Abstract
Purpose
Psoriasis vulgaris is one of the most common skin disorders. Patients with psoriasis carry an excessive risk of atrial fibrillation (AF). The differences between the maximum (Pmax) and the minimum (Pmin) P-wave duration on ECG are defined as P-wave dispersion (PWD). Prolongation of PWD is an independent risk factor for the development of AF. The aim of this the study was to investigate P-wave duration and PWD in patients with psoriasis.
Methods
Sixty-one adult patients with psoriasis vulgaris (group 1) and 58 age and sex-matched healthy individuals (group 2) were included in this study. ECG recordings were obtained, and the P-wave variables were calculated. Results were reported as mean ± standard deviation and percentages. Continuous variables were analysed using Student's t test. A value of P < 0.05 was considered statistically significant.
Results
Pmax and PWD were significantly higher in group 1 than in group 2 (108.8 ± 21.3 ms versus 93.3 ± 13.0 ms, P < 0.001; 67.4 ± 22.9 ms versus 45.0 ± 19.6 ms, P < 0.001, respectively). Also, Pmin was significantly lower in group 1 (41.3 ± 12.3 ms versus 48.3 ± 14.3 ms, P = 0.04). The psoriasis disease activity score and hsCRP correlated with PWD (P < 0.01).
Conclusions
Atrial conduction of sinus impulses was impaired in patients with psoriasis vulgaris. It was more prominent in patients with severe disease. Physicians caring for patients with psoriasis vulgaris should screen them for AF development.
Keywords: Atrial fibrillation, psoriasis vulgaris, P-wave dispersion
Introduction
Psoriasis vulgaris is one of the most prevalent T-cell-mediated, chronic, multisystemic, inflammatory diseases and is characterized by epidermal hyperproliferation, abnormal keratinocyte differentiation, T-lymphocyte infiltration, and increased expression of cytokines, which results in the formation of inflamed plaque affecting the skin, scalp, nails, and joints (1). Although the pathogenesis of psoriasis is still not fully understood, systemic inflammatory response and oxidative stress are considered the most important mechanisms in the disease's development. Some factors such as infection, drugs, trauma, alcohol consumption, smoking, cold weather, sunlight deprivation, and emotional stress have been acknowledged as triggering and/or aggravating the disease (2).
Chronic inflammation and oxidative stress are also thought to be responsible for the increased prevalence of cardiovascular diseases. Patients with psoriasis have a much higher risk of developing hypertension, atherosclerosis, and heart valve abnormalities (3). Recently, Ahlehoff et al. demonstrated that psoriasis is associated with increased risk of atrial fibrillation (AF) (4). As the most prevalent arrhythmia, AF is associated with increased risks of ischaemic stroke, heart failure, coronary artery disease, and cardiovascular death. An assumption that inflammation and oxidative stress in patients with psoriasis contributed to the development of AF seems only natural.
The differences between the maximum (Pmax) and minimum (Pmin) P-wave duration on standard 12-lead electrocardiogram (ECG) are defined as P-wave dispersion (PWD). PWD can be easily measured using a single ECG and is regarded as an electrocardiographic marker of prolongation of intra-atrial and inter-atrial conduction time in addition to heterogeneous and discontinuous propagation of sinus impulses (5). Prolongation of PWD has been demonstrated to be an independent risk factor for the development of atrial fibrillation (AF), which is the most common sustained arrhythmia in the general population that increases cardiovascular morbidity and mortality and decreases quality of life (6). To date, maximum P-wave duration and PWD are commonly used to determine the risk of AF in several patient populations with inflammatory diseases (7,8). This study aims to investigate P-wave duration and PWD in patients with long-lasting psoriasis.
Materials and methods
Patients
We selected 61 patients over the age of 18 with a minimum psoriasis vulgaris disease duration of 3 years and who were previously diagnosed via biopsy and admitted to the Dermatology Department, Faculty of Medicine, BezmiÂlem Foundation University, Istanbul, Turkey (group 1). Also, 58 age- and sex-matched healthy individuals were included in this study as the control group (group 2). Patients and control subjects with past or concurrent diseases like hypertension (HT), diabetes mellitus, coronary artery disease (CAD), lung diseases and/or pulmonary HT, valvular heart diseases, liver or kidney diseases, collagen vascular diseases, rhythms other than sinus, any cardiovascular drug use, abnormal thyroid function, or serum electrolyte values were excluded from the study. Also, obese subjects (BMI ≥30 kg/m2) were excluded from the study. All subjects gave informed consent, and the study was approved by the local ethics committee. Patients with coexisting psoriatic arthritis (diagnosed by a rheumatologist) were excluded due to possible adverse effects on heart rhythm.
The age, gender, age of onset, duration of the disease, and drug history of each patient were recorded. Height, weight, body mass index (BMI), and waist circumference were assessed in all patients. Biochemical variables such as fasting glucose levels and lipid panel were analysed. Also, the serum high-sensitivity C-reactive protein (hsCRP) level was obtained with the nephelometric method, using a Dade Behring Cardio Phase kit (Dade Behring Inc. Newark, Delaware, USA). Transthoracic echocardiography was performed using a Philips Envisor C echocardiograph (Philips Medical Systems, Andover, MA, USA), and a 3.5 MHz transducer was employed to measure the left ventricular dimensions, left ventricular ejection fraction (LVEF), left atrial (LA) diameter, and diastolic function analysis. Echocardiographic examinations were performed by two experienced echocardiographers blinded to the study.
Evaluation of patients' disease activity
The diagnosis of psoriasis vulgaris was based on a dermatologist's diagnosis and/or description of characteristic lesions. The disease duration of the patients ranged from 3 to 26 years (mean 12.8 ± 7.0). Clinical severity was determined according to the Psoriasis Area and Severity Index (PASI) (9). The PASI assesses four body regions: the head, trunk, upper extremities, and lower extremities. For each region, the surface area involved is graded from 0 to 6, and each of the three variables (erythema, thickness, and scaling of the plaques) is graded from 0 to 4. The scores from the regions were added to determine a PASI score ranging from 0 to 72. The Psoriasis Severity Index (PSI) was also used to evaluate clinical signs (erythema, thickness, and scaling) on a scale of 0 (absent) to 3 (severe) (10). Affected body surface area (BSA) was also evaluated. The Nail Psoriasis Severity Index (NAPSI)—a simple numerical tool for evaluation of nail psoriasis—was used to quantify the degree of nail changes (11). The NAPSI was assessed separately for each finger-nail and toe-nail. This scale is used to evaluate the severity of nail-bed psoriasis and nail-matrix psoriasis. Each nail is evaluated for the presence or absence of nail-matrix disease (pitting, leukonychia, red spots in lunula, nail-plate crumbling) and nail-bed disease (oil drop/salmon patch discoloration, onycholysis, nail-bed hyperkeratosis, and splinter haemorrhage). The sum of the scores for all of the nails is the patient's NAPSI. Nail involvement was considered for patients with a NAPSI score ≥1. The disease-specific characteristics (age of onset, duration of psoriasis, mean PASI, mean PSI, mean NAPSI, and number of patients with nail involvement) of the psoriasis patients are summarized in Table I. Most of the patients had mild psoriasis and received local treatment.
Table I.
Disease-specific characteristics of patients with psoriasis vulgaris.
| Mean ± SD | |
|---|---|
| Mean age of onset of psoriasis (years ± SD) | 20.1 ± 7.7 (range: 9–35) |
| Mean duration of disease (years ± SD) | 12.8 ± 7.0 (range: 3–26) |
| Mean PASI score | 3.5 ± 3.7 (range: 0.4–16.8) |
| Mean PSI score | 1.6 ± 0.9 (range: 0–3) |
| Mean Affected BSA (%) | 6.2 ± 14.5 (range: 0–90) |
| Mean NAPSI score | 21.4 ± 25.2 (range: 0–93) |
| % of patients with nail involvement | 75.6 (31/41) |
BSA = body surface area; NAPSI = Nail Psoriasis Severity Index; PASI = Psoriasis Area and Severity Index; PSI = Psoriasis Severity Index; SD = standard deviation.
Twelve-lead electrocardiogram and P-wave duration analysis
A twelve-lead ECG of all patients was recorded in the supine position (Montara Instrument EU 250 Electrocardiograph, Milwaukee, WI, USA). ECG recordings were obtained at a paper speed of 50 mm/s and 10 mm/mV amplitude. The beginning of the P-wave was defined as the point where the first atrial deflection crossed the isoelectric line, and the end of the P-wave was defined as the point where the atrial deflection returned to the isoelectric line. The P-wave durations (Pmax, Pmin) were calculated in all 12 ECG leads. The difference between Pmax and Pmin was defined as PWD (12). Two ECG readers, who were blinded to the study, evaluated the ECGs. Initially, the measurements were performed manually with the help of callipers and a magnifying glass to define the electrocardiographic deflection.
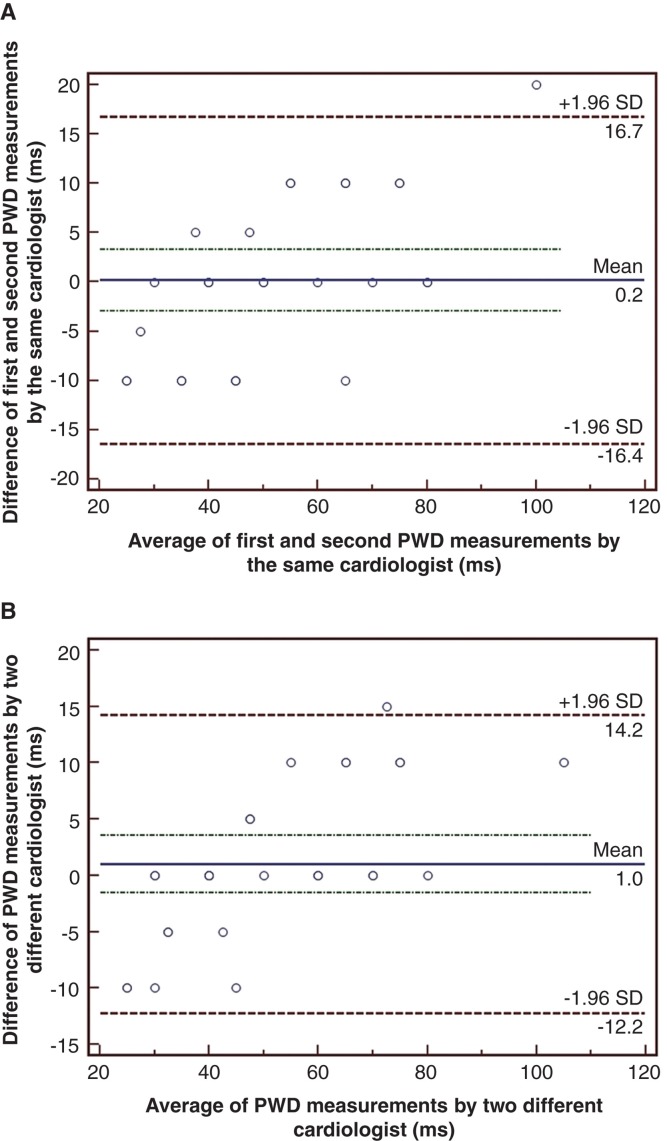
After completing the manual measurements, P-wave variables were tested to identify intra-observer variability in 30 randomly selected patients using the Bland–Altman method (13). The 95% limits of agreement for PWD and Pmax were acceptable (–12.2 and 14.2 ms; –8.6 and 8.9 ms; respectively) (Figure 1A). The same ECGs were then scanned at 300 dpi and measured by another cardiologist on a high-resolution computer screen to ascertain inter-observer variability. The 95% limits of agreement for PWD were –16.4 and 16.7 ms, and for Pmax –21 and 16 ms, respectively (Figure 1B).
Figure 1.
Bland–Altman plots demonstrating the 95% limits of agreement between (A) the repeated measurements of PWD by the same observer and (B) between the manual and digital measurements of PWD by two different observers, in 30 randomly selected patients.
Statistical analysis
Continuous variables were reported as mean ± standard deviations (SD), and categorical variables were expressed as percentages. The categorical and continuous variables between the two groups were compared using the chi-square test and unpaired t test, respectively. The correlations between P-wave variables and clinical variables were assessed using the Pearson correlation test. A binary logistic regression analysis was performed to identify the independent relationships of PWD with clinical and echocardiographic variables. A value of P < 0.05 was considered statistically significant. SPSS 19.0 for Windows statistical software package program was used for statistical analysis.
Results
The descriptive demographical characteristics of the groups are shown in Table II. There were no statistically significant differences in terms of age, gender, cigarette smoking, family history of CAD, height, weight, BMI, and waist circumference between groups. Also, the mean systolic and diastolic blood pressures and heart rate were similar. Echocardiographic variables including LVEF, LA dimension, and diastolic Doppler indexes were not statistically different between groups (Table III). Biochemical data including serum glucose, LDL-cholesterol, and triglyceride levels were not significantly different between groups, but hsCRP levels were significantly higher in patients with psoriasis compared to controls (1.3 ± 0.3 versus 0.3 ± 0.3, P = 0.001).
Table II.
Demographic characteristics of the study population. Data were presented as mean ± standard deviation.
| Group 1 (Psoriatic) (n = 61) |
Group 2 (Control) (n = 58) |
P | |
|---|---|---|---|
| Age (years) | 33.3 ± 6.7 | 35.0 ± 6.4 | NS |
| Male gender (%) | 34 (55.7%) | 35 (60.3%) | NS |
| Current smoker (%) | 42.6 (26/61) | 34.5 (20/58) | NS |
| Family history of CAD (%) | 16.4 (10/61) | 15.5 (9/58) | NS |
| BMI (kg/m2) | 25.7 ± 3.4 | 26.0 ± 2.6 | NS |
| Waist circumference (cm) | 95.4 ± 8.2 | 93.5 ± 7.9 | NS |
| Office SBP (mmHg) | 118.9 ± 10.6 | 119.1 ± 10.5 | NS |
| Office DBP (mmHg) | 73.8 ± 8.7 | 73.5 ± 10.6 | NS |
| HR (bpm) | 80.5 ± 9.9 | 83.1 ± 11.5 | NS |
| Fasting glucose (mg/dL) | 89.2 ± 10.1 | 92.3 ± 12.7 | NS |
| Serum LDL cholesterol (mg/dL) | 114.6 ± 35.3 | 117.7 ± 25.7 | NS |
| Serum triglycerides (mg/dL) | 113.7 ± 51.4 | 126.1 ± 60.1 | NS |
| HsCRP (mg/L) | 1.3 ± 0.3 | 0.3 ± 0.3 | 0.001 |
BMI = body mass index; CAD = coronary artery disease; DBP = diastolic blood pressure; HR = heart rate; HsCRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; NS = statistically non-significant; SBP = systolic blood pressure.
Table III.
Echocardiographic measurements of study population. Data were presented as mean ± standard deviation.
| Group 1 (n = 61) | Group 2 (n = 58) | P | |
|---|---|---|---|
| 2D echocardiography | |||
| LVESD (cm) | 3.6 ± 0.5 | 3.5 ± 0.7 | NS |
| LVEDD (cm) | 4.9 ± 0.4 | 5.1 ± 0.5 | NS |
| LV Ejection fraction (%) | 62.6 ± 4.4 | 63.2 ± 3.6 | NS |
| LA (cm) | 3.7 ± 0.2 | 3.8 ± 0.3 | NS |
| Doppler echocardiography | |||
| E (cm/s) | 81.7 ± 18.8 | 83.2 ± 16.1 | NS |
| A (cm/s) | 61.1 ± 14.3 | 63.4 ± 16.0 | NS |
| EDT (ms) | 162.8 ± 51.4 | 167.7 ± 48.9 | NS |
| IRT (ms) | 83.3 ± 14.5 | 84.7 ± 12.9 | NS |
A = the peak mitral valve flow velocity during atrial contraction; E = the peak mitral valve flow velocity during the early rapid filling phase; EDT= deceleration time of early phase of mitral valve flow; IRT = isovolumetric relaxation time; LA = left atrial diameter; LV = left ventricle; LVEDD = left ventricle end-diastolic diameter; LVESD = left ventricle end-systolic diameter.
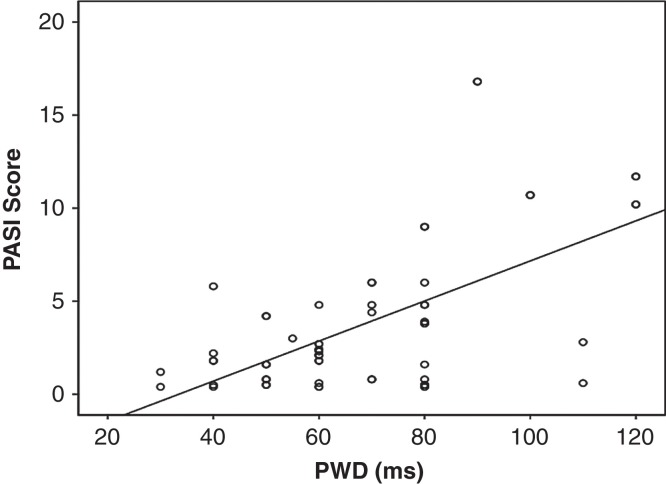
Pmax and PWD were significantly higher in group 1 than in group 2 (112.6 ± 22.7 versus 93.0 ± 12.8 ms, P < 0.001; 69.1 ± 22.6 versus 45.6 ± 19.4 ms, P < 0.001, respectively) (Table IV). Pmin was significantly lower in group 1 compared with group 2 (42.2 ± 12.5 ms versus 47.4 ± 14.3 ms, P = 0.04) (Table IV). The PASI score was the only psoriasis disease activity index that correlated with Pmax and PWD (P = 0.002 and P = 0.005, respectively) (Figure 2). In addition, there was a significant positive correlation between hsCRP and PWD (r = 0.229, P = 0.001).
Table IV.
Comparison of P-wave values of the groups. Data were presented as mean ± standard deviation.
| Group 1 (n = 61) | Group 2 (n = 58) | P | |
|---|---|---|---|
| Pmax (ms) | 112.6 ± 22.7 | 93.0 ± 12.8 | <0.001 |
| Pmin (ms) | 42.2 ± 12.5 | 47.4 ± 14.3 | 0.04 |
| PWD (ms) | 69.1 ± 22.6 | 45.6 ± 19.4 | <0.001 |
Pmax = maximum P-wave duration; Pmin = minimum P-wave duration; PWD = P-wave dispersion.
Figure 2.
Scatter plot of the PASI score against PWD (ms) (r = 0.367, P = 0.005).
Discussion
The principal new finding of this study is that the ECG markers of atrial conduction are influenced in patients with psoriasis vulgaris. Enhancement of PWD strengthens the role of chronic inflammation for the provocation of atrial conduction abnormalities and, consequently, the creation of the necessary prerequisite of the development of atrial arrhythmia in these patients. The association between PWD and both PASI score and hsCRP verified this result.
Psoriasis vulgaris is a chronic inflammatory skin disease affecting nearly 3% of the global population, including 125 million persons worldwide (14). Papulosquamous plaques symmetrically located on extensor surfaces of the joints are the most common clinical manifestations. It is not just a skin disease: it has also been shown to be a systemic inflammatory condition, similar to other inflammatory immune disorders such as rheumatoid arthritis and systemic lupus erythematosus (15). Most of the cardiovascular disorders, including atherosclerosis, hypertension, insulin resistance, and arrhythmias, share the same pathogenetic mechanisms: chronic inflammation, endothelial dysfunction, and increased oxidative stress (16). Studies have shown that psoriasis vulgaris is associated with excessive cardiovascular morbidity and mortality (4,14–18). This association is not only the result of the fact that the cardiovascular risk factors of hypertension, diabetes mellitus, obesity, smoking, and dyslipidaemia have been found to be more prevalent in patients with psoriasis vulgaris, but even after these risk factors are compensated for, psoriasis confers an independent risk (19).
Cerebrovascular disease and stroke have also been reported as being more frequent in these patients, with a prevalence ranging from 3.1% to 6.5% (4,19–22). Ischemic stroke constitutes a large portion of strokes, mainly due to thromboembolic occlusion of cranial vessels (23). AF is responsible for up to 20% of all ischemic strokes, and it increases the patient's risk of an ischemic stroke 5-fold (24). Furthermore, AF-related strokes are more disabling, recurrent, and fatal. Recently, Ahlehoff et al. demonstrated that patients with psoriasis vulgaris had an increased risk of AF (4). Although the risk was augmented in all psoriatic patients, it seemed to be highest in younger (<50 years old) patients with more severe disease (4). They concluded that the association between psoriasis and AF was independent of age, gender, co-morbidity, concomitant medication, and socio-economic status. We demonstrate in the present study that patients with psoriasis vulgaris had impaired atrial conduction of sinus impulses. Prolongation of PWD, which is an independent risk factor for the development of AF, could be the previous stage in these patients. To date, a clinical evaluation of PWD has been performed in the assessment of the risk for AF in patients without apparent heart disease, such as smokers and patients with obstructive sleep apnoea, metabolic syndrome, and prehypertension (25-28). The estimation of PWD, which is a reliable, non-invasive, and feasible variable with good reproducibility of intra- and inter-atrial heterogenicity, could be a method useful to differentiate the group of patients prone to suffer from AF.
In general, early detection and appropriate treatment of these diseases are important in terms of preventing progression to more advanced stages and irreversible damage. Accordingly, it is important to detect psoriasis patients at increased risk of AF and related disorders. In this context, we evaluated the P-wave variables in these patients and found that PWD and Pmax values were significantly higher and Pmin values were significantly lower in psoriasis vulgaris compared with normal individuals. Because these variables demonstrate the prolongation of intra- and inter-atrial conduction time and the heterogeneous propagation of sinus impulses in the left atrium, which are well-known electrophysiological characteristics in patients with paroxysmal AF, these data could be used to classify psoriasis vulgaris patients with an increased risk of AF. Although atrial remodelling induced by chronic inflammation and oxidative stress in psoriasis seems to facilitate the development of AF, other possible underlying mechanisms should be considered to explain susceptibility to AF in these patients; the LA left atrial dimensions were similar in patients with psoriasis compared to controls. Also, patients with diastolic dysfunction were excluded from the study.
Clinicians caring for patients with psoriasis vulgaris should inform their patients that they may have an increased risk of cardiovascular disease. Risk factors such as smoking, dyslipidaemia, hypertension, and obesity should be assessed rigorously in every such patient. Lifestyle modification, including diet, exercise, and smoking cessation, should be advised, and medical therapy should be prescribed in appropriate cases. An ECG as a part of suggested screening for cardiovascular risk factors should be ordered, particularly in patients on systemic treatment. It should be noted that younger patients with moderate or severe forms benefit most when preventive strategies are implemented early.
Study limitations
A major limitation of the present study is its cross-sectional design. In addition, since all patients were in sinus rhythm during the study, we did not perform a Holter examination to investigate the presence of atrial arrhythmia. In other words, the value of a prolonged PWD in predicting future arrhythmic events in patients with psoriasis has not been evaluated. Moreover, we do not have data from before and after initiation of therapy to see whether this treatment affects atrial conduction time. For these reasons, large-scale and long-term follow-up prospective studies are required to establish the predictive value of atrial conduction variables for development of AF in patients with psoriasis.
Conclusion
In conclusion, we have shown that patients with psoriasis vulgaris have higher PWD, indicating an increased risk for AF in contrast to control subjects without psoriasis. Although the exact mechanism still remains unclear, chronic inflammation rather than atrial remodelling may be responsible for increased PWD in these patients. Further long-term prospective studies are needed to clarify the clinical utility and prognostic importance of PWD in patients with psoriasis vulgaris.
Acknowledgements
The authors would like to thank Professor Dr Omer Goktekin for all his input and guidance on this manuscript. The authors contributed as follows: Bacaksiz: A, B, C, D, E, F; Erdogan: B, D, F; Vatankulu: D, E, F; remaining authors: B, C, D (A = study design; B = data collection; C = statistical analysis; D = data interpretation; E = manuscript preparation; F = literature search).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 2.Rosa DJ, Machado RF, Matias FA, Cedrim SD, Noronha FL, Gaburri D, et al. Influence of severity of the cutaneous manifestations and age on the prevalence of several cardiovascular risk factors in patients with psoriasis. J Eur Acad Dermatol Venereol. 2012;26:348–53. doi: 10.1111/j.1468-3083.2011.04076.x. [DOI] [PubMed] [Google Scholar]
- 3.Markuszeski L, Bissinger A, Janusz I, Narbutt J, Jedrzejowska AS, Zalewska A. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64–9. doi: 10.1016/j.arcmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33:2054–64. doi: 10.1093/eurheartj/ehr285. [DOI] [PubMed] [Google Scholar]
- 5.Centurion OA. Clinical implications of the P wave duration and dispersion: relationship between atrial conduction defects and abnormally prolonged and fractionated atrial endocardial electrograms. Int J Cardiol. 2009;134:6–8. doi: 10.1016/j.ijcard.2008.12.072. [DOI] [PubMed] [Google Scholar]
- 6.Dilaveris PE, Gialafos EJ, Sideris SK, Theopistou AM, Andrikopoulos GK, Kyriakidis M, et al. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733–8. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- 7.Dogdu O, Yarlioglues M, Kaya MG, Ardic I, Kilinc Y, Elcik D, et al. Assessment of atrial conduction time in patients with systemic lupus erythematosus. J Investig Med. 2011;59:281–6. doi: 10.231/JIM.0b013e318207050a. [DOI] [PubMed] [Google Scholar]
- 8.Acar G, Akcay A, Sayarlioglu M, Sokmen A, Sokmen G, Koroglu S, et al. Assessment of atrial conduction time in patients with familial Mediterranean fever. Pacing Clin Electrophysiol. 2009;32:308–13. doi: 10.1111/j.1540-8159.2008.02237.x. [DOI] [PubMed] [Google Scholar]
- 9.Louden BA, Pearce DJ, Lang W, Feldman SR. A Simplified Psoriasis Area Severity Index (SPASI) for rating psoriasis severity in clinic patients. Dermatol Online J. 2004;10:7. [PubMed] [Google Scholar]
- 10.Amornpinyokeit N, Asawanonda P. 8-Methoxypsoralen cream plus targeted narrowband ultraviolet B for psoriasis. Photodermatol Photoimmunol Photomed. 2006;22:285–9. doi: 10.1111/j.1600-0781.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 11.Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49:206–12. doi: 10.1067/s0190-9622(03)00910-1. [DOI] [PubMed] [Google Scholar]
- 12.Michelucci A, Bagliani G, Colella A, Pieragnoli P, Porciani MC, Gensini G, et al. P wave assessment: state of the art update. Card Electrophysiol Rev. 2002;6:215–20. doi: 10.1023/a:1016368723033. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 14.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–3. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 15.Rico T, Marchione R, Kirsner RS. Vascular disease in psoriasis. J Invest Dermatol. 2009;129:2327. doi: 10.1038/jid.2009.268. [DOI] [PubMed] [Google Scholar]
- 16.Flammer AJ, Ruschitzka F. Psoriasis and atherosclerosis: two plaques, one syndrome? Eur Heart J. 2012;33:1989–91. doi: 10.1093/eurheartj/ehr425. [DOI] [PubMed] [Google Scholar]
- 17.Patel RV, Shelling ML, Prodanovich S, Federman DG, Kirsner RS. Psoriasis and vascular disease-risk factors and outcomes: a systematic review of the literature. J Gen Intern Med. 2011;26:1036–49. doi: 10.1007/s11606-011-1698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daudén E, Castañeda S, Suárez C, García-Campayo J, Blasco AJ, Aguilar M. D, et al. [Integrated approach to comorbidity in patients with psoriasis] Actas Dermosifiliogr. 2012;103:1–64. doi: 10.1016/S0001-7310(12)70001-7. [DOI] [PubMed] [Google Scholar]
- 19.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 20.Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411–18. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case–control analysis. Br J Dermatol. 2009;160:1048–56. doi: 10.1111/j.1365-2133.2008.09020.x. [DOI] [PubMed] [Google Scholar]
- 22.Kimball AB, Guerin A, Latremouille-Viau D, Yu AP, Gupta S, Bao Y, et al. Coronary heart disease and stroke risk in patients with psoriasis: retrospective analysis. Am J Med. 2010;123:350–7. doi: 10.1016/j.amjmed.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Shiber JR, Fontane E, Adewale A. Stroke registry: hemorrhagic vs ischemic strokes. Am J Emerg Med. 2010;28:331–3. doi: 10.1016/j.ajem.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Go AS. The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol. 2005;14:56–61. doi: 10.1111/j.1076-7460.2005.02278.x. [DOI] [PubMed] [Google Scholar]
- 25.Akturk E, Yağmur J, Açıkgöz N, Ermiş N, Cansel M, Karakuş Y, et al. Assessment of atrial conduction time by tissue Doppler echocardiography and P-wave dispersion in smokers. J Interv Card Electrophysiol. 2012;34:247–53. doi: 10.1007/s10840-011-9658-x. [DOI] [PubMed] [Google Scholar]
- 26.Yagmur J, Yetkin O, Cansel M, Acikgoz N, Ermis N, Karakus Y, et al. Assessment of atrial electromechanical delay and influential factors in patients with obstructive sleep apnea. Sleep Breath. 2012;16:83–8. doi: 10.1007/s11325-010-0477-6. [DOI] [PubMed] [Google Scholar]
- 27.Yasar AS, Bilen E, Bilge M, Ipek G, Ipek E, Kirbas O. P-wave duration and dispersion in patients with metabolic syndrome. Pacing Clin Electrophysiol. 2009;32:1168–72. doi: 10.1111/j.1540-8159.2009.02460.x. [DOI] [PubMed] [Google Scholar]
- 28.Celik T, Yuksel UC, Bugan B, Celik M, Fici F, Iyisoy A, et al. P-wave dispersion and its relationship to aortic elasticity in young prehypertensive patients. Am J Hypertens. 2009;22:1270–5. doi: 10.1038/ajh.2009.157. [DOI] [PubMed] [Google Scholar]