Abstract
Background and purpose
Giant cell tumor of bone (GCT) is sometimes difficult to distinguish from other giant-cell-rich tumors such as chondroblastoma (CHB) and aneurysmal bone cyst (ABC). The usefulness of p63 as a diagnostic marker for GCT is controversial. While there have been no reports about p63 as a prognostic marker for local recurrence, various p63-positive rates in GCT have been reported. The purpose of this study was to investigate retrospectively whether p63 is useful as a diagnostic marker and/or a prognostic marker for local recurrence of GCT.
Methods
This study included 36 patients diagnosed with either GCT (n = 16), CHB (n = 9), ABC (n = 7), or non-ossifying fibroma (NOF) (n = 4). p63 immunostaining was performed for all specimens. The mean p63-positive rate was compared with the four diseases and between the recurrent and non-recurrent cases of GCT.
Results
Although the mean p63-positive rate for GCT (36.3%) was statistically higher than that of all other diseases examined (CHB: 15.2%; ABC: 5.8%; NOF: 3.4%), p63 was not specific for GCT. The mean p63-positive rate for recurrent GCT cases (73.6%) was statistically higher than that for non-recurrent cases (29.1%).
Conclusion
In the diagnosis of GCT, p63 is a useful but not a conclusive marker. However, p63 did appear to indicate the biological aggressiveness of GCT. Therefore, p63 may help surgeons to estimate the risk of recurrence after surgery and help them to choose the best treatment for each GCT case.
Keywords: Giant cell tumor of bone, local recurrence, p63, prognostic marker
Introduction
Giant cell tumor of bone (GCT) is a primary bone tumor that occurs predominantly in young adults. Although GCT is classified as a benign neoplasm, it has a wide clinical spectrum and is similar to malignant tumors: it sometimes behaves more aggressively with a high recurrence rate and occasional metastasis to the lungs. Recurrence rates after curettage and bone grafting are approximately 50% (1-3). Although several types of adjuvant therapies have improved the prognosis, recurrence rates are still relatively high, and control of local GCT recurrence remains an important issue. In addition, no reliable prognostic factor or marker for risk of local recurrence has been identified. While there is controversy about the usefulness of p63 as a diagnostic marker for GCT, p63 has recently been identified to be highly expressed in this disease (4-6). In addition, in a clinicopathological study of GCT in small bones, p63 was found to be highly expressed in the case of a patient with two episodes of local recurrence, suggesting that p63 expression indicates GCT recurrence (7). The purpose of this study was to investigate retrospectively whether p63 is useful for the differential diagnosis of GCT or for the prediction of aggressiveness and risk of local GCT recurrence.
Patients and methods
Patients and initial characterization
Patients treated for GCT, chondroblastoma (CHB), aneurysmal bone cyst (ABC), and non-ossifying fibroma (NOF) at Hirosaki National Hospital, Hirosaki University Hospital, and various other institutions between 1982 and 2009 were selected for this study. The final diagnosis and the instances of recurrences were confirmed using medical records. Patients with no recorded recurrence but a follow-up period of less than 2 years were excluded from this study. To make the conditions of the primary surgery as unified as possible, we excluded cases of GCT treated by en bloc- resection or curettage followed by bone cementing as well as a case in which a pathological fracture was present at the time of first presentation. Paraffin-embedded specimens (5-μm-thick sections) were stained with hematoxylin and eosin (H+E). Cases of possible malignant tumors, as suggested by H+E staining, were excluded from this study.
Immunohistochemical staining of tumors and calculations
Immunohistochemical staining was performed using an anti-p63 mouse monoclonal antibody (clone4A4, 1:50 dilution; Dako, Glostrup, Denmark). Staining was performed on the Ventana Benchmark XT system autostainer (Ventana Medical Systems, Tucson, AZ, USA) using the iView DAB staining protocol according to the manufacturer's instructions. Epitopes were retrieved by a heat-induced epitope retrieval method. For each case, ten microscopic images (BX41,DP25; Olympus Co, Tokyo, Japan) were taken at a magnification of 400×. Total and p63-positive mononuclear cells were manually counted for each case. The p63-positive rate was defined as shown in Figure 1. The mean p63-positive rate for each of the four diseases examined was then calculated. These calculations were randomly performed, such that the investigators did not know which disease group the samples belonged to or whether the cases showed a recurrence.
Figure 1.
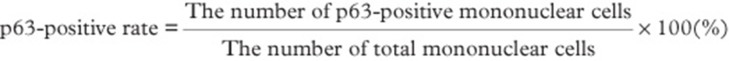
Definition of p63-positive rate.
Statistical analysis
Data input and calculations were performed using SPSS ver. 12.0J (SPSS Inc., Chicago, IL, USA). Comparison of the mean p63-positive rate between recurrent and non-recurrent GCT cases was performed using the Mann–Whitney U test. One-way analysis of variance and Tukey test for post hoc analysis were used to analyze the p63-positive rate among GCT, CHB, ABC, and NOF cases. Values of P < 0.05 were considered to be statistically significant.
Results
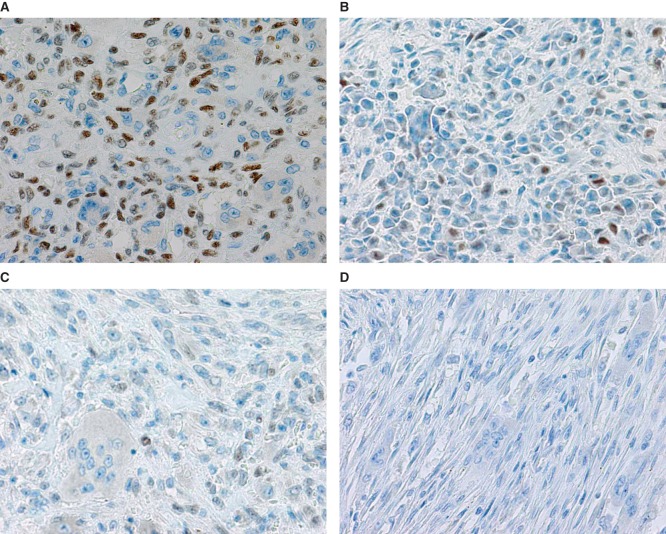
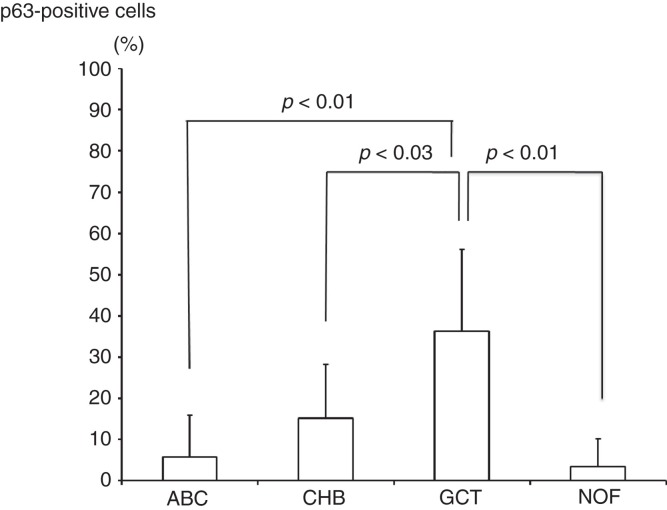
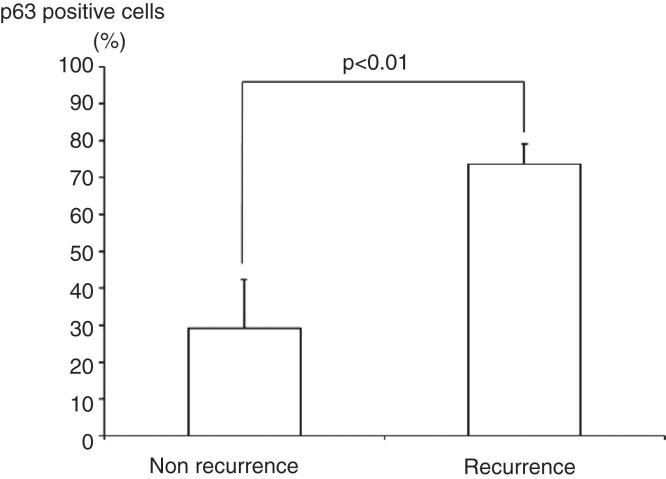
The study included 36 patients with either GCT (n = 6), CHB (n = 9), ABC (n = 7), or NOF (n = 4). Clinical data of GCT cases are shown in Table I. The mean p63-positive rates for GCT, CHB, ABC, and NOF were 36.3% (8.7%–79.8%), 15.2% (3.4%–38.2%), 5.8% (0%–27.6%), and 3.4% (0%–13.5%), respectively. A representative image of immunostaining in each disease is shown in Figure 2. The mean p63-positive rate for GCT was statistically higher than that observed in all other diseases (P < 0.04, Figure 3). In addition, the mean p63-positive rate for recurrent GCT cases (73.6%) was statistically higher than that for non-recurrent GCT cases (29.1%, P < 0.01, Figure 4). Representative images of the non-recurrent cases and recurrent cases are shown in Figure 5. Although we made no objective measurements, the intensity of staining was stronger in recurrent GCT cases than in non-recurrent GCT cases.
Table I.
Characteristics of the 16 GCT cases.
| Case | Sex | Age | Location | Stage | p63-positive rate (%) | Initial treatment (adjuvant therapy) | Recurrence |
|---|---|---|---|---|---|---|---|
| 1 | F | 27 | Distal femur | - | 21.0 | Curettage/bone grafting (N) | No |
| 2 | F | 34 | Proximal tibia | - | 9.2 | Curettage/bone grafting (N) | No |
| 3 | F | 44 | Proximal tibia | 2 | 8.7 | Curettage/bone grafting (E+P) | No |
| 4 | M | 19 | Femoral neck | 3 | 72.0 | Curettage/bone grafting (E+P) | Yes (twice) |
| 5 | F | 18 | Humeral neck | 2 | 69.1 | Curettage/bone grafting (E+P) | Yes |
| 6 | M | 56 | Distal femur | 2 | 43.3 | Curettage/bone grafting (E+P) | No |
| 7 | M | 55 | Ilium | 2 | 30.5 | Curettage/bone grafting (E+P) | No |
| 8 | M | 33 | Proximal femur | 2 | 53.1 | Curettage/bone grafting (E+P) | No |
| 9 | M | 38 | Proximal tibia | 2 | 44.1 | Curettage/bone grafting (E+P) | No |
| 10 | M | 19 | Cuboid | 2 | 34.6 | Curettage/bone grafting (O) | No |
| 11 | F | 20 | Middle phalanx | 2 | 79.8 | Curettage/bone grafting (E+P) | Yes (twice) |
| 12 | F | 60 | Metatarsal | 2 | 29.7 | Curettage/bone grafting | No |
| 13 | F | 15 | Distal femur | 1 | 28.6 | Curettage/bone grafting (E+P) | No |
| 14 | M | 61 | Distal femur | 1 | 37.9 | Curettage/bone grafting (E+P) | No |
| 15 | M | 32 | Proximal tibia | 2 | 23.7 | Curettage/bone grafting (E+P) | No |
| 16 | M | 29 | Distal femur | 3 | 22.5 | Curettage/bone grafting (E+P) | No |
Stage: Image evaluation by Campanacci stage classification. Adjuvant therapy: E+P = ethanol + phenol; N = liquid nitrogen; O = others.
Figure 2.
Representative microscopic images of p63 immunostaining in GCT (A), CHB (B), ABC (C), and NOF (D) cases.
Figure 3.
The mean p63-positive rate for GCT (36.3%) was significantly higher than that detected for CHB (15.2%), ABC (5.8%), and NOF (3.4%).
Figure 4.
The mean p63-positive rate for recurrent GCT cases (73.6%) was significantly higher than that observed in non-recurrent GCT cases (29.1%).
Figure 5.
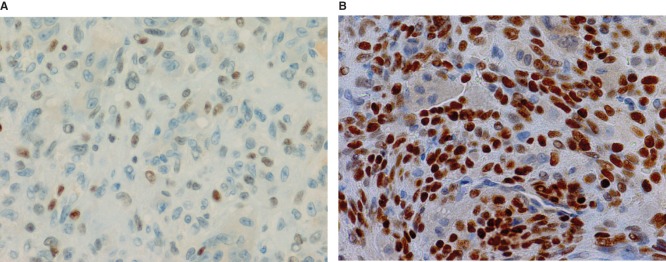
Representative microscopic images of p63 immunostaining for non-recurrent (A) and recurrent GCT cases (B).
Discussion
GCT is a primary bone tumor occurring predominantly in young adults. Although these tumors are histologically classified as benign neoplasms, they may be locally aggressive and destructive. Despite the high risk of local recurrence, intralesional excision is preferred as the primary surgery because of improved postoperative functional preservation (2). As the recurrence rate is approximately 50% following surgery with only curettage and bone grafting (1-3), adjuvant treatments using high-speed burr (8), phenol (9), liquid nitrogen (10), zinc chloride (11), and bone cement (12-14) have been used to prevent recurrence. However, the reported recurrence rates after surgeries with these adjuvant therapies are inconsistent. Curettage with high-speed burring and bone cementing appears to be the best intralesional surgery for GCT from the viewpoint of tumor recurrence (12,14). However, since GCT usually occurs in the epiphyses of the long bones, possible effects on the joint cartilage and potential difficulties in handling degenerative diseases or injuries are worrying. En bloc resection may be most effective in preventing local recurrence (3,15,16). However, serious functional loss may occur in the adjacent joint after surgery (17). Thus, each type of therapy has both advantages and disadvantages.
To date, no prognostic marker for the risk of GCT recurrence has been identified (18). The histological grading system of Jaffe and the X-ray grading system using Campanacci classification are considered to have no correlation with clinical outcome (19). However, if there were reliable prognostic markers for the risk of local recurrence, surgeons could consider the best treatment option for each individual case. Two groups have shown that mononuclear cells in GCT express p63, which is useful for the differential diagnosis of GCT from other giant-cell-rich tumors such as ABC and CHB (4,5). On the other hand, de la Roza showed no difference in p63 positivity by immunostaining among the giant-cell-rich lesions such as GCT and CHB (6). In this study, the mean p63-positive rate was statistically higher in GCT than in CHB, ABC, and NOF. However, p63 cannot be an essential factor for the differential diagnosis of GCT because the p63-positive rates overlapped to a varying degree among these diseases. Alberghini et al. also showed no difference in p63 positivity by immunostaining between primary GCT and secondary metastatic GCT to the lung (20). However, no one has verified the usefulness of p63 as a prognostic marker with respect to local recurrence. Wülling et al. suggested that the stromal cells are neoplastic components of GCT while the monocytes and multinucleated giant cells are just reactive components (21). They also reported that the stromal cells in GCT can develop into both mesenchymal stem cells and osteoblasts (22). As p63 immunostaining is positive only for the mononuclear cells in GCT, it may be indicative of the nature of individual tumors. In the study of GCT of small bones, p63 was found to be highly expressed in a case with two episodes of recurrence (7). This finding led us to consider the usefulness of p63 as a prognostic marker.
p63 is a member of the gene family that includes p53 and p73; p63 plays a role in skeletal development, and its mutation in humans leads to limb abnormalities (limb-mammary syndrome) (5). Unlike p53, an important tumor suppressor that is frequently inactivated in many tumor types, the precise role of p63 in oncogenesis remains unclear. It has been suggested that p63 may be a bifunctional cancer gene (23). Although the mechanisms underlying p63 expression in GCT remain unknown, stronger specific nuclear p63 immunoreactivity is found in GCT in comparison with other mesenchymal tumors (5,21). p63 encodes for at least six protein isoforms with or without a transactivation (TA) domain (TA isotypes or ΔN isotypes respectively). While the TA isotypes show a p53-like tumor-suppressor function, ΔNp63 is considered to exert oncogenic effects in some epithelial cancers (24-26). Although it is not clear why, it has been found that TAp63 is expressed in GCT (4,5). In this study, the p63 immunoreactivity of mononuclear cells in GCT was statistically higher than that observed in CHB, ABC, and NOF, in agreement with the reports mentioned above. In addition, the p63-positive rate for recurrent cases of GCT was statistically higher than that for non-recurrent GCT cases. This suggests that p63 immunoreactivity in GCT may indicate not only the specificity of the tumor but also its biological activity. Therefore, in GCT, p63 appears to play a role in oncogenesis or progression, even if the subtype was TAp63. Further investigations are needed to clarify the role of TAp63 in oncogenesis or progression in GCT.
In summary, although the expression of p63 in GCT is higher than that in other giant-cell-rich tumors such as CHB, ABC, and NOF, its expression is not a conclusive factor for the diagnosis of GCT. However, p63 may be useful as a prognostic marker for the risk of local recurrence of GCT. Reference to the p63-positive rate may help surgeons to decide the best treatment for each case of GCT.
Acknowledgements
We thank Takahiro Tajino, Tadashi Hasegawa, and Ryo Inoue for their additional information.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Persson BM, Ekelund L, Lövdahl R, Gunterberg B. Favourable results of acrylic cementation for giant cell tumors. Acta Orthop Scand. 1984;55:209–14. doi: 10.3109/17453678408992339. [DOI] [PubMed] [Google Scholar]
- 2.Capanna R, Fabbiri N, Bettelli G. Currettage of giant cell tumor of bone: the effect of surgical technique and adjuvants on local recurrence rate. Chir Organi Mov. 1990;75:206. [PubMed] [Google Scholar]
- 3.Szendöi M, Kiss J, Antal I. Surgical treatment and prognostic referrals in giant-cell tumor of bone. Acta Chir Orthop Traumatol Chech. 2003;70:142–50. [PubMed] [Google Scholar]
- 4.Dickson B, Li S, Wunder J, Ferguson P, Eslami B, Werier J, et al. Giant cell tumor of bone express p63. Mod Pathol. 2008;21:369–75. doi: 10.1038/modpathol.2008.29. [DOI] [PubMed] [Google Scholar]
- 5.Lee C, Espinosa I, Jensen K, Subramanian S, Zhu S, Varma S, et al. Gene expression profiling identifies p63 as a diagnostic marker for giant cell tumor of the bone. Mod Pathol. 2008;21:531–9. doi: 10.1038/modpathol.3801023. [DOI] [PubMed] [Google Scholar]
- 6.de la Roza G. p63 expression in giant cell-containing lesion of bone and soft tissue. Arch Pathol Lab Med. 2010;135:776–9. doi: 10.5858/2010-0291-OA.1. [DOI] [PubMed] [Google Scholar]
- 7.Yanagisawa M, Okada K, Tajino T, Tirugoe T, Kawai A, Nishida J, et al. Clinicopathological study of giant cell tumor of small bones. Upsala J Med Sci. 2011;116:265–8. doi: 10.3109/03009734.2011.596290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg Am. 1999;81:811–20. doi: 10.2106/00004623-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima H, Arai T, Shimizu K, Beppu M, Takagi M, Kazama A. Intra-lesional excision with local adjuvant treatment utilizing phenol cauterization for giant cell tumor of bone. Rinsho Seikei Geka. 2006;41:1093–8. [Google Scholar]
- 10.Malawer M, Bickels J, Meller I, Buch R, Henshaw R, Kollender Y. Cryosurgery in the treatment of giant cell tumor. Clin Orthop. 1999;359:176–88. doi: 10.1097/00003086-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Zhen W, Yaortian H, Songjian L, Ge L, Qingliang W. Giant-cell tumor of bone. J Bone Joint Surg Br. 2004;86:212–16. doi: 10.1302/0301-620x.86b2.14362. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell R, Springfield D, Motowani H, Ready J, Gebhardt M, Mankin H. Recurrence of giant-cell tumors of long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76:1827–33. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Remedios D, Saifuddin A, Pringle J. Radiological and clinical recurrence of giant-cell tumor of bone after the use of cement. J Bone Joint Surg Br. 1997;79:26–30. doi: 10.1302/0301-620x.79b1.7102. [DOI] [PubMed] [Google Scholar]
- 14.Wada T, Kaya M, Nagoya S, Kawaguchi S, Isu K, Yamashita T, et al. Complications associated with bone cementing for the treatment of giant cell tumor of bone. J Orthop Sci. 2002;7:194–8. doi: 10.1007/s007760200033. [DOI] [PubMed] [Google Scholar]
- 15.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–4. [PubMed] [Google Scholar]
- 16.Su Y, Chen W, Chen T. Giant-cell tumors of bone: an analysis of 87 cases. Int Orthop. 2004;28:239–43. doi: 10.1007/s00264-004-0564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en block resection for giant-cell tumors of bone. J Bone Joint Surg Am. 1993;75:1648–55. doi: 10.2106/00004623-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 18.McDonald D, Sim F, McLeod R, Dahlin D. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68:235–42. [PubMed] [Google Scholar]
- 19.Unni KK, Inward CY. Bone tumors. 6th ed. New York: Lippincott-Raven; 2010. [Google Scholar]
- 20.Alberghini M, Kliskey K, Krenacs T, Picci P, Kindblom L, Forsyth R, et al. Morphological and immunophenotypic features of primary and metastatic giant cell tumour of bone. Virchows Arch. 2010;456:97–103. doi: 10.1007/s00428-009-0863-2. [DOI] [PubMed] [Google Scholar]
- 21.Wülling M, Engels C, Jesse N, Werner M, Delling G, Kaiser E. The nature of giant cell tumor of bone. J Cancer Res Clin Oncol. 2001;127:467–74. doi: 10.1007/s004320100234. [DOI] [PubMed] [Google Scholar]
- 22.Wülling M, Delling G, Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003;34:983–93. doi: 10.1053/s0046-8177(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 23.Mills A. p63: oncogene or tumor suppressor? Curr Opin Genet Dev. 2006;16:38–44. doi: 10.1016/j.gde.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Como C, Urist M, Babayan I, Drobnjak M, Hedvat C, Teruya-Feldstein J, et al. p63 expression profiles in human normal and tumor tissues. Clin Cancer Res. 2002;8:494–501. [PubMed] [Google Scholar]
- 25.Geddert H, Kiel S, Heep H, Gabbert H, Sarbia M. The role of p63 and ΔNp63 (p40) protein expression and gene amplification in esophageal carcinogenesis. Hum Pathol. 2003;34:850–6. doi: 10.1016/s0046-8177(03)00342-3. [DOI] [PubMed] [Google Scholar]
- 26.Koga F, Kawakami A, Fujii Y, Saito K, Ohtsuka Y, Iwai A, et al. Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res. 2003;9:5501–7. [PubMed] [Google Scholar]