected by those in the Psa-positive group with transmissible strains with the potential for consequent clinical deterioration.8 It therefore follows that in order for any cohorting policy to be effective, the clinician needs to be aware of the strains of Psa in their CF clinic population in realtime. This can only be achieved by Psa genotyping on a regular basis, allowing those with transmissible strains to be segregated from all other individuals.
It is this policy we adopted in 2003 in our developing adult CF clinic, where increasing numbers of patients already infected with transmissible Psa (LES) were arriving from the local paediatric centre as they reached adulthood, and a cross-sectional survey had shown a high prevalence among our patients. Our results show that by using regular genotypic surveillance of Psa strains and segregating patient groups accordingly, we have prevented infection by nosocomial contact. The very few patients who have developed super-infection all did so through well-documented social contact outside the hospital environment, despite advice to the contrary. It is of note that other units undertaking Psa genotypic analysis, but without effective patient segregation measures in place, have noted a high cross-infection rate with LES during this period.37 Furthermore, although we have not separated those without Psa infection from those infected with sporadic Psa strains, there has been only one new case of chronic Psa infection in this group (with a unique strain), underlining that it is unnecessary to separate these patient cohorts. A further six cases of acute Psa infection with unique strains occurred, all of which were successfully eradicated – although previous studies4,39,40 reported a higher incidence of such acute infections, these were carried out in a largely paediatric population where the prevalence of chronic Psa infection is much lower.
There are financial consequences to adopting this genotypic surveillance protocol: each test costs approximately £20, and with the addition of technician time the yearly cost to our clinic is currently £22,000. This is likely to increase further, since although the relative numbers of patients infected with Psa strains is diminishing with time due to better cross-infection control and eradication therapy (particularly in paediatric practice), the absolute numbers continue to grow as more patients live longer. Nevertheless, we believe that not only is the cost of this testing outweighed by the clinical benefit, but there are strong economic arguments for its use. Firstly, segregating patient by other methods would necessitate an alteration in clinic and ward infrastructure, which would be costly and for some units impossible, and secondly cross-infection with transmissible strains has been shown to confer an increased healthcare cost burden.41 Finally, the emerging medicolegal consequences of allowing cross-infection between CF patients within the hospital environment, which can be costly, are also avoided.
In conclusion, we recommend the use of genotypic surveillance of Psa strains, to allow rational segregation of CF patients. Using such a method, we have halted the epidemic in our clinic of LES, the most prevalent and important transmissible Psa strain within the CF community.
DECLARATIONS
Competing interests
None declared
Funding source
None
Ethical approval
Ethical approval was not sought or necessary for this study because it was an observational study following a change in clinical practice.
Guarantor
MJW
Contributorship
AA collected all the data, collated the results drafted and edited the manuscript. MS collated and analysed the data and edited the manuscript. LH provided the data and edited the manuscript. CW analysed the results, microbiological methods and edited the manuscript. MW reviewed the results, drafted, edited and proofread the manuscript
Acknowledgments
None
Reviewer
Frank Edinborough
References
- 1.UK CF Registry Annual data report 2010 UK CF Trust, Bromley, Kent, UK
- 2.Belkin RA, Henig NR, Singer LG, et al. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med 2006;173:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry RL, Mellis CM, Petrovic L Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol 1992;12:158–61 [DOI] [PubMed] [Google Scholar]
- 4.Frederiksen B, Koch C, Hoiby N Changing epidemiology of Pseudomonas aeruginosa infection in Danish cystic fibrosis patients (1974–1995). Pediatr Pulmonol 1999;28:159–66 [DOI] [PubMed] [Google Scholar]
- 5.Kerem E, Corey M, Gold R, Levison H Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr 1990;116:714–9 [DOI] [PubMed] [Google Scholar]
- 6.Speert DP, Campbell ME Hospital epidemiology of Pseudomonas aeruginosa from patients with cystic fibrosis. J Hosp Infect 1987;9:11–21 [DOI] [PubMed] [Google Scholar]
- 7.Cheng K, Smyth RL, Govan JR, et al. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 1996;348:639–42 [DOI] [PubMed] [Google Scholar]
- 8.McCallum SJ, Corkill J, Gallagher M, Ledson MJ, Hart CA, Walshaw MJ Superinfection with a transmissible strain of Pseudomonas aeruginosa in adults with cystic fibrosis chronically colonised by P. aeruginosa. Lancet 2001;358:558–60 [DOI] [PubMed] [Google Scholar]
- 9.McCallum S, Gallagher M, Corkill J, Hart C, Ledson M, Walshaw M Spread of an epidemic Pseudomonas aeruginosa strain from a patient with cystic fibrosis (CF) to non-CF relatives. Thorax 2002;57:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan K, Fothergill JL, Storrar J, Ledson MJ, Winstanley C, Walshaw MJ Transmission of Pseudomonas aeruginosa epidemic strain from a patient with cystic fibrosis to a pet cat. Thorax 2008;63:839–40 [DOI] [PubMed] [Google Scholar]
- 11.Scott FW, Pitt TL Identification and characterization of transmissible Pseudomonas aeruginosa strains in cystic fibrosis patients in England and Wales. J Med Microbiol 2004;53(Pt 7):609–15 [DOI] [PubMed] [Google Scholar]
- 12.Aaron S, Vandemheen K, Ramotar K, et al. Epidemic strains of Pseudomonas aeruginosa in adult CF patients in Ontario, Canada – prevalence and epidemiology. Pediatr Pulmonol 2008;43(s 31):327 [Google Scholar]
- 13.Jones AM, Govan JR, Doherty CJ, et al. Spread of a multiresistant strain of Pseudomonas aeruginosa in an adult cystic fibrosis clinic. Lancet 2001;358:557–8 [DOI] [PubMed] [Google Scholar]
- 14.Al-Aloul M, Crawley J, Winstanley C, Hart CA, Ledson MJ, Walshaw MJ Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax 2004;59:334–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edenborough F, Stone H, Kelly S, Zadik P, Doherty C, Govan J Genotyping of Pseudomonas aeruginosa in cystic fibrosis suggests need for segregation. J Cyst Fibros 2004;3:37–44 [DOI] [PubMed] [Google Scholar]
- 16.Tubbs D, Lenney W, Alcock P, Campbell CA, Gray J, Pantin C Pseudomonas aeruginosa in cystic fibrosis: cross-infection and the need for segregation. Respir Med 2001;95:147–52 [DOI] [PubMed] [Google Scholar]
- 17.Denton M, Kerr K, Mooney L, et al. Transmission of colistin-resistant Pseudomonas aeruginosa between patients attending a paediatric cystic fibrosis center. Pediatr Pulmonol 2002;34:257–61 [DOI] [PubMed] [Google Scholar]
- 18.Armstrong D, Bell S, Robinson M, et al. Evidence for spread of a clonal strain of Pseudomonas aeruginosa among cystic fibrosis clinics. J Clin Microbiol 2003;41:2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths AL, Jamsen K, Carlin JB, et al. Effects of segregation on an epidemic Pseudomonas aeruginosa strain in a cystic fibrosis clinic. Am J Respir Crit Care Med 2005;171:1020–5 [DOI] [PubMed] [Google Scholar]
- 20.O'Carroll MR, Syrmis MW, Wainwright CE, et al. Clonal strains of Pseudomonas aeruginosa in paediatric and adult cystic fibrosis units. Eur Respir J 2004;24:101–6 [DOI] [PubMed] [Google Scholar]
- 21.Bradbury R, Champion A Poor clinical outcomes associated with a multi-drug resistant clonal strain of Pseudomonas aeruginosa in the Tasmanian cystic fibrosis population. Respirology 2008;13:886–92 [DOI] [PubMed] [Google Scholar]
- 22.Speert DP, Campbell ME, Henry DA, et al. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. Am J Respir Crit Care Med 2002;166:988–93 [DOI] [PubMed] [Google Scholar]
- 23.Dinesh S, Grundmann H, Pitt T, Römling U European-wide distribution of Pseudomonas aeruginosa clone C. Clin Microbiol Infect 2003;9:1228–33 [DOI] [PubMed] [Google Scholar]
- 24.Panagea S, Winstanley C, Parsons YN, Walshaw MJ, Ledson MJ, Hart CA PCR-based detection of a cystic fibrosis epidemic strain of Pseudomonas aeruginosa. Mol Diagn 2003;7:195–200 [DOI] [PubMed] [Google Scholar]
- 25.Mahenthiralingam E, Campbell ME, Foster J, Lam JS, Speert DP Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J Clin Microbiol 1996;34:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fothergill JL, Upton AL, Pitt TL, Hart CA, Winstanley C Diagnostic multiplex PCR assay for the identification of the Liverpool, Midlands 1 and Manchester CF epidemic strains of Pseudomonas aeruginosa. J Cyst Fibros 2008;7:258–61 [DOI] [PubMed] [Google Scholar]
- 27.Pegues DA, Carson LA, Tablan OC, et al. Acquisition of Pseudomonas cepacia at summer camps for patients with cystic fibrosis. J Pediatr 1994;124:694–702 [DOI] [PubMed] [Google Scholar]
- 28.Whiteford M, Wilkinson J, McColl J, et al. Outcome of Burkholderia (Pseudomonas) cepacia colonisation in children with cystic fibrosis following a hospital outbreak. Thorax 1995;50:1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen HK, Kovesi TA, Koch C, Corey M, Høiby N, Levison H Pseudomonas aeruginosa and Burkholderia cepacia infection in cystic fibrosis patients treated in Toronto and Copenhagen. Pediatr Pulmonol 1998;26:89–96 [DOI] [PubMed] [Google Scholar]
- 30.Ledson M, Gallagher M, Corkill J, Hart C, Walshaw M Cross infection between cystic fibrosis patients colonised with Burkholderia cepacia. Thorax 1998;53:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhdi K, Edenborough F, Gumery L, et al. Outcome for patients colonised with Burkholderia cepacia in a Birmingham adult cystic fibrosis clinic and the end of an epidemic. Thorax 1996;51:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen SS, Koch C, Hoiby N, Rosendal K An epidemic spread of multiresistant Pseudomonas aeruginosa in a cystic fibrosis centre. J Antimicrob Chemother 1986;17:505–16 [DOI] [PubMed] [Google Scholar]
- 33.Armstrong DS, Nixon GM, Carzino R, et al. Detection of a widespread clone of Pseudomonas aeruginosa in a pediatric cystic fibrosis clinic. Am J Respir Crit Care Med 2002;166:983–7 [DOI] [PubMed] [Google Scholar]
- 34.Mahadeva R, Webb K, Westerbeek RC, et al. Clinical outcome in relation to care in centres specialising in cystic fibrosis: cross sectional study. BMJ 1998;316:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panagea S, Winstanley C, Walshaw M, Ledson M, Hart C Environmental contamination with an epidemic strain of Pseudomonas aeruginosa in a Liverpool cystic fibrosis centre, and study of its survival on dry surfaces. J Hosp Infect 2005;59:102–7 [DOI] [PubMed] [Google Scholar]
- 36.Saiman L, Siegel J, Cystic Fibrosis Foundation Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Infect Control Hosp Epidemiol 2003;24(Suppl):S6–52 [DOI] [PubMed] [Google Scholar]
- 37.Jones AM, Dodd ME, Morris J, Doherty C, Govan JR, Webb AK Clinical outcome for cystic fibrosis patients infected with transmissible Pseudomonas aeruginosa: an 8-year prospective study. Chest 2010;137:1405–9 [DOI] [PubMed] [Google Scholar]
- 38.Davies G, McShane D, Davies J, Bush A Multiresistant Pseudomonas aeruginosa in a pediatric cystic fibrosis center: Natural history and implications for segregation. Pediatr Pulmonol 2003;35:253–6 [DOI] [PubMed] [Google Scholar]
- 39.Lee TWR, Brownlee KG, Denton M, Littlewood JM, Conway SP Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatr Pulmonol 2004;37:104–10 [DOI] [PubMed] [Google Scholar]
- 40.McKay KO, Cooper PJ, van Asperen PP Segregation of children with CF diagnosed via newborn screening and acquisition of Pseudomonas aeruginosa. J Cyst Fibros 2009;8:400–4 [DOI] [PubMed] [Google Scholar]
- 41.Ashish A, Nazreth D, Tan H, Jordan T, Ledson M, Walshaw M The increased healthcare economic burden associated with chronic infection with transmissible Pseudomonas aeruginosa strains in CF. J Cyst Fibros 2010;9(Suppl. 1):S116 [Google Scholar]