Abstract
Background
The risk factors for No. 12p and No. 12b lymph node (LN) metastases in advanced gastric cancer (GC) remain controversial. The aim of this study was to investigate the risk factors for No. 12p and No. 12b LN metastases in advanced GC.
Methods
From January 1999 to December 2005, a retrospective analysis of 163 patients with advanced GC who underwent D2 lymphadenectomy in addition to No. 12p and No. 12b LN dissections was conducted. Potential clinicopathological factors that could influence No. 12p and No. 12b LN metastases were statistically analyzed.
Results
There were 15 cases (9.2%) with No. 12p LN metastases and 5 cases (3.1%) with synchronous No. 12b LN metastases. A logistic regression analysis revealed that the Borrmann type (III/IV versus I/II, P = 0.029), localization (lesser/circular versus greater, P = 0.025), and depth of invasion (pT4 versus pT2/pT3, P = 0.009) were associated with 11.1-, 3.8-, and 5.6-fold increases, respectively, for risk of No. 12p and No. 12b LN metastases. A logistic regression analysis also showed that No. 5 (P = 0.006) and No. 12a (P = 0.004) LN metastases were associated with 6.9- and 11.3-fold increases, respectively, for risk of No. 12p and No. 12b LN metastases. In addition, significant differences in 5-year survival of patients with and without No. 12p and No. 12b LN metastases were observed (13.3% versus 35.1%, P = 0.022).
Conclusion
We conclude that Borrmann type, localization, and depth of invasion are significant variables for identifying patients with No. 12p and No. 12b LN metastases. Individuals with No. 5 or No. 12a LN metastases should be on high alert for the possibility of additional metastases to the No. 12p and No. 12b LNs.
Keywords: Gastrectomy, gastric cancer, lymphadenectomy, metastasis, risk factor
Introduction
Although the incidence of gastric cancer (GC) has been declining in recent years, it remains one of the most important causes of cancer-related death in China (1–3). Curative surgery is the treatment of choice for GC, and radical lymph node (LN) dissection is an important part of curative resection (4,5). However, mortality for those diagnosed with GC remains high, because many patients are diagnosed with advanced-stage disease. At present, advanced GC accounts for 92%–95% of cases in China, 40%–60% in Japan, and 80%–90% in Europe (2,6,7).
However, the extent of LN dissections in GC remains controversial. East Asian surgeons, especially Japanese surgeons, routinely perform gastrectomy with D2 dissection. However, most Western surgeons perform D1 dissection, because D1 was associated with less mortality and morbidity than D2 in prospective randomized trials performed in the Netherlands and the UK, leading to the conclusion that there was no survival benefit for D2 over D1 dissection (8,9). However, more recent studies have demonstrated that Western surgeons at experienced centers can be trained to perform D2 dissection for selected Western patients with low morbidity and mortality (10–12). Fifteen-year follow-up results of the Dutch trial were recently reported (13). They reported that D2 dissection was associated with lower loco-regional recurrence and GC-related death rates than D1. They concluded that D2 dissection is the recommended surgical approach for patients with resectable GC. A meta-analysis of 5 randomized trials involving 1642 patients (845 D1 and 797 D2) showed that earlier trials with D2 have higher operative mortality while recent trials have similar rates, and the 5-year overall survival was similar between D1 versus D2 trials (14).
It is generally accepted that D2 lymphadenectomy is the standard procedure for patients with advanced GC in China. According to the Japanese Gastric Cancer Association N-classification, all LNs are numbered as stations (No. 1 to No. 112) and grouped by their anatomical position (15). Based on this grouping system, the LNs closest to the stomach (the perigastric LNs, No. 1 to No. 6) are defined as group 1, the more extraperigastric LNs (No. 7 to No. 11), including No. 12a (along the hepatic artery in the hepatoduodenal ligament), are defined as group 2, and all LNs more distal to the stomach than No. 12b are defined as group 3. In this classification system, no evidence of LN metastasis is defined as N0, metastasis only to LNs in group 1 is defined as N1, metastasis to group 2 but not to group 3 LNs is defined as N2, and metastasis to group 3 LNs is defined as N3. Generally, D1 dissections have involved the resection of perigastric LNs, and D2 dissections are typically more extended and include dissections of more distant LNs including No. 12a. According to the Japanese classification system, the hepatoduodenal LNs are sub-classified as No. 12a when they occur along the hepatic artery, No. 12b when they are present along the bile duct, and No. 12p when they are found behind the portal vein. However, most studies have focused on LN metastasis only in the No. 12a or in No. 12 LNs as a whole. In contrast, there have been few studies regarding metastasis to the No. 12p and No. 12b LNs (16–18).
The aim of this study was to investigate the risk factors for No. 12p and No. 12b LN metastases in advanced GC.
Patients and methods
Patients
From January 1999 to December 2005, a retrospective analysis was conducted of 163 consecutive patients with advanced GC who underwent D2 lymphadenectomy with additional dissections of No. 12p and No. 12b LNs at the Department of Oncological Surgery, Zhejiang Cancer Hospital (Hangzhou, China) and Department of General Surgery, Southeast Hospital Affiliated to Xiamen University (Xiamen, China). The inclusion criteria were as follows: 1) advanced GC; 2) adenocarcinoma confirmed by histopathology; 3) physical fitness suitable for surgery; 4) D2 lymphadenectomy with additional dissections of No. 12p and No. 12b LNs; and 5) no prior history of any type of adjunctive therapy. The exclusion criteria were as follows: 1) older than 80 years of age; 2) previous or concomitant other cancer; 3) previous or concomitant gastrectomy for benign disease; 4) previous chemotherapy or radiotherapy; 5) esophageal involvement; or 6) distant metastatic disease.
All of the above patients were followed up by posting letters or by telephone interviews. The last follow-up was 30 September 2009. The clinicopathological and follow-up findings were collected and recorded in the database. All subjects gave written informed consent to the study protocol, which was approved by the Ethical Committees of Zhejiang Cancer Hospital and Xiamen University.
Surgery
All patients in the study underwent standard total or distal gastrectomy, depending on the location and macroscopic appearance of the primary tumor (Table I). In the present study, distal gastrectomies were performed principally for tumors located in the lower third of the stomach. For tumors in the middle third, either distal or total gastrectomies were performed, depending on the direction of tumor invasion. Total gastrectomies were used for tumors in the upper third of the stomach and those occupying the entire stomach. The strategy for LN dissections was determined using a standardized technique according to the guidelines of the 2010 Japanese Classification of Gastric Cancer and Gastric Cancer Treatment Guidelines edited by the Japanese Gastric Cancer Association (19). LN dissections consisted of the removal of the perigastric nodes (stations 1–6) and extraperigastric nodes, including those along the left gastric artery (station 7), the common hepatic artery (station 8a), the celiac axis (station 9), and the splenic artery (station 11), as well as those in the splenic hilum (station 10) and the hepatoduodenal ligament (station 12, including 12a, 12p, and 12b).
Table I.
LN dissections in standard distal and total gastrectomies.
| Distal gastrectomy | Total gastrectomy | |
|---|---|---|
| D1 dissection | 1, 3, 4sb, 4d, 5, 6, 7 | 1, 2, 3, 4d, 4sa, 4sb, 5, 6, 7 |
| D2 dissection | D1 + 8a, 9, 11, 12a | D1 + 8a, 9, 10, 11, 12a |
Distal gastrectomies were performed principally for tumors located in the lower third of the stomach. For tumors in the middle third, either distal or total gastrectomies were performed, depending on the direction of tumor invasion. Total gastrectomies were used for tumors in the upper third of the stomach and those occupying the entire stomach.
Clinicopathological characteristics
The clinicopathological findings, including depth of tumor invasion and LN metastases, were used to stage tumors according to the 7th edition of the International Union Against Cancer classification system (20). LNs were dissected and described according to the Japanese Classification of Gastric Carcinoma (19), which was also used to classify the location, histological type, and lymphatic invasion of tumors. The gross appearance of each tumor was classified using Borrmann's classification (21). According to this classification, tumors can be divided into superficial tumors, well-defined tumors, and ill-defined tumors. Furthermore, they can be classified as well-defined tumors (polypoid or fungating type, Borrmann's type I) and circumscribed excavating type (Borrmann's type II). The ill-defined tumors are ulcerated and infiltrating type (Borrmann's type III) and diffusely thickened type (Borrmann's type IV).
Statistical analysis
Statistical analyses were conducted using Statistical Product for Social Sciences (SPSS) 17.0 software (SPSS, Inc., Chicago, IL, USA). The distribution of baseline characteristics between patients with and without No. 12p and No. 12b LN metastases was compared by using either Fisher's exact test or the chi-square test. Significant factors were extracted for further analysis, which was conducted by using the logistic regression method. Furthermore, LN metastasis was evaluated for any independent factor with regard to No. 12p and No. 12b LN metastases, using similar methods as above. The overall cumulative probability of survival was calculated by the Kaplan–Meier method, and differences were assessed by using the log-rank test. A P value less than 0.05 was considered to be statistically significant.
Results
Clinicopathological characteristics
Among the 163 patients, 112 (68.7%) were men and 51 (31.3%) were women. The mean age was 62.5 ± 13.7 years, with an age range from 38 to 79 years. There were 15 cases (9.2%) with No. 12p LN metastases and 5 cases (3.1%) with synchronous No. 12b LN metastases. There were no statistically significant differences between the metastastic rates of the lower stomach compared to cancer of the middle or upper third of the stomach (Table II). The clinicopathological characteristics are shown in Table III. From the variables considered to be potentially associated with No. 12p and No. 12b LN metastases, age (P = 0.017), Borrmann type (P = 0.005), localization (P = 0.024), tumor size (P = 0.007), and depth of invasion (P = 0.000) were found to differ significantly between patients with and without No. 12p and No. 12b LN metastases (Table III). There were no significant differences between these two groups in terms of gender, tumor location, or histological type. A logistic regression analysis showed that the Borrmann type (III/IV versus I/II, P = 0.029), localization (lesser/circular versus greater, P = 0.025), and depth of invasion (pT4 versus pT2/pT3, P = 0.009) were associated with 11.1-, 3.8-, and 5.6-fold increases, respectively, for risk of No. 12p and No. 12b LN metastases (Table IV).
Table II.
Frequency of No. 12 LN metastases according to the tumor location.
| No. 12a (n, %) | No. 12p (n, %) | No. 12b (n, %) | |
|---|---|---|---|
| Upper (n = 60) | 4 (6.7) | 3 (5.0) | 0 (0.0) |
| Middle (n = 53) | 9 (17.0) | 6 (11.3) | 2 (3.8) |
| Lower (n = 26) | 5 (19.2) | 4 (15.4) | 2 (7.7) |
| Total (n = 24) | 3 (12.5) | 2 (8.3) | 1 (4.2) |
The metastatic rate of the lower stomach was higher than that of cancer of the middle or upper third of the stomach; no statistically significant differences in lymphatic metastases were found between different tumor locations of the stomach.
Table III.
Characteristics in 163 patients with and without No. 12p and No. 12b LN metastases.
| Positive (n, %) | Negative (n, %) | Total (n, %) | P value | |
|---|---|---|---|---|
| Age (years) | 0.017 | |||
| ≤ 60 | 4 (26.7) | 87 (58.8) | 91 (55.8) | |
| > 60 | 11 (73.3) | 61 (41.2) | 72 (44.2) | |
| Gender | 0.445 | |||
| Male | 9 (60.0) | 103 (69.6) | 112 (68.7) | |
| Female | 6 (40.0) | 45 (30.4) | 51 (31.3) | |
| Borrmann type | 0.005 | |||
| I/II | 1 (6.7) | 65 (43.9) | 66 (40.5) | |
| III/IV | 14 (93.3) | 83 (56.1) | 97 (59.5) | |
| Tumor location | 0.358 | |||
| Upper | 3 (20.0) | 57 (38.5) | 60 (36.8) | |
| Middle | 6 (40.0) | 47 (31.7) | 53 (32.5) | |
| Lower | 4 (26.7) | 22 (14.9) | 26 (16.0) | |
| Total | 2 (13.3) | 22 (14.9) | 24 (14.7) | |
| Localization | 0.024 | |||
| Greater | 2 (13.3) | 43 (29.1) | 45 (27.6) | |
| Lesser | 7 (46.7) | 85 (57.4) | 92 (56.4) | |
| Circular | 6 (40.0) | 20 (13.5) | 26 (16.0) | |
| Tumor size (cm) | 0.007 | |||
| ≤ 4 | 1 (6.7) | 25 (16.9) | 26 (16.0) | |
| > 4,< 8 | 3 (20.0) | 75 (50.7) | 78 (47.8) | |
| ≥ 8 | 11 (73.3) | 48 (32.4) | 59 (36.2) | |
| Depth of invasion | 0.000 | |||
| pT2 | 1 (6.7) | 22 (14.9) | 23 (14.1) | |
| pT3 | 3 (20.0) | 91 (61.5) | 94 (57.7) | |
| pT4 | 11 (73.3) | 35 (23.6) | 46 (28.2) | |
| Histological type | 0.143 | |||
| Differentiated | 3 (20.0) | 58 (39.2) | 61 (37.4) | |
| Undifferentiated | 12 (80.0) | 90 (60.8) | 102 (62.6) |
Positive = patients with No. 12p and No. 12b LN metastases; Negative = patients without No. 12p and No. 12b LN metastases.
Table IV.
Logistic regression analysis of characteristics for No. 12p and No. 12b LN metastases.
| B value | SE | Wald | P value | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Age | 1.295 | 0.687 | 3.556 | 0.059 | 3.651 (0.950–14.029) |
| Borrmann type | 2.409 | 1.105 | 4.754 | 0.029 | 11.127 (1.276–97.034) |
| Localization | 1.335 | 0.597 | 4.994 | 0.025 | 3.800 (1.178–12.255) |
| Tumor size | 0.917 | 0.593 | 2.385 | 0.122 | 2.501 (0.781–8.002) |
| Depth of invasion | 1.730 | 0.662 | 6.826 | 0.009 | 5.641 (1.541–20.655) |
A logistic regression analysis was conducted for five variables (age, Borrmann type, localization, tumor size, and depth of invasion) that had been found to be significant using either Fisher's exact or chi-square tests. A logistic regression analysis showed that Borrmann type, localization, and depth of invasion were each a significant variable of No. 12p and No. 12b LN metastases (P = 0.029, P = 0.025, and P = 0.009, respectively).
LNs metastases
All patients underwent radical gastrectomies, including total gastrectomies in 117 cases (71.8%) and distal gastrectomies in 46 cases (28.2%). The metastatic rates for each LN are shown in Table V. The presence of metastasis in the No. 3 (P = 0.015), No. 5 (P = 0.000), No. 7 (P = 0.003), No. 9 (P = 0.027), and No. 12a (P = 0.000) LNs was found to differ significantly between patients with and without No. 12p and No. 12b LN metastases (Table V). A logistic regression analysis also showed that metastases to the No. 5 (P = 0.006) and No. 12a (P = 0.004) LNs were associated with 6.9- and 11.3-fold increases, respectively, for risk of No. 12p and No. 12b LN metastases (Table VI).
Table V.
Regional LNs metastases in 163 patients with No. 12p and No. 12b metastases.
| LNs metastases (n, %) | No. 12p/b metastases (n, %) | P value | |
|---|---|---|---|
| No. 1 | 56/163 (34.4) | 7/15 (46.7) | 0.292 |
| No. 2 | 33/117 (28.2) | 5/15 (33.3) | 0.869 |
| No. 3 | 93/163 (57.1) | 13/15 (86.7) | 0.015 |
| No. 4sa | 17/117 (14.5) | 3/15 (20.0) | 0.801 |
| No. 4sb | 23/163 (14.1) | 5/15 (33.3) | 0.064 |
| No. 4d | 35/163 (21.5) | 6/15 (40.0) | 0.133 |
| No. 5 | 27/163 (16.6) | 9/15 (60.0) | 0.000 |
| No. 6 | 12/163 (7.4) | 3/15 (20.0) | 0.148 |
| No. 7 | 45/163 (27.6) | 9/15 (60.0) | 0.003 |
| No. 8a | 19/163 (11.7) | 4/15 (26.7) | 0.139 |
| No. 9 | 8/163 (4.9) | 3/15 (20.0) | 0.027 |
| No. 10 | 18/117 (15.4) | 3/15 (20.0) | 0.883 |
| No. 11 | 16/163 (9.8) | 2/15 (13.3) | 0.631 |
| No. 12a | 21/163 (12.9) | 8/15 (53.3) | 0.000 |
No. 1 = right paracardial; No. 2 = left paracardial; No. 3 = lesser curvature; No. 4a = along short gastric vessels; No. 4sb = along left gastroepiploic vessels; No. 4d = along right gastroepiploic vessels; No. 5 = suprapyloric; No. 6 = infrapyloric; No. 7 = along left gastric artery; No. 8a = anterosuperior group; No. 9 = around celiac axis; No. 10 = splenic hilum; No. 11 = along splenic artery; No. 12a = along hepatic artery in the hepatoduodenal ligament.
Table VI.
Logistic regression analysis of LN metastasis for No. 12p and No. 12b LN metastases.
| B value | SE | Wald | P value | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| No. 3 | 1.442 | 0.813 | 3.141 | 0.076 | 4.227 (0.859–20.815) |
| No. 5 | 1.935 | 0.702 | 7.590 | 0.006 | 6.921 (1.748–27.409) |
| No. 7 | 0.764 | 0.682 | 1.254 | 0.263 | 2.147 (0.564–8.177) |
| No. 9 | -0.083 | 1.020 | 0.007 | 0.935 | 0.920 (0.125–6.789) |
| No. 12a | 2.428 | 0.843 | 8.304 | 0.004 | 11.340 (2.174–59.146) |
Significant LNs were extracted for further analysis, carried out using a logistic regression method. A logistic regression analysis showed that the metastasis to the No. 5 and No. 12a LNs were each a significant variable for No. 12p and 12b LN metastases (P = 0.006 and P = 0.004, respectively).
Survival
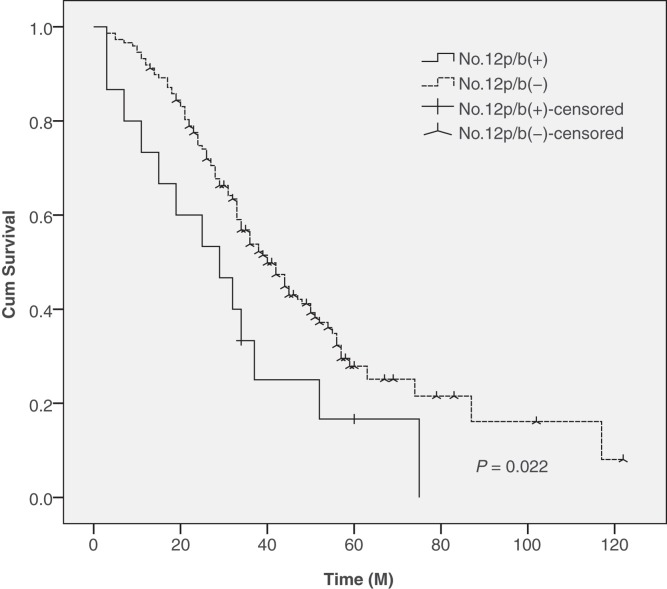
The 5-year survival rates were 13.3% in patients with No. 12p and No. 12b LN metastases and 35.1% in patients without No. 12p and No. 12b LN metastases, a difference that was found to be statistically significant between these two groups (Figure 1).
Figure 1.
Survival in 163 patients with and without No. 12p and No. 12b LN metastases. There were significant differences in the 5-year survival rate between patients with and without No. 12p and No. 12b LN metastases (13.3% versus 35.1%, P = 0.022).
Discussion
Surgical resection is a curative treatment that is available for advanced GC, and lymphadenectomy is an important part of curative resection (22,23). Theoretically, radical dissections of LNs by extended lymphadenectomy increase the possibility of curing advanced GC. However, the extent of LN dissection is also a significant risk factor for complications and death (24,25). D2 dissection is a standard procedure for patients with GC in Japan, and since the 1990s it has been increasingly employed to treat patients with GC in China (2,3,6). The D2 gastrectomy procedure is known as ‘extended' in Europe, while Japanese surgeons employ D2 as the standard technique, and use the term ‘extended' for para-aortic dissection (26,27). The mortality of D2 gastrectomy is approximately 5% in Western countries, whereas it is less than 2% in Japan, and less than 1% in specialized institutions (26,28). Here, we examined the clinicopathological characteristics of advanced GC patients with No. 12p and No. 12b LN metastases to clarify their associated risk factors.
Our study showed that metastases to the No. 12p and No. 12b LNs occurred in 9.2% (15/163) and 3.1% (5/163) of cases, respectively. Maruyama et al. (16) calculated the risk for LN metastasis in each station based on the location of the primary tumor and showed that the metastatic rate to No. 12 LNs was 4.2%. They also reported that GCs in the lower one-third of the stomach had a higher incidence of metastasis to No. 12 LNs (6.8%) when compared to cancers of the middle (2.6%) and upper thirds (2.7%) of the stomach. Bollschweiler et al. (17) showed that the metastatic rate of No. 12 LNs was 8.9%, but the rate of LN metastases for cancers in the middle third of the stomach was higher (11.0%) than those in the upper (3.3%) and lower thirds (6.7%) of the stomach. Wu et al. (18) showed that metastasis to No. 12 LNs occurs at a rate of approximately 11.6% and reported a significantly increased frequency of N3 metastases, which is largely the result of metastases to the No. 12 LNs (P = 0.0012). The results of our study were similar but did not reach statistical significance (Table II). In our study, the rate of metastasis to No. 12 LNs was higher primarily because the patients in our study were diagnosed with advanced-stage disease. The percentages of N0 stage in GC patients were 50% and 30.2% in Maruyama's (16) and Bollschweiler's (17) studies, respectively.
Our study also showed that Borrmann type (III/IV versus I/II) and depth of invasion (pT4 versus pT2/pT3) were associated with 11.1- and 5.6-fold increases, respectively, for risk of No. 12p and No. 12b LN metastases. Zhang et al. (29) showed that the factors that independently correlated with poor survival in GC patients included advanced stage, location in the upper third of the stomach, poor differentiation, and Borrmann type. Xu et al. (30) studied the risk factors of early GC patients with LN metastases, and the results showed that depth of invasion, histological type, lymphatic invasion, and tumor size were independent risk factors for LN involvement. In our opinion, Borrmann type and depth of invasion should be carefully observed during operations because they are independent predictors for No. 12p and No. 12b LN metastases. Our study also showed that localization is a significant variable. In our study, the metastatic rate of No. 12p and No. 12b LNs was higher for cancers located in the lesser curvature than in the greater curvature (46.7% versus 13.3%). It was previously suggested that the number of metastatic LNs is a prognostic factor for GC (25,31). However, recent studies, most of which have been carried out in Western populations, have demonstrated that the metastatic LN ratio is a more reliable prognostic factor (26,32). Our study showed that the No. 5 and No. 12a LNs are associated with 6.9- and 11.3-fold increases, respectively, for risk of No. 12p and No. 12b LN metastases. Based on these results, we conclude that individuals with No. 5 or No. 12a LN metastases should be on high alert for the possibility of No. 12p LN and No. 12b LN metastases.
The potential limitations of the present study include the relatively small number of patients, the use of a retrospective analysis, and the short duration of the mean follow-up duration. In addition, due to the limited number of patients with No. 12p and No. 12b LN metastases, our analysis may suffer from type I or type II error. The results of the study should therefore be regarded with caution. Further studies are needed to explore its long-term effect.
In conclusion, our study showed that Borrmann type, localization, and depth of invasion are significant variables for predicting No. 12p and No. 12b LN metastases. As a result, patients with No. 5 or No. 12a LN metastases should be examined carefully for the possibility of No. 12p and No. 12b LN metastases.
Acknowledgments
Declaration of interest: The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Yeoh KG. How do we improve outcomes for gastric cancer? J Gastroenterol Hepatol. 2007;22:970–2. doi: 10.1111/j.1440-1746.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- 2.Sun XD, Mu R, Zhou YS, Dai XD, Zhang SW, Huangfu XM, et al. Analysis of mortality rate of stomach cancer and its trend in twenty years in China. Zhonghua Zhongliu Zazhi. 2004;26:4–9. [PubMed] [Google Scholar]
- 3.Chen L, Zhang Y, Wei B, Zhao XY, Li T. Surgical treatment for patients with gastric cancer: report of 2335 cases. ZhonghuaWeichang Waike Zazhi. 2007;10:421–4. [PubMed] [Google Scholar]
- 4.Kubota H, Tabara H, Kotoh T, Kumar DD, Monden N, Watanabe R, et al. Prognostic factors and rational approach in the treatment of submucosal cancer of the stomach. J Surg Res. 1998;80:304–8. doi: 10.1006/jsre.1998.5423. [DOI] [PubMed] [Google Scholar]
- 5.Nitti D, Marchet A, Mammano E, Ambrosi A, Belluco C, Mencarelli R, et al. Extended lymphadenectomy (D2) in patients with early gastric cancer. Eur J Surg Oncol. 2005;31:875–81. doi: 10.1016/j.ejso.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000;3:9–18. doi: 10.1007/pl00011692. [DOI] [PubMed] [Google Scholar]
- 7.Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol. 2005;2:98–107. doi: 10.1038/ncponc0099. [DOI] [PubMed] [Google Scholar]
- 8.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–8. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 9.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–9. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 10.Roviello F, Marrelli D, Morgagni P, de Manzoni G, Di Leo A, Vindigni C, et al. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol. 2002;9:894–900. doi: 10.1007/BF02557527. [DOI] [PubMed] [Google Scholar]
- 11.Degiuli M, Sasako M, Ponti A, Soldati T, Danese F, Calvo F. Morbidity and mortality after D2 gastrectomy for gastric cancer: results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. J Clin Oncol. 1998;16:1490–3. doi: 10.1200/JCO.1998.16.4.1490. [DOI] [PubMed] [Google Scholar]
- 12.Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, et al. Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303–8. doi: 10.1016/j.ejso.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 14.Seevaratnam R, Bocicariu A, Cardoso R, Mahar A, Kiss A, Helyer L, et al. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer. 2012:60–9. doi: 10.1007/s10120-011-0110-9. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer Asociation Japanese classification of gastric carcinoma. 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama K, Okabayashi K, Kinoshita T. Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg. 1987;11:418–25. doi: 10.1007/BF01655804. [DOI] [PubMed] [Google Scholar]
- 17.Bollschweiler E, Boettcher K, Hoelscher AH, Sasako M, Kinoshita T, Maruyama K, et al. Preoperative assessment of lymph node metastases in patients with gastric cancer: Evaluation of the Maruyama computer program. Br J Surg. 1992;79:156–60. doi: 10.1002/bjs.1800790221. [DOI] [PubMed] [Google Scholar]
- 18.Wu CW, Hsieh MJ, Lo SS, Tsay SH, Lui WY, Peng FK. Lymph node metastasis from carcinoma of the distal one-third of the stomach. Cancer. 1994;73:3109–14. doi: 10.1002/1097-0142(19940615)73:12<3109::aid-cncr2820731234>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–23. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. American Joint Committee on Cancer. Stomach; pp. 117–26. p. [Google Scholar]
- 21.Borrmann R. Geschwulste des Magens und Duodenums. In: Henke F, Lubarsch O, editors. Handbuch der speziellen pathologischen Anatomie und Histologie. Vol. IV/I. Berlin: Springer Verlag; 1926. pp. 865–1095. p. [Google Scholar]
- 22.Shiu MH, Moore E, Sanders M, Huvos A, Freedman B, Goodbold J, et al. Influence of the extent of resection on survival after curative treatment of gastric carcinoma: a retrospective multivariate analysis. Arch Surg. 1987;122:1347–52. doi: 10.1001/archsurg.1987.01400230135024. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa H, Imamura H, Kodera Y. The role of surgery in the current treatment of gastric carcinoma. Gastric Cancer. 2002;5:13–16. doi: 10.1007/s10120-002-0207-2. [DOI] [PubMed] [Google Scholar]
- 24.Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110–12. doi: 10.1002/bjs.1800750206. [DOI] [PubMed] [Google Scholar]
- 25.Bostanci EB, Kayaalp C, Ozogul Y, Aydin C, Atalay F, Akoglu M. Comparison of complications after D2 and D3 dissection for gastric cancer. Eur J Surg Oncol. 2004;30:20–5. doi: 10.1016/j.ejso.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy—Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–73. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi T, Sawada H, Yamada Y, Fujimoto H, Emoto K, Takayama T, et al. Indication of splenectomy for gastric carcinoma involving the proximal part of the stomach. Hepatogastroenterology. 2001;48:603–5. [PubMed] [Google Scholar]
- 28.Fujii M, Sasaki J, Nakajima T. State of the art in the treatment of gastric cancer: from the 71st Japanese Gastric Cancer Congress. Gastric Cancer. 1999;2:151–7. doi: 10.1007/s101200050039. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XF, Huang CM, Lu HS, Wu XY, Wang C, Guang GX, et al. Surgical treatment and prognosis of gastric cancer in 2613 patients. World J Gastroenterol. 2004;10:3405–8. doi: 10.3748/wjg.v10.i23.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu YY, Huang BJ, Sun Z, Lu C, Liu YP. Risk factors for lymph node metastasis and evaluation of reasonable surgery for early gastric cancer. World J Gastroenterol. 2007;13:5133–8. doi: 10.3748/wjg.v13.i38.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichikura T, Tomimatsu S, Okusa Y, Uefuji K, Tamakuma S. Comparison of the prognostic significance between the number of metastatic lymph nodes and nodal stage based on their location in patients with gastric cancer. J Clin Oncol. 1993;11:1894–900. doi: 10.1200/JCO.1993.11.10.1894. [DOI] [PubMed] [Google Scholar]
- 32.Hyung WJ, Noh SH, Yoo CH, Huh JH, Shin DW, Lah KH, et al. Prognostic significance of metastatic lymph node ratio in T3 gastric cancer. World J Surg. 2002;26:323–9. doi: 10.1007/s00268-001-0227-9. [DOI] [PubMed] [Google Scholar]