Abstract
High-density lipoprotein (HDL) plays a major role in vasodilation and in the reduction of low-density lipoprotein (LDL) oxidation, inflammation, apoptosis, thrombosis, and infection; however, HDL is now less functional in these roles under certain conditions. This paper focuses on HDL, its anti-inflammation behavior, and the mechanisms by which HDL interacts with components of the innate and adaptive immune systems. Genome-wide association studies (GWAS) and proteomic studies have elucidated important molecules involved in the interaction between HDL and the immune system. An understanding of these mechanisms is expected to be useful for the prevention and treatment of chronic inflammation due to metabolic syndrome, atherosclerosis, or various autoimmune diseases.
1. Introduction
High-density lipoprotein (HDL) contains free or esterified cholesterol, phospholipids, triglycerides, and various proteins, including apolipoproteins, enzymes, and transfer proteins. The most abundant HDL apolipoproteins are apoA-I and apoA-II; less abundant are apoC, apoE, apoD, and apoJ. HDL enzymes include lecithin:cholesterol acyltransferase (LCAT), serum paraoxonase-1 (PON1) [1–3], and platelet-activating factor acetylhydrolase (PAF-AH) [4]. Transfer proteins include cholesteryl ester transfer protein (CETP) and phospholipid transfer protein (PLTP). Furthermore, chromatography and mass spectrometry have revealed many other proteins in HDL [5, 6]. HDL particles can be subclassified into small discoidal HDL (pre-β 1 HDL and pre-β 2 HDL), intermediate spherical HDL3 (HDL3c, HDL3b, and HDL3a), and large, cholesterol-rich spherical HDL2 (HDL2a and HDL2b) [7–10] (Figure 1). Large HDL2 particles interact with liver scavenger receptors class B type 1 (SR-B1), which ensures the delivery of cholesterol to the liver [11]. Intermediate HDL3 induces cholesterol efflux through the ATP-binding cassette transporter G1 (ABCG1) [12]. Small HDL particles promote cholesterol efflux through the ATP-binding cassette transporter A1 (ABCA1) [13]. Accumulating evidence suggests that in addition to reverse transport of cholesterol from the periphery to the liver, HDL plays a major role in vasodilation and in the reduction of LDL oxidation [14], inflammation, apoptosis, thrombosis, and infection [15]. During infection, both innate and adaptive immunities are involved in the inflammatory process and the immune response. Innate immunity is a nonspecific defense mechanism comprising cellular and humoral responses. The cellular response includes antigen-presenting cells such as macrophage and dendritic cells. The humoral response includes various effectors, such as the complement cascade or soluble pattern recognition receptors (PRRs). Adaptive immunity is an antigen-specific defense mechanism against foreign antigens or pathogens. The principal effectors of adaptive immunity are B lymphocytes (humoral response) and T lymphocytes (cellular response). This paper focuses on the role of HDL in the immune system [16, 17] and in the pathogenesis of atherosclerosis and other types of immune-mediated disease.
Figure 1.
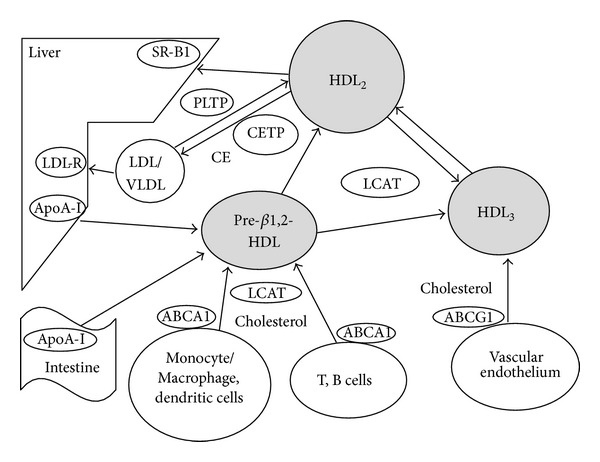
Dynamics of HDL particles and immune cells. SR-BI: scavenger receptor type B1, LDL-R: LDL receptor, CETP: cholesterol ester transfer protein, PLTP: phospholipid transfer protein, LCAT: lecithin:cholesterol acyltransferase, ABCA1: ATP binding cassette transporter A1, ABCG1: ATP binding cassette transporter G1.
2. HDL and Innate Immunity
Innate immunity is an ancient defense mechanism that humans inherited from invertebrates and that they use against a variety of pathogens. The main cells involved in innate immunity are monocyte-derived macrophage and dendritic precursor cells. Additional cells include natural killer cells, neutrophiles, eosinophiles, mast cells, basophiles, and epithelial cells. These cells use PRRs to recognize pathogen-associated molecular patterns (PAMPs). These PRRs include c-type lectins, leucin-rich proteins, macrophage scavenger receptors, pentraxins, lipid transferase, integrins, and inflammasome proteins [18, 19]. PAMP recognition leads to activation and production of the complement cascade, cytokines, and antimicrobial peptides [20]. In addition, PAMPs stimulate the differentiation of dendritic precursors into antigen-presenting, mature dendritic cells and trigger the adaptive immune system [20].
2.1. Acute Phase
During the acute phase of inflammation, mediators such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) induce serum amyloid A (SAA) and group IIA secretary phospholipase A (sPLA2-IIA), which markedly change the composition of HDL apolipoproteins and lipids [21, 22]. ApoA-1 gene expression and plasma half-life decrease [23, 24]. SAA rapidly becomes the most abundant protein in association with HDL [25]. PON1 enzyme activity decreases and, thereby, the antioxidant properties of HDL are reduced [26]. PAF-AH is increased, thus leading to increased levels of proatherogenic lipids [27, 28]. The altered composition of HDL lipids includes decreased levels of cholesteryl ester and phospholipids and increased levels of triglycerides, free cholesterol, ceramides, and glucosylceramides [29].
Acute phase HDL is associated with disease activity; a decreased number of small HDL particles is inversely associated with the disease activity score and C-reactive protein (CRP) level [30].
2.2. Protection from Sepsis
Lipopolysaccharide (LPS) is the primary cause of sepsis induced by gram-negative bacteria. LPS, LPS-binding protein, CD14, and the toll-like receptor 4 (TLR4) complex induce macrophage activation [31]. HDL, particularly apoA-I, decreases macrophage activation by binding and neutralizing LPS [32]. The HDL receptor in the liver, SR-B1 also provides important protection against sepsis [33, 34]. SR-B1 deficiency results in a reduced rate of survival following sepsis [33]. SR-B1 also modulates TLR4 signaling in macrophages and helps facilitate LPS removal from circulation [33, 34]. HDL2 modulates SR-B1 function by reverse transporting core cholesteryl ester to the liver via SR-B1, enabling the production of pre-β HDL which effectively removes cholesterol from macrophages, dendritic cells, and lymphocytes.
In clinical sepsis, a positive correlation is evident between PLTP activity and acute-phase markers such as CRP and LPS-binding proteins. During human experimental endotoxemia, PLTP activity decreases at the time of LPS infusion and transiently increases during reconstituted HDL infusion. PLTP can accelerate the disturbance of lipoprotein homeostasis, thereby playing a role in the attenuation of the acute-phase response [35].
2.3. Cellular Innate Response
Macrophages and dendritic cells are antigen-presenting cells that are crucial to innate immunity. The cell surfaces of macrophage and dendritic cells express costimulatory molecules, which are required for stimulation of the adaptive cellular immune system, and lipid rafts, which are microdomains that contain high concentrations of cholesterol, sphingolipids, and proteins integral to signaling, protein transport, and adhesion [36, 37]. The shifting composition of lipid rafts particularly decreases in cholesterol, downregulates some cellular functions [38], including the activation, adhesion, spread, and migration of neutrophiles. HDL, or particularly apoA-I, is involved in interaction with ABCA1 or ABCG1 and removes cholesterol from the lipid rafts in macrophages and dendritic cells [39, 40] (Figure 2). Thus, HDL negatively regulates T-cell activation and the expression of inflammatory mediators in macrophages and dendritic cells. In macrophages, T-cell inactivation is caused by decreased macrophage expression of major histocompatibility complex class II (MHC II), which is a lipid raft component critical to antigen presentation [41–43]. ApoA-I of HDL inhibits the differentiation of monocytes to dendritic cells by increasing monocyte secretion of prostaglandin E2 (PGE2) and IL-10 [44]. It also inhibits T-lymphocyte activation by decreasing antigen presentation in differentiated dendritic cells [45].
Figure 2.
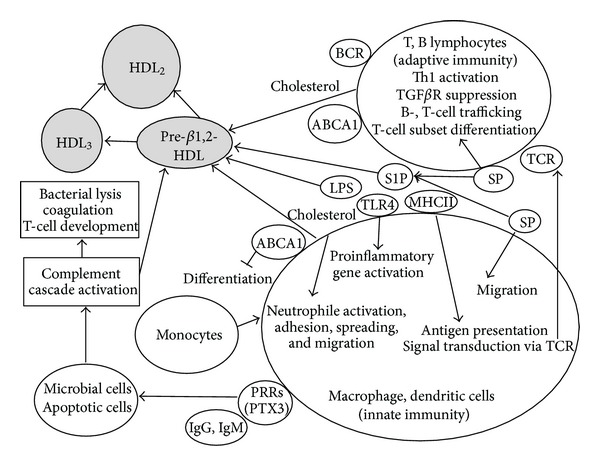
HDL and innate as well as adaptive immune cell functions. LPS: lipopolysaccharide, TLR4: Toll-like receptor 4, MHC II: major histocompatibility complex class II, SP: sphingolipid, S1P: sphingosine-1-phosphate, PRRs: pattern recognition receptors, PTX3: pentraxin 3, TCR: T-cell receptor, BCR; B-cell receptor.
Receptors from the TLR family are expressed on the surface of macrophages and dendritic cells. TLRs are involved in the innate immune response to infections. In rodent and human atherosclerotic lesions, TLRs, particularly TLR1, TLR2, and TLR4, play a role in T-lymphocyte activation by recruiting and activating leucocytes, regulating foam cell formation, and controlling antigen presentation [46, 47]. Some phospholipids in HDL function directly in immunoregulation by modulating dendritic cells for their ability to activate T helper type 1 (Th1) cells [48]. A well-characterized TLR ligand, LPS, upregulates a large number of proinflammatory genes in macrophages. Through TLR4 interaction, HDL inhibits LPS-induced antiviral response in macrophages [49, 50]. Lipid raft integrity is crucial to LPS-induced monocyte activation. ApoA-I and its mimetic peptide deplete cholesterol from lipid rafts of monocytes and thereby reduce TLR4 expression [51].
The other major class of lipid rafts is sphingolipids, which are metabolized to ceramide and subsequently to sphingosine, a metabolite that becomes phosphorylated by sphingosine kinase (SPHK) to generate sphingosine-1-phosphate (S1P) [52]. The S1P receptor 2 (SIP2) inhibits macrophage migration. Free or albumin-bound S1P rapidly degrades in most tissues, but HDL-bound S1P is less susceptible to degradation [53]. The mechanism by which HDL removes S1P from lipid rafts remains unclear but may involve specific molecules such as ABCA1. HDL-bound S1P is enriched with small, dense HDL3 and positively correlates with serum levels of HDL cholesterol, apoA-I, and apoA-II [52]. The central role of S1P and SPHK in the pathogenesis of several inflammatory disorders, including rheumatoid arthritis (RA), asthma, and atherosclerosis, is well known [54]; however, additional studies are required to clarify the role of HDL-bound S1P.
2.4. Humoral Innate Response
Innate immunity consists of a highly regulated immune surveillance system comprising several humoral factors, including soluble PRRs, such as collectins, ficolins, and pentraxins [32, 33], and the complement cascade [34]. PRRs, IgG, and IgM clusters recognize microbial or apoptotic cells and activate the complement cascade, which leads to the assembly of a terminal complement complex, bacterial lysis, and activation of several nonlethal signals that promote opsonization, chemotaxis, and TLR signaling [34] (Figure 2). The complement cascade coordinates innate defenses and potentiates coagulation to provide a mechanical barrier against bacterial spread. Activation of the complement also modulates antigen-presenting cells, macrophages, and dendritic cells, resulting in the regulation of T-lymphocyte development. Recent proteomic analyses in healthy subjects [5, 6] revealed several types of HDL particles, including complement components C4a, C4b, C9, and vitronectin. In contrast, HDL particles detected in patients with coronary artery disease include complement C3 [5]. In vitro experiments on endothelial cells have shown that HDL inhibits the formation of the terminal attack complex of the complement [55, 56]. Another study has shown that plasma HDL levels inversely correlate with terminal complex C5b–C9 levels [57]. This evidence suggests that HDL binds complements and enhances complement clearance.
A member of the pentraxin subfamily, PTX3, is soluble PRR. PTX3 deficiency leads to invasive pulmonary aspergillosis due to the defective recognition of conidia by alveolar macrophages and dendritic cells. PTX3 deficiency also causes inappropriate induction of an adaptive type 2 response [58] and some types of cardiovascular disease, including atherosclerosis [59, 60]. HDL induces mRNA expression and protein release of PTX3. This HDL effect is dependent on lysosphingolipid receptors, the PI3K/Akt axis, and is mimicked by S1P [61]. PTX mRNA increase in the aorta of transgenic mice that overexpress human apoA-I, whereas PTX mRNA decreasess in the aorta of apoAI knockout mice. HDL injection results in increase in plasma PTX3 levels in C57BL/6 mice [61]. Thus, the anti-inflammatory mechanism of HDL likely involves PTX3 activation.
3. HDL and Adaptive Immunity
The adaptive immune system is found only in vertebrates and is characterized by antigen-specific responses to pathogens. The principle components of adaptive humoral immunity are B lymphocytes that originate in the bone marrow. The principal components of cellular immunity are T lymphocytes that originate from hematopoietic cells and mature in the thymus. Gene rearrangement generates the antigen-specific receptors expressed in lipid rafts on the surface of T or B cells. Therefore, T and B lymphocytes incorporate specificity and immune memory in vertebrate host defenses.
The key receptor in B cells is the B-cell receptor (BCR), and the key receptor of T cells is the T-cell receptor (TCR). BCR and TCR are located in lipid rafts. Removal of cholesterol from BCR lipid rafts by HDL affects several modes of B-cell activation, including BCR-initiated signal transduction, endocytosis of BCR-antigen complexes, loading of antigenic peptides onto MHC-II, MHC-II-associated antigen presentation to T cells, and detection of helper signals via the CD40 receptor [62]. The HDL-induced cholesterol efflux from macrophages also affects antigen presentation to T cells as well as TCR signaling [63–65] (Figure 2).
S1P regulates B- and T-cell trafficking as well as differentiation of T cell subsets. S1P inhibits forkhead box P3 (FoxP3)+ in regulatory T cells (Tregs) but stimulates the development of Th1 cells [65]. S1P controls the dichotomy between these two T-cell lineages by antagonizing transforming growth factor β (TGF-β) [66]. ApoA-I suppresses inflammation by stimulating Tregs in the lymph nodes and by inhibiting effectors such as memory T cells [67].
4. HDL and Immune-Mediated Disease
4.1. Autoimmune Disease
Plasma HDL cholesterol (HDL-C) levels are elevated in multiple sclerosis and reduced in autoimmune diseases such as systemic lupus erythematosus (SLE), RA [68], Sjögren's syndrome [69], ankylosing spondylitis [70], psoriatic arthritis, and inflammatory bowel disease [71]. Proinflammatory HDL is detected in 45% of SLE patients and 20% of RA patients [72]. Relative to HDL, proinflammatory HDL is less capable of reverse cholesterol transport, antioxidation and other anti-inflammatory roles because it contains lower levels of apoA-1 and higher levels of monocyte chemoattractant protein-1 (MCP-1) and cellular adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [73]. Thus, it is important to quantify HDL-C and measure HDL quality. The representative methods used to measure HDL quality are the monocyte chemotaxis assay or the cell-free assay developed by Navab et al. [74, 75]. In addition to SLE and RA, factors that promote proinflammatory HDL include coronary atherosclerosis, diabetes mellitus, hemodialysis, a high saturated fat diet, infection, and surgery [73].
In SLE and RA, B and T cells are the main components of pathogenesis. In SLE, antibodies against apoA-I or HDL are associated with persistent disease activity [76]. In RA patients, the oxidative LDL antibody correlates positively with CRP and negatively with plasma HDL [77].
4.2. Metabolic Disease and Atherosclerosis
Genome-wide association studies (GWAS) using high throughput techniques have uncovered significant genetic variation in association with plasma HDL-C levels [78–81]. Among these genetic variations, CETP, lipoprotein lipase (LPL), ABCA1, hepatic lipase (LIPC), and endothelial lipase (LIPG) exhibit highly significant associations with plasma HDL-C levels. Newly identified loci, including GALNT2, are associated with plasma HDL-C levels.
A reduced plasma HDL-C level is a factor of metabolic syndrome, which causes obesity and chronic inflammation [82, 83]. We have previously shown that two single-nucleotide polymorphisms (SNPs) of the promoter region of the neuropeptide Y (NPY) receptor Y2 gene are associated with altered levels of plasma HDL-C [84]. Kuo et al. reported that stress plus a diet high in fat and sugar cause increased NPY secretion from the sympathetic nerve terminal and thereby trigger metabolic syndrome through the NPY receptor Y2 [85]. It is interesting to speculate whether the SNPs of the NPY receptor Y2 affect HDL-C expression levels directly or plasma HDL-C expression indirectly through certain molecules such as CETP, LPL, LIPC, and apoA-1 in the liver; ABCA1 in monocyte/macrophages and dendritic cells; or LIPG, ABCG1, and LPL in the endothelium. Furthermore, in obese subjects, a number of metabolic and immune genes that exhibited expression in subcutaneous adipose tissues correlating with plasma HDL-C levels were identified [86]. Reduced levels of plasma HDL-C are one of the risk factors for atherosclerosis-induced cardiovascular events. Treg cells play an important role in adaptive immunity and become elevated in acute myocardial infarction. A study demonstrated significant inverse correlations between levels of Treg cells and plasma HDL-C [87]. HDL3 induces in vitro and in vivo anti-inflammatory signals such as TGF-β 2 expression in endothelial cells or various signals in transgenic mice overexpressing human apoA-1 and apoA-1 knockout mice [88].
PLTP is a protein involved in HDL remodeling. Vergeer et al. reported that 2 PLTP SNPs are associated with lower PLTP transcription and activity, an increased number of HDL particles, smaller HDL size, and decreased risk of cardiovascular disease [89].
Carriers with a functional mutation in SR-B1 yield higher plasma levels of HDL-C and reduced efflux of cholesterol from macrophages, but no significant increases in atherosclerosis [90]. Reduced SR-B1 function associates with altered platelet function and decreased adrenal steroidogenesis [89]. Low levels of plasma HDL as a result of heterozygosity for loss-of-function mutations in ABCA1 do not associate with an increased prevalence of ischemic heart disease [91].
Tangier disease is a genetic disorder that results from ABCA1 deficiency and results in extremely low levels of HDL-C and premature atherosclerosis. The immunological features of this disease are not well defined. The results of an in vitro study on Tangier fibroblasts indicate that ABCA1 forms a complex with syntaxin 13 and flotillin-1, which resides the plasma membrane and phagosomes partially located in raft microdomains [92].
In contrast, CETP deficiency is a genetic disorder that results in extremely high levels of HDL-C. However, the long life span of these patients is not still evident. In accordance with this evidence, recent randomized prospective study resulted that CETP inhibitor dalcetrapib increased HDL-C levels but did not reduce the risk of recurrent cardiovascular events in patient who had had a recent acute coronary syndrome [93].
5. Conclusions
Accumulating evidence suggests that HDL or a specific apolipoprotein associated with HDL, such as apoA-I, is involved in the innate and adaptive immune responses primarily through the modulation of lipid raft components in monocytes/macrophages, dendritic cells, and T and B lymphocytes. Plasma HDL-C is usually reduced in chronic inflammation. These findings suggest that HDL protect against inflammation. However, chronic inflammation modifies HDL from a molecule with anti-inflammatory properties to one with proinflammatory properties, which leads to complex interpretation of plasma HDL-C levels. Although recent genetic and proteomic studies have unveiled important molecular players in HDL metabolism and immune activity, the mechanism for HDL regulation by these molecules remains unclear. Additional studies are required to answer several questions about HDL-C and inflammatory disease with regard to reduced plasma HDL-C levels as potential pathogenic cause of inflammatory diseases; HDL-C consumption and its consequences versus benefits for protection against these diseases; and altered HDL function in these diseases.
Acknowledgment
This study was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
- 1.Dragnov DL, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn-Schmiedeberg's Archives of Pharmacology. 2004;369(1):78–88. doi: 10.1007/s00210-003-0833-1. [DOI] [PubMed] [Google Scholar]
- 2.Luis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih DM, Gu L, Xia YR, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394(6690):284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 4.Navab M, Ananthramaiah GM, Reddy ST, et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. Journal of Lipid Research. 2004;45(6):993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the anti-inflammatory properties of HDL. The Journal of Clinical Investigation. 2007;117(3):746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon SM, Jingyuan D, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. Journal of Proteome Research. 2010;9(10):5239–5249. doi: 10.1021/pr100520x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Eckardstein A, Huang Y, Assmann G. Physiological role and clinical relevance of high-density lipoprotein subclasses. Current Opinion in Lipidology. 1994;5(6):404–416. doi: 10.1097/00041433-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Mowri HO, Patsch W, Smith LC, Gotto AM, Patsch JR. Different reactivities of high density lipoprotein2 subfractions with hepatic lipase. Journal of Lipid Research. 1992;33(9):1269–1279. [PubMed] [Google Scholar]
- 9.Miida T, Kawano M, Fielding CJ, Fielding PE. Regulation of the concentration of preβ high-density lipoprotein in normal plasma by cell membranes and lecithin-cholesterol acyltransferase activity. Biochemistry. 1992;31(45):11112–11117. doi: 10.1021/bi00160a022. [DOI] [PubMed] [Google Scholar]
- 10.Gu F, Jones MK, Chen J, et al. Structures of discoidal high density lipoproteins: a combined computational-experimental approach. The Journal of Biological Chemistry. 2010;285(7):4652–4665. doi: 10.1074/jbc.M109.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connelly MA, Williams DL. Scavenger receptor BI: a scavenger receptor with a mission to transport high density lipoprotein lipids. Current Opinion in Lipidology. 2004;15(3):287–295. doi: 10.1097/00041433-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy MA, Barrera GC, Nakamura K, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metabolism. 2005;1(2):121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Rosenson RS, Brewer HB, Jr., Chapman MJ, et al. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardio-vascular events. Clinical Chemistry. 2011;57(3):392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 14.Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(10):1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 15.Murphy AJ, Chin-Dusting JPF, Sviridov D, Woollard KJ. The anti inflammatory effects of high density lipoproteins. Current Medicinal Chemistry. 2009;16(6):667–675. doi: 10.2174/092986709787458425. [DOI] [PubMed] [Google Scholar]
- 16.Norata GD, Pirillo A, Catapano AL. HDLs, immunity, and atherosclerosis. Current Opinion in Lipidology. 2011;22(5):410–416. doi: 10.1097/MOL.0b013e32834adac3. [DOI] [PubMed] [Google Scholar]
- 17.Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220(1):11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 18.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annual Review of Immunology. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 19.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annual Review of Immunology. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 20.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature Immunology. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabana VG, Siegel JN, Sabesin SM. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. Journal of Lipid Research. 1989;30(1):39–49. [PubMed] [Google Scholar]
- 22.Menschikowski M, Hagelgans A, Siegert G. Secretory phospholipase A2 of group IIA: is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins and Other Lipid Mediators. 2006;79(1-2):1–33. doi: 10.1016/j.prostaglandins.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Navarro MA, Carpintero R, Acín S, et al. Immune-regulation of the apolipoprotein A-I/C-III/A-IV gene cluster in experimental inflammation. Cytokine. 2005;31(1):52–63. doi: 10.1016/j.cyto.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Tape C, Kisilevsky R. Apolipoprotein A-I and apolipoprotein SAA half-lives during acute inflammation and amyloidogenesis. Biochimica et Biophysica Acta. 1990;1043(3):295–300. doi: 10.1016/0005-2760(90)90030-2. [DOI] [PubMed] [Google Scholar]
- 25.Coetzee GA, Strachan AF, Van Der Westhuyzen DR. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. The Journal of Biological Chemistry. 1986;261(21):9644–9651. [PubMed] [Google Scholar]
- 26.Feingold KR, Memon RA, Moser AH, Grunfeld C. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis. 1998;139(2):307–315. doi: 10.1016/s0021-9150(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Stafforini DM, Zimmerman GA, McIntyre TM, Prescott SM. Expression of plasma platelet-activating factor acetylhydrolase is transcriptionally regulated by mediators of inflammation. The Journal of Biological Chemistry. 1998;273(7):4012–4020. doi: 10.1074/jbc.273.7.4012. [DOI] [PubMed] [Google Scholar]
- 28.Memon RA, Fuller J, Moser AH, Feingold KR, Grunfeld C. In vivo regulation of plasma platelet-activating factor acetylhydrolase during the acute phase response. American Journal of Physiology. 1999;277(1, part 2):R94–R103. doi: 10.1152/ajpregu.1999.277.1.R94. [DOI] [PubMed] [Google Scholar]
- 29.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. Journal of Lipid Research. 2004;45(7):1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Chung CP, Oeser A, Raggi P, et al. Lipoprotein subclasses determined by nuclear magnetic resonance spectroscopy and coronary atherosclerosis in patients with rheumatoid arthritis. The Journal of Rheumatology. 2010;37(8):1633–1638. doi: 10.3899/jrheum.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nature Reviews Immunology. 2008;8(10):776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. The Journal of Experimental Medicine. 1994;180(3):1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai L, Ji A, De Beer FC, Tannock LR, Van Der Westhuyzen DR. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. The Journal of Clinical Investigation. 2008;118(1):364–375. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L, Song Z, Li M, et al. Scavenger receptor BI protects against septic death through its role in modulating inflammatory response. The Journal of Biological Chemistry. 2009;284(30):19826–19834. doi: 10.1074/jbc.M109.020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levels JHM, Pajkrt D, Schultz M, et al. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochimica et Biophysica Acta. 2007;1771(12):1429–1438. doi: 10.1016/j.bbalip.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Brown DA, London E. Functions of lipid rafts in biological membranes. Annual Review of Cell and Developmental Biology. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh TJ, Vidal A, Simon SA. Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts. Biophysical Journal. 2003;85(3):1656–1666. doi: 10.1016/S0006-3495(03)74595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy AJ, Woollard KJ, Suhartoyo A, et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(6):1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- 39.Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. The Journal of Biological Chemistry. 2006;281(47):36091–36101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- 40.Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(11):2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 41.Hiltbold EM, Poloso NJ, Roche PA. MHC class II-peptide complexes and APC lipid rafts accumulate at the immunological synapse. Journal of Immunology. 2003;170(3):1329–1338. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 42.Poloso NJ, Roche PA. Association of MHC class II-peptide complexes with plasma membrane lipid microdomains. Current Opinion in Immunology. 2004;16(1):103–107. doi: 10.1016/j.coi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Setterblad N, Roucard C, Bocaccio C, Abastado JP, Charron D, Mooney N. Composition of MHC class II-enriched lipid microdomains is modified during maturation of primary dendritic cells. Journal of Leukocyte Biology. 2003;74(1):40–48. doi: 10.1189/jlb.0103045. [DOI] [PubMed] [Google Scholar]
- 44.Kim KD, Lim HY, Lee HG, et al. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochemical and Biophysical Research Communications. 2005;338(2):1126–1136. doi: 10.1016/j.bbrc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 45.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annual Review of Immunology. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 46.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105(10):1158–1161. [PubMed] [Google Scholar]
- 47.Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104(25):3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 48.Perrin-Cocon L, Diaz O, Carreras M, et al. High-density lipoprotein phospholipids interfere with dendritic cell Th1 functional maturation. Immunobiology. 2012;217(1):91–99. doi: 10.1016/j.imbio.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nature Reviews Immunology. 2008;8(7):559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki M, Pritchard DK, Becker L, et al. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 2010;122(19):1919–1927. doi: 10.1161/CIRCULATIONAHA.110.961193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smythies LE, Roger White C, Maheshwari A, et al. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. American Journal of Physiology. 2010;298(6):C1538–C1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scanu AM, Edelstein C. HDL: bridging past and present with a look at the future. The FASEB Journal. 2008;22(12):4044–4054. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yatomi Y. Plasma sphingosine 1-phosphate metabolism and analysis. Biochimica et Biophysica Acta. 2008;1780(3):606–611. doi: 10.1016/j.bbagen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Weigert A, Weis N, Brüne B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology. 2009;214(9-10):748–760. doi: 10.1016/j.imbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Rosenfeld SI, Packman CH, Leddy JP. Inhibition of the lytic action of cell-bound terminal complement components by human high density lipoproteins and apoproteins. The Journal of Clinical Investigation. 1983;71(4):795–808. doi: 10.1172/JCI110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamilton KK, Zhao J, Sims PJ. Interaction between apolipoproteins A-I and A-II and the membrane attack complex of complement. Affinity of the apoproteins for polymeric C9. The Journal of Biological Chemistry. 1993;268(5):3632–3638. [PubMed] [Google Scholar]
- 57.Pasqui AL, Puccetti L, Bova G, et al. Relationship between serum complement and different lipid disorders. Clinical and Experimental Medicine. 2002;2(1):33–38. doi: 10.1007/s102380200004. [DOI] [PubMed] [Google Scholar]
- 58.Garianda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420(6912):182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 59.Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends in Cardiovascular Medicine. 2010;20(2):35–40. doi: 10.1016/j.tcm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Norata GD, Marchesi P, Pulakazhi Venu VK, et al. Deficiency of the long pentraxin ptx3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120(8):699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 61.Norata GD, Marchesi P, Pirillo A, et al. Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(5):925–931. doi: 10.1161/ATVBAHA.107.160606. [DOI] [PubMed] [Google Scholar]
- 62.Gupta N, DeFranco AL. Lipid rafts and B cell signaling. Seminars in Cell and Developmental Biology. 2007;18(5):616–626. doi: 10.1016/j.semcdb.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruaz L, Delucinge-Vivier C, Descombes P, Dayer JM, Burger D. Blockade of T cell contact-activation of human monocytes by high-density lipoproteins reveals a new pattern of cytokine and inflammatory genes. PLoS ONE. 2010;5(2) doi: 10.1371/journal.pone.0009418.e9418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norata GD, Catapano AL. HDL and adaptive immunity: a tale of lipid rafts. Atherosclerosis. 2012;225(1):34–35. doi: 10.1016/j.atherosclerosis.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Wang SH, Yuan SG, Peng DQ, Zhao S-P. HDL and apoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis. 2012;225(1):105–114. doi: 10.1016/j.atherosclerosis.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 66.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P 1-mTOR axis directs the reciprocal differentiation of T (H) 1 and T (reg) cells. Nature Immunology. 2010;11(11):1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilhelm AJ, Zabalawi M, Owen JS, et al. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr -/-, ApoA-I-/- mice. The Journal of Biological Chemistry. 2010;285(46):36158–36169. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borba EF, Carvalho JF, Bonfá E. Mechanisms of dyslipoproteinemias in systemic lupus erythematosus. Clinical and Developmental Immunology. 2006;13(2–4):203–208. doi: 10.1080/17402520600876945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cruz W, Fialho S, Morato E, et al. Is there a link between inflammation and abnormal lipoprotein profile in Sjögren’s syndrome? Joint Bone Spine. 2010;77(3):229–231. doi: 10.1016/j.jbspin.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Mathieu S, Gossec L, Dougados M, Soubrier M. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care & Research. 2011;63(4):557–563. doi: 10.1002/acr.20364. [DOI] [PubMed] [Google Scholar]
- 71.Van Leuven SI, Hezemans R, Levels JH, et al. Enhanced atherogenesis and altered high density lipoprotein in patients with Crohn’s disease. Journal of Lipid Research. 2007;48(12):2640–2646. doi: 10.1194/jlr.M700176-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Hahn BH, Grossman J, Ansell BJ, Skaggs BJ, McMahon M. Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Research and Therapy. 2008;10(4, article 213) doi: 10.1186/ar2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ansell BJ. The two faces of the ’good’ cholesterol. Cleveland Clinic Journal of Medicine. 2007;74(10):697–705. doi: 10.3949/ccjm.74.10.697. [DOI] [PubMed] [Google Scholar]
- 74.Navab M, Hama SY, Anantharamaiah GM, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. Journal of Lipid Research. 2000;41(9):1495–1508. [PubMed] [Google Scholar]
- 75.Navab M, Hama SY, Hough GP, Subbanagounder G, Reddy ST, Fogelman AM. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of inactivating oxidized phospholipids. Journal of Lipid Research. 2001;42(8):1308–1317. [PubMed] [Google Scholar]
- 76.O’Neill SG, Giles I, Lambrianides A, et al. Antibodies to apolipoprotein A-I, high-density lipoprotein, and C-reactive protein are associated with disease activity in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 2010;62(3):845–854. doi: 10.1002/art.27286. [DOI] [PubMed] [Google Scholar]
- 77.Peters MJL, Van Halm VP, Nurmohamed MT, et al. Relations between autoantibodies against oxidized low-density lipoprotein, inflammation, subclinical atherosclerosis, and cardiovascular disease in rheumatoid arthritis. The Journal of Rheumatology. 2008;35(8):1495–1499. [PubMed] [Google Scholar]
- 78.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nature Genetics. 2008;40(2):189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiura Y, Shen CS, Kokubo Y, et al. Identification of genetic markers associated with high-density lipoprotein-cholesterol by genome-wide screening in a Japanese population—the Suita study. Circulation Journal. 2009;73(6):1119–1126. doi: 10.1253/circj.cj-08-1101. [DOI] [PubMed] [Google Scholar]
- 80.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population releavance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Igl W, Johansson A, Wilson JF, et al. Modeling of environmental effects in genome-wide association studies identifies SLC2A2 and HP as novel loci influencing serum cholesterol levels. PLoS Genetics. 2010;6(1):1–10. doi: 10.1371/journal.pgen.1000798.e1000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 83.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation Research. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 84.Takiguchi E, Fukano C, Kimura Y, Tanaka M, Tanida K, Kaji H. Variation in the 5′-flanking region of the neuropeptide Y2 receptor gene and metabolic parameters. Metabolism. 2010;59(11):1591–1596. doi: 10.1016/j.metabol.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 85.Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nature Medicine. 2007;13(7):803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 86.Wolfs MG, Rensen SS, Bruin-Van Dijk EJ, et al. Co-expressed immune and metabolic genes in visceral and subcutaneous adipose tissue from severely obese individuals are associated with plasma HDL and glucose levels: a microarray study. BMC Medical Genomics. 2010;3, article 34:1–15. doi: 10.1186/1755-8794-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ammirati E, Cianflone D, Banfi M, et al. Circulating CD4+CD25hiCD127lo regulatory T-cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(9):1832–1841. doi: 10.1161/ATVBAHA.110.206813. [DOI] [PubMed] [Google Scholar]
- 88.Norata GD, Callegari E, Marchesi M, Chiesa G, Eriksson P, Catapano AL. High-density lipoproteins induce transforming growth factor-β 2 expression in endothelial cells. Circulation. 2005;111(21):2805–2811. doi: 10.1161/CIRCULATIONAHA.104.472886. [DOI] [PubMed] [Google Scholar]
- 89.Vergeer M, Boekholdt SM, Sandhu MS, et al. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation. 2010;122(5):470–477. doi: 10.1161/CIRCULATIONAHA.109.912519. [DOI] [PubMed] [Google Scholar]
- 90.Vergeer M, Korporaal SJA, Franssen R, et al. Genetic variant of the scavenger receptor BI in humans. The New England Journal of Medicine. 2011;364(2):136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- 91.Frikke-Schmidt R, Nordestgaard BG, Stene MCA, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. Journal of the American Medical Association. 2008;299(21):2524–2532. doi: 10.1001/jama.299.21.2524. [DOI] [PubMed] [Google Scholar]
- 92.Bared SM, Buechler C, Boettcher A, et al. Association of ABCA1 with syntaxin 13 and flotillin-1 and enhanced phagocytosis in tangier cells. Molecular Biology of the Cell. 2004;15(12):5399–5407. doi: 10.1091/mbc.E04-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England Journal of Medicine. 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]


