Abstract
Background
Harmaline and harmine are tremorigenic β-carbolines that, on administration to experimental animals, induce an acute postural and kinetic tremor of axial and truncal musculature. This drug-induced action tremor has been proposed as a model of essential tremor. Here we review what is known about harmaline tremor.
Methods
Using the terms harmaline and harmine on PubMed, we searched for papers describing the effects of these β-carbolines on mammalian tissue, animals, or humans.
Results
Investigations over four decades have shown that harmaline induces rhythmic burst-firing activity in the medial and dorsal accessory inferior olivary nuclei that is transmitted via climbing fibers to Purkinje cells and to the deep cerebellar nuclei, then to brainstem and spinal cord motoneurons. The critical structures required for tremor expression are the inferior olive, climbing fibers, and the deep cerebellar nuclei; Purkinje cells are not required. Enhanced synaptic norepinephrine or blockade of ionic glutamate receptors suppresses tremor, whereas enhanced synaptic serotonin exacerbates tremor. Benzodiazepines and muscimol suppress tremor. Alcohol suppresses harmaline tremor but exacerbates harmaline-associated neural damage. Recent investigations on the mechanism of harmaline tremor have focused on the T-type calcium channel.
Discussion
Like essential tremor, harmaline tremor involves the cerebellum, and classic medications for essential tremor have been found to suppress harmaline tremor, leading to utilization of the harmaline model for preclinical testing of antitremor drugs. Limitations are that the model is acute, unlike essential tremor, and only approximately half of the drugs reported to suppress harmaline tremor are subsequently found to suppress tremor in clinical trials.
Keywords: Tremor, harmaline, harmine, inferior olive, cerebellum, animal model
Introduction
Harmaline induces action tremor in mammals, and as an easily elicited model has attracted increasing interest from workers searching for new therapies for essential tremor (ET). In view of this interest, we review what is known about harmaline's actions. We describe the model and review the anatomy and physiology of the olivocerebellar circuitry underlying harmaline tremor. We consider proposed mechanisms by which harmaline produces tremor and survey the pharmacology of harmaline tremor. We discuss the limitations of the model and consider how well harmaline predicts drug efficacy for ET.
Methods
We surveyed literature obtained via PubMed using the search words “harmaline” and “harmine”, examining papers describing mechanisms, properties, or tremor. We also consulted related papers on cerebellum physiology and ET. Only a fraction of these publications could be cited.
Results
β-Carbolines
The basic structure of β-carboline alkaloids is similar to tryptamine, a two-ring indole, but the ethylamine side chain is reconnected to the indole via a carbon atom, forming a third ring. β-Carbolines differ according to the degree of saturation of the third ring, the third ring side chain, and the side chains on the benzene ring (Figure 1).
Figure 1. β-Carboline Structures.
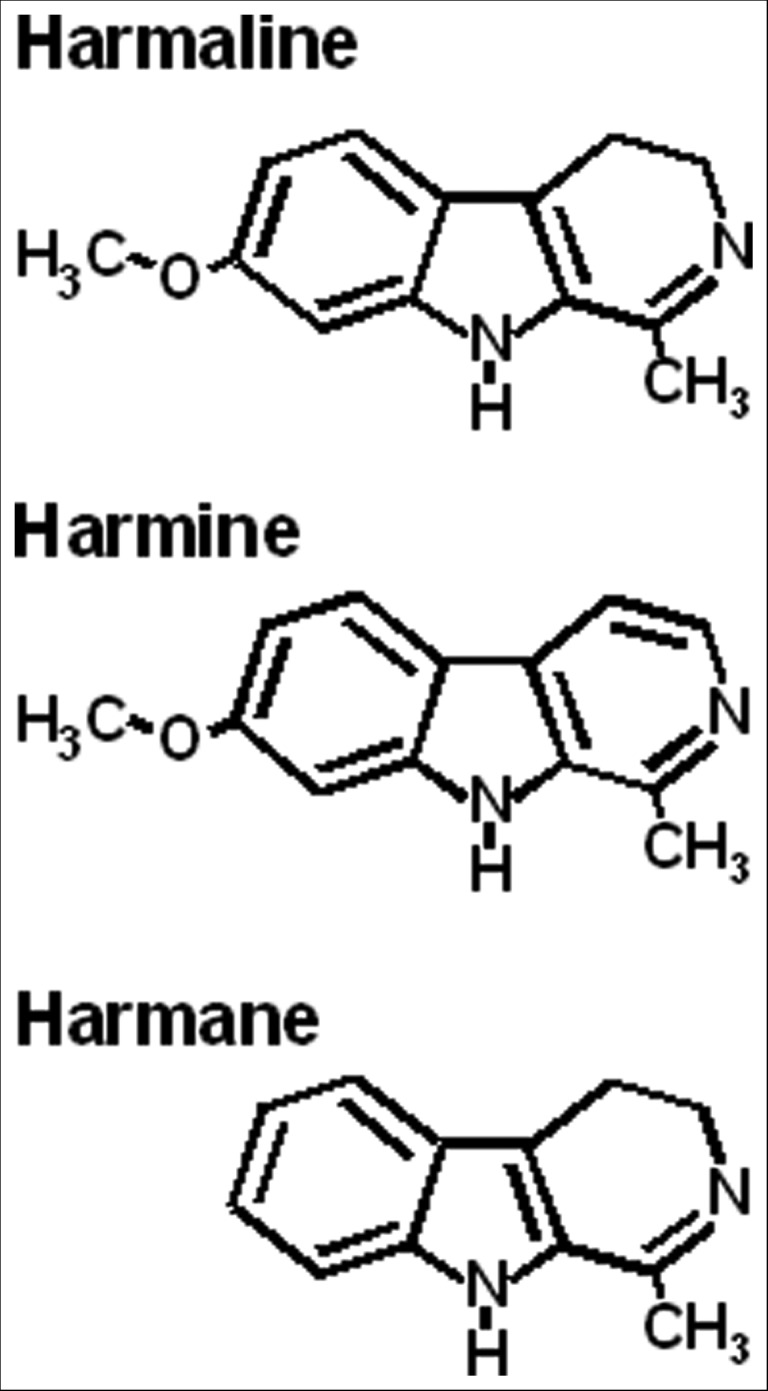
Harmaline and harmine are used in animal models of tremor. Harmane, found in human tissue, can be converted to harmine.
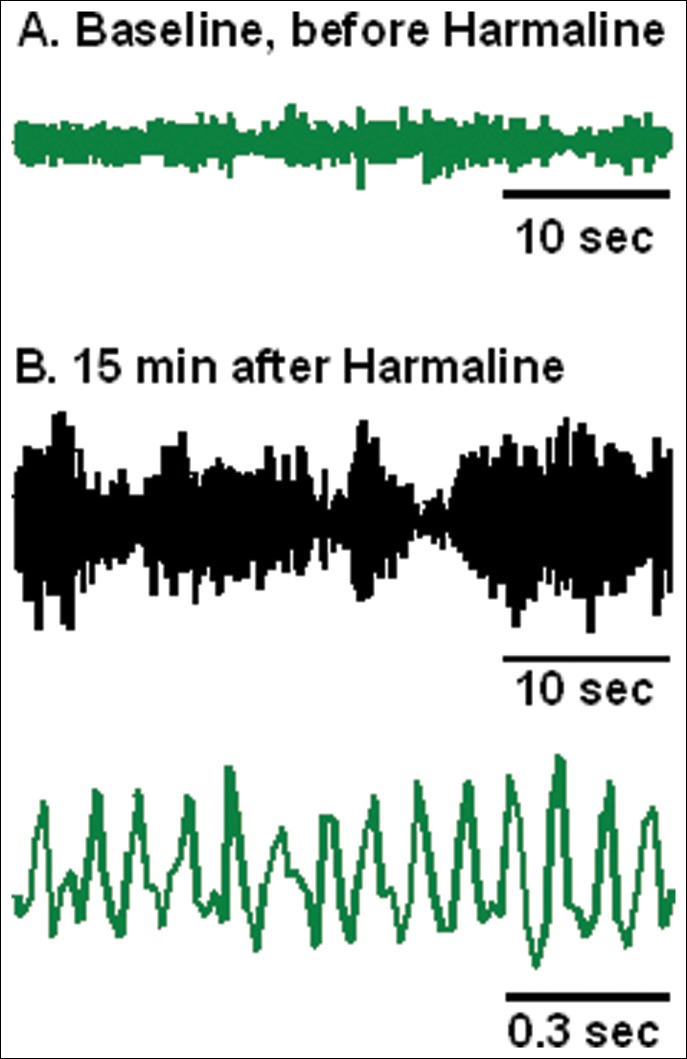
Figure 2. Recorded Motion.
Data are shown for example mouse prior to (A), and after intraperitoneal harmaline administration (B). The slow recording speed demonstrates tremor that fluctuates according to general motor activity. The fast recording speed shows that motion is dominated by tremor at 10-16 Hz. Motion between 0 and 34 Hz was detected with a load sensor under a platform and digitized, with filters at 1 and 70 Hz and sampling frequency of 128 Hz.
Nutritionally the main source of β-carbolines is animal protein, but they are also found in cereals, corn, beverages (wine, whiskey, beer, sake), and in tobacco.1 β-Carbolines are also formed endogenously from the condensation of tryptophan-derived indolealkylamines with simple aldehydes or with pyruvic acid. Thus some β-carbolines, such as harmane and norharman, are normal constituents of human tissue. Because ethanol is converted to aldehyde in tissues and in the stomach,2 a question is whether alcohol ingestion elevates β-carboline levels. Rats chronically administered alcohol display elevated plasma and brain norharman,3 but no change in brain or lung harmane.4 In humans, elevated plasma norharman is associated with heavy smoking rather than alcohol intake.5
The rate of elimination also affects β-carboline levels. In humans, CYP 1A2 and 2D6 are the major cytochrome enzymes metabolizing harmaline and harmine, converting these by O-demethylation to non-tremorigenic harmalolol and harmol.6 Human Purkinje cells express 2D6, and this expression is upregulated in alcoholics.7 Smoking also increases 2D6 expression in the human brain, as does nicotine administration in animals.8 Cytochrome 2D6 may play a role in defending against β-carboline derivatives that have potential 1-methyl-4-phenylpyridine (MPP)-like neurotoxicity. Endogenous tetrahydro-β-carbolines can undergo methylation by N-methyltransferases to form N(2)-methyl- and N(2,9)-dimethyl-tetrahydro-β-carbolines, which are similar to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).9 Such compounds are found in rat brain and are oxidized by heme oxidases to the corresponding β-carboliniums, structurally analogous to MPP+,10 and similarly neurotoxic. N(2)- and N(9)-methylnorharmanium ions induce bradykinesia and dopamine loss in mice.11 As an alternative to forming β-carbolinium products, N-methylated β-carbolines are removed by CYP 2D6, at a rate highly dependent on the 2D6 polymorphism subtype.12
Such observations have led to speculation that brain 2D6 may protect against neurotoxins derived from β-carbolines or other toxins, in view of evidence that smoking protects against Parkinson's and ET.13 However, persons who inherit a 2D6 polymorphism with low activity have an inconsistent or weak increase in risk for Parkinson's disease.14
Do β-carbolines cause ET?
ET patients have higher harmane blood levels, especially in familial cases,15 which is not due to higher dietary intake.16 Harmane in high doses may cause tremor,17 or do so via a metabolite, harmine. A role of harmane could also be from tissue damage that gives rise to tremor. Indeed, among ET patients, blood harmane levels are significantly correlated with the decline in a spectroscopic magnetic resonance imaging measure of viable neurons in the cerebellar cortex.18
Harmaline tremor: an acute model
The harmala alkaloids harmine, harmaline, and tetrahydroharmine are especially rich in the seeds of Peganum harmala (Syrian Rue) and in the Banisteriopsis caapi vine. Extracts from the latter are combined with leaves from Psychotria viridis, containing dimethyltryptamine (DMT), to create the ayahuasca sacramental beverage used in shamanic rituals. The principal purpose of the harmala compounds is to inactivate gastrointestinal monoamine oxidase (MAO-A), enabling enough DMT to elude first-pass metabolism so as to produce cognitive/affective effects. The amount of harmala alkaloids absorbed may be small or negligible.19 Large doses of harmine or preparations of B. caapi induce in human volunteers a transient coarse tremor.20
Among β-carbolines, ibogaine, harmaline and harmine are especially tremorigenic. Of these, harmaline (7-methoxy-3.4-dihydro-β-carboline) has been most frequently utilized experimentally, but harmine acts similarly, and similar doses are employed. Harmaline produces an 8–16 Hz tremor in mice,21 rats, cats,22 and monkeys.23 The tremor involves appendicular and axial musculature during posture and kinesis. In a mouse or rat the tremor visibly involves all four limbs, the tail, trunk, and head, including whiskers. The tremor is particularly visible when the animal ambulates, and is less when it lies down.
The peak tremor frequency varies according to the species, ranging from 8–10 Hz in monkey to 11–14 Hz in mice.24 After subcutaneous or intraperitoneal injection, tremor develops within minutes and lasts up to several hours before subsiding. Tremor may be assessed with rating scales,25 electromyographic recordings, or digitally quantified with systems that detect motion through force or magnetic field transduction.26, 27 Because harmaline-induced tremor is an action tremor, the amount of tremor will vary according to the motor activity level. This source of variation can be greatly reduced by normalizing the data to overall motion.28
Whole-body tremor is dose-dependent with harmaline doses at 4 mg and above26 in rats. Doses of 10–20 mg/kg are frequently employed in rats; mice require approximately twice that. If rats are trained to press a disk connected to a transducer, forelimb tremor can be detected at a dose as low as 1.0 mg/kg.29
Repeated daily administration of harmaline to rats, 4–16 mg/kg, results in a loss of the tremor response (tolerance) after three to seven treatments, lasting at least 7–10 days.26, 30 In contrast, tolerance is not observed with four daily doses of a low dose at 1.0 mg/kg,29 or if tremor is prevented with diazepam or morphine during the initial exposure.30 Mice also develop harmaline tolerance.24
Inferior olive (IO) neurons in tolerant rats fail to show harmaline-associated sustained rhythmic activity, and vermal Purkinje neurons do not show expected rhythmic climbing fiber responses, suggesting that tolerance may reflect physiological changes.31, 32
Another explanation for tolerance may be neuronal damage, but rats and mice show different morphological alterations after harmaline. In rats, harmaline causes Purkinje neuron loss in narrow parasagittal vermis zones, possibly due to excitotoxic climbing fiber hyperactivity. The IO is unaffected33, 34 In contrast, mice show no cell loss or gliosis in the cerebellar cortex but instead show microgliosis in IO without cell loss, with the medial and dorsal accessory regions most affected.34
The anatomic and physiologic basis of harmaline tremor
Harmaline activates circuits within the olivocerebellar system to produce tremor. Before discussing this circuitry we briefly review selected aspects of olivocerebellar physiology.
Normal olivocerebellar system functioning
Subthreshold oscillation
IO neurons in brainstem slices normally demonstrate rhythmic membrane voltage subthreshold oscillations (STOs) that involve serial ion conductances. A high threshold calcium spike is followed by a depolarizing shoulder that is terminated by a potassium conductance that leads to an afterhyperpolarization, which in turn deinactivates a low-threshold (T-type) calcium current. That causes a rebound spike which triggers the high-threshold calcium spike, and may or may not be enough to trigger a sodium spike. The oscillation frequency is approximately 10 Hz, whereas individual IO cells fire at 1 Hz.35–38 Spontaneous STOs are suppressed by apamin, serotonin via 5HT2a receptors,39 NMDA receptor antagonists, and L-, P-, and T-type calcium channel blockers.40
STOs are synchronized among ensembles of IO neurons via electrical coupling.35–37, 41 Hundreds of IO neurons oscillate coherently in discrete clusters, with moment-to-moment variation in cluster size. Addition of picrotoxin, a GABAA receptor antagonist, induces clusters to merge, forming larger units.42 IO neurons form dendritic tangles (glomeruli) containing abundant gap junctions.43 Addition of a gap junction blocker disrupts synchrony.44 GABAergic afferents terminate near gap junctions, where they can control electrotonic coupling.36
IO–Purkinje ensembles
IO neurons project climbing fibers to Purkinje cell dendrites, with collaterals to deep cerebellar nuclei (DCN) non-GABAergic and GABAergic neurons.45 Purkinje cells respond to climbing fibers with complex spikes, whereas parallel fibers from granule cells mediate simple spikes. Because each Purkinje cell receives a single climbing fiber from a dedicated IO neuron, the behavior of multiple IO neurons in vivo can be studied by recording complex spikes from many Purkinje cells simultaneously. Such studies reveal that Purkinje cell firing is synchronized at 10 Hz within small vertical cortical bands, indicating that they are controlled by electrically coupled IO ensembles.46 The intra-band synchrony and band size, and by inference that of the projecting IO ensemble, are not fixed but modulated by afferents to IO. Intra-IO picrotoxin injection or lesions of the dentate nucleus, which projects GABA to the IO, increases Purkinje cell within-band synchrony and synchronous band width.47, 48 Glutamate also modulates IO/Purkinje synchrony. Intra-IO injection of 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), an α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid (AMPA) receptor antagonist, reduces synchronous Purkinje cell band width, indicating that glutamate expands synchrony in the IO.48
In summary, IO neurons do not fire often, but when they do, the timing is precisely timed according to the depolarization phase of STOs that are tightly synchronized within an IO ensemble. The aggregate firing is thus highly rhythmic. The IO ensembles are not fixed, but sculpted on a moment-to-moment basis.49 IO gap junctions and the activity of DCN that control IO coupling via GABA projections play critical roles.
Gap junctions
IO neurons richly express the gap junction protein connexin 36 (Cx36).41 Cx36-null mice appear to lack IO gap junctions,50 and IO slices show rare and weak electrical coupling, so that IO STOs and action potentials are not synchronized.51 Loss of IO coupling also occurs with transduction of inactive mutant Cx36 and the addition of the gap junction blocker carbenoxolone.52, 53 Purkinje cell complex spike synchrony is lost in mice receiving intra-IO carbenoxolone injection and in Cx36-null mice.54, 55
Deep cerebellar nuclei
DCN neurons send excitatory efferents to extracerebellar structures and a massive GABAergic projection to the IO. Climbing fibers are excitatory and send collaterals to GABAergic DCN neurons, which are coupled with Cx36 gap junctions,41 and project back to IO, and to non-GABAergic DCN neurons. Climbing fiber-activated Purkinje cells project GABAergic terminals to both DCN non-GABA (glutamate) and GABAergic neurons.56
GABA DCN neurons show strong rebound discharges after AHP, due to Cav3.1 T-type channels,57 compatible with phasic firing, in which timing rather than intensity modulation is important.58 In contrast, large non-GABA DCN neurons express a linear firing-to-stimulation relationship, functioning as a linear transducer of spike frequency, suitable as output neurons. These neurons express weaker rebound discharges,58 and may correspond to excitatory neurons observed not to express Cav3.1 T-type channels.57
What happens to this system when harmaline is administered?
When harmaline is added to the brainstem slice, IO neurons exhibit increased rebound low threshold (T-type) calcium spikes, so that each rebound is now associated with bursts of sodium action potentials. Thus the IO neurons are made more excitable by harmaline and convert from STO to rhythmic 9–12 Hz burst-firing.36, 37 In animals, IO harmaline microinjection elicits rhythmic local bursting only when the medial accessory olive (MAO) and dorsal accessory olive (DAO) are injected.59 Intravenous harmaline in cats causes IO neurons confined to MAO and DAO to fire rhythmically and synchronously at 6–12 Hz, generating rhythmic Purkinje cell complex spikes, whereas simple spikes are suppressed.22. In addition, neurons in DCN, lateral reticular nucleus, red nucleus, nucleus reticularis tegmenti pontis, spinal cord interneurons, and motoneurons also fire at the tremor frequency.60, 61 In cat, harmaline increases glucose utilization in the MAO, caudolateral DAO, the molecular layer of the vermis and paravermis cerebellar cortex, and the same three brainstem nuclei shown to fire at the tremor frequency.62 Fos mapping demonstrates IO activation 15 minutes after harmaline administration, followed by DCN at 30 minutes, cerebellar cortex at 1 hour, and vertical bands of vermal Purkinje cells at 2–6 hours.63 The delay in DCN recruitment has also been found with field potential recordings.64
The harmaline-responsive Purkinje cells are mainly found in the vermis and paravermis regions in the rat and cat,65 to which MAO and DAO project. These cortical regions project to the fastigial and interpositus DCN, which also receive climbing fiber collaterals from MAO and DAO. The DCN send reciprocating GABAergic projections back to the IO subnuclei. These connections are highly organized with somatotopic precision.66, 67
Climbing fibers are required for tremor expression
The destruction of IO by systemic 3-acetylpyridine injection eliminates the tremor response.68 If the cerebellar peduncles are cut to sever climbing fibers, the MAO and DAO still show bursting and increased glucose utilization by harmaline, whereas Purkinje cell and fastigial nucleus bursting is abolished, and metabolic activation of other medulla structures fails to occur.22, 61 The importance of climbing fibers is also illustrated in genetically dystonic rats, which do not show normal climbing fiber-induced complex spike responses.69 On harmaline administration, IO neurons but not Purkinje cells, fire rhythmically, and tremor does not occur.32, 70
Are Purkinje cells required for tremor?
Cooling of the cerebellar cortex does not abolish harmaline-induced motoneuron firing, suggesting that the olivo–DCN loop may be sufficient for tremor.61 Mice with Purkinje cell degeneration (pcd) still manifest harmaline tremor despite the complete absence of Purkinje cells, although the tremor is of lower frequency and amplitude than controls. Lurcher mice also have no Purkinje neurons, but mount no harmaline tremor. The difference between these two strains of mice is that pcd mice have intact climbing fibers capable of contacting DCN neurons, whereas lurcher mice appear not to.71
What is the role of DCN in tremor?
DCN neurons, as the sole output of the cerebellum, are required for the expression of harmaline tremor. On the other hand, lesions of the dentate nucleus or DCN outflow pathways are well known to induce action tremor in humans,72 and lesions or inhibitory injections of the interpositus nucleus or combined lesions of interpositus and dentate nucleus can induce action tremor in monkeys.73–75 Thus DCN paradoxically can express and suppress tremor. On adding harmaline to a guinea pig cerebellum–brainstem in vitro preparation, one group of DCN neurons responds with an excitatory post-synaptic potential, an inhibitory post-synaptic potential (IPSP), then a rebound discharge, whereas another group responds with an initial IPSP followed by a rebound discharge. These responses have been interpreted to indicate that harmaline induces phasic rhythmic activity in excitatory DCN output neurons, thereby expressing tremor, and in inhibitory nucleo-olivary neurons, thereby modulating rhythmicity and synchronicity.76
Are the brainstem and spinal cord sufficient for harmaline tremor?
Cooling of the motor cerebral cortex and lesions of the ventrolateral thalamus or globus pallidus reportedly do not affect harmaline tremor in intact monkeys.77, 78 Not even intercollicular decerebration abolishes tremor in cats or monkeys.78, 79 These observations suggest that the brainstem and spinal cord are sufficient to express harmaline tremor, even in primates. On the other hand, high-frequency stimulation simulating deep brain stimulation (DBS) of the ventrolateral thalamus, and intrathalamic infusion of muscimol or an adenosine A1 receptor agonist suppress harmaline tremor in mice,80 indicating a potential role of the thalamus in modulating harmaline tremor.
Summary
Harmaline induces rhythmic bursting in accessory IO neurons that then recruits medial regions of cerebellar cortex and DCN. The end result is rhythmic activation of the spinal gamma and alpha motoneurons and tremor.
Proposed mechanisms of harmaline tremor
Here we consider the question how harmaline acts at the cellular level to induce tremor.
Serotonin
Initial ligand binding studies indicated that harmaline does not bind significantly to 5-hydroxytryptamine (HT)1a-d, 5-HT2 or 5-HT3 receptors (Ki>100 μM).81 Subsequently harmaline was found to have affinity to the 5-HT2a (Ki = 7.8, 42.5 μM) and 5-HT2c (Ki = 9.4 μM) receptors,82, 83 comparable to the cerebellar harmaline level of 18.2 μM after 15 mg/kg in mice.84 Acting through 5-HT2a receptors, intra-IO 5-HT injection increases IO neuronal firing rates and improves coherence, while increasing intra-band Purkinje cell synchrony, similar to harmaline, but harmaline's action is not blocked by a 5-HT2a antagonist.85
NMDA receptor channel
Harmaline competitively displaces tritiated MK801 (dizocilpine) from the NMDA receptor in rabbit IO fractions (IC50 60 μM),86 leading to the suggestion that harmaline induces tremor by acting as an NMDA receptor inverse agonist. However this action would produce depolarization, whereas harmaline hyperpolarizes IO neurons.
Benzodiazepine receptor
Harmaline displaces tritiated flunitrazepam from brain tissue only at high IC50 concentrations: 126–600 μM.84, 87 The benzodiazepine antagonists flumazenil and CGS8216 do not affect harmaline tremor in mice.88 Moreover, binding of 3H-flunitrazepam in IO of adult rodents is very sparse.89 Harmaline is thus not likely to induce tremor via benzodiazepine receptors.
Sodium and high-voltage calcium conductances
Harmaline does not significantly displace ligands at adrenergic, dopamine, opiate, muscarinic, nicotinic, GABA receptors or the chloride channel. An affinity for the voltage-gated sodium channel, (Ki = 13.9 μM), suggested this as a potential mechanism of tremor.81 However, very high levels of harmaline are needed to affect the action potential (>0.5 mM).90 At 100 μM, harmaline inhibits sodium conductance by 23% in dorsal root ganglia neurons. High-voltage calcium channels (L- and N-type) are more sensitive, with an IC50 by harmaline of 100 μM. However harmane, which is less tremorigenic, is more potent (IC50 = 76 μM),91 thus this channel is not likely to mediate tremor.
The Cav3.1 T-type calcium channel
IO slices from Cav3.1-null mice fail to show STOs or low-threshold calcium spikes. Harmaline fails to produce rhythmic firing in IO slices from Cav3.1 null mice.64 Park et al.64 studied harmaline effects on Cav3.1 currents in vitro and found a complex set of actions that in combination leads to enhanced rebound spikes. They postulate that harmaline engenders tremor by effects on the Cav3.1 channel.64 Effects on Cav3.2 or Cav3.3 channels were not studied.
Other actions
Harmaline inhibits sodium-dependent transport of substances into various tissues, such as choline into striatal synaptosomes (Ki = 36 μM),92 and gamma-hydroxybutyrate into whole-brain synaptosomes (Ki = 94 μM).93 Harmaline is a potent inhibitor of MAO-A, with IC50 values as low as 4–8 nM. Activity against MAO-B is negligible.94 Harmaline inhibits synaptosomal GABA uptake (IC50 47 μM)95 and dopamine uptake (IC50 = 8.1 μM),96 and increases dopamine release from striatal slices at 6 μM.94 Low doses enhance levodopa-induced stereotypy in mice.97 These observations are compatible with reports that Parkinson's motor symptoms are ameliorated by extracts of B. caapi.94
Harmaline also potently inhibits cerebral histamine N-methyltransferase (IC50 = 4.4 μM), which may raise histamine levels,98 and displaces tritiated tryptamine is brain tissue (IC50 = 25 nM).99 Harmaline potently binds the imidazoline 2B receptor (Ki = 177 nM).100
Summary
Several potential mechanisms by which harmaline could produce tremor have been investigated. At present the most likely mechanism appears to be modulation of T-type calcium channels. It is not clear whether this action is restricted to Cav3.1 channels or also involves Cav3.2 and Cav3.3. Harmaline is a potent inhibitor of MAO-A, and has significant effects on dopamine and histamine processing.
The pharmacology of harmaline tremor
Serotonin (5-HT)
Serotonergic fiber innervation in IO is highest in caudal MAO and caudolateral DAO, correlating with high sensitivity to harmaline-induced rhythmicity.101, 102 Lesions of 5-HT fibers to the IO, or of the medial and dorsal raphe nuclei, reduce the harmaline tremor response.101, 103 The genetically epilepsy-prone rat (GEPR) has reduced serotonergic IO innervation, and manifests poor harmaline tremor.104 Harmine tremor is exacerbated by the 5-HT precursor 5-hydroxytryptophan,103 an effect reduced by raphe lesions.103 Conversely, the broad-spectrum 5-HT antagonist methysergide and the 5-HT synthesis inhibitor para-chlorophenylalanine reduce harmaline tremor.105, 106 The serotonin uptake inhibitor citalopram (10–40 mg/kg) enhances harmaline tremor in rats,107 as does imipramine.106
Norepinephrine
When norepinephrine is added to guinea pig brainstem slices, harmaline-induced rhythmic IO oscillations ceases.37 Systemic injection of the norepinephrine precursor l-threo-3,4-dihydroxyphenylserine (l-threo-DOPS, 50–200 mg/kg) suppresses harmaline tremor in rats.108 Intraventricular l-threo-DOPS also suppresses harmaline tremor, as does electrical stimulation of locus ceruleus.109 In contrast, the tyrosine hydroxylase inhibitor alpha-methyl-p-tyrosine, 200 mg/kg; 6-hydroxydopamine injections that reduce cerebellar norepinephrine; and locus ceruleus destruction each exacerbate harmaline tremor.108–110
Beta-adrenergic blockers such as propranolol suppress harmaline and harmine tremor in rodents.111, 112 Selective beta1- and beta2-adrenergic antagonists can each suppress harmaline tremor in rats, but beta2-blockade may be more effective.113 Although propranolol may act in part peripherally,113 it also acts directly by antagonizing the electrophysiological effects of harmaline on IO neurons in brainstem slices.114 In contrast, the alpha-adrenergic antagonist phenoxybenzamine does not suppress harmine tremor,115 and is ineffective for ET.116
Glutamate
Intracisternal 2-amino-5-phosphonovalerate (2-APV), an NMDA receptor antagonist, suppresses harmaline tremor in mice.115 Similarly the competitive NMDA antagonist d-CPPene suppresses harmaline tremor in mice and rats.117, 118 The non-competitive NMDA antagonist dizocilpine (MK-801) potently suppresses harmaline tremor in mice and rabbits,118, 119 as does phencyclidine in mice.118 Memantine has only a weak antitremor effect in rats,120 comparable to a weak or non-existent effect on ET.121 However, memantine confers striking protection against harmaline-induced cell loss in the cerebellum and IO.120
The AMPA receptor antagonist RPR117824 suppresses harmaline tremor,122 as does NBQX disodium salt.118
The mGluR1 antagonist JNJ 16259685-a strongly enhances harmaline tremor in rats, suggesting an agonist at this receptor should suppress tremor.123 In contrast, the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) has no effect in mice.118
GABA
The benzodiazepine diazepam, 1.5–5 mg/kg, suppresses harmaline tremor in rodents.88, 124 The GABAA receptor agonist muscimol also suppresses harmaline tremor in mice.118 The GABAB receptor agonist baclofen, 2.5–10 mg/kg, dose-dependently suppress harmaline tremor in rats,125 and reduces harmine tremor in alcohol-withdrawing rats.126 However Paterson et al.118 did not find tremor suppression by baclofen in mice.
Dopamine (DA)
Harmine tremor is reduced by levodopa and by the agonists apomorphine and piribedil in rats and mice.103, 118 Similarly the dopamine uptake inhibitor GBR12909 reduces tremor.118 Apomorphine is a D1/D2 agonist. Tremor is not reduced by the D1 agonist SKF82958, but is by the D2/D3 agonist quinpirole.118
Alcohol
Suppression of harmaline tremor by ethanol has been well replicated,28, 127 but the site of action and mechanism remain unclear. Ethanol reduces harmaline tremor in mice at low doses that do not suppress harmaline-induced cerebellar cyclic GMP elevations.127 This observed dissociation between ethanol's climbing fiber-mediated and behavioral effects raised the possibility of an extra-olivary localization of the antitremor action. In rats anesthetized with agents other than urethane, or immobilized and given local anesthesia, ethanol increases IO firing rates.128 A moderate ethanol dose (1 g/kg) increases vermal Purkinje cell complex spike rhythmicity and synchrony in ketamine-anesthetized rats, and by inference IO ensemble rhythmicity and synchrony. Moreover ethanol fails to affect the rhythmicity of harmaline-induced complex spike activity.129 These observations suggest that alcohol does not suppress but instead increases IO firing with potentially excitotoxic effects. This inference is supported by the finding that although ethanol, 1.5 g/kg, effectively suppresses tremor in rats, histology 24 hours later reveals that ethanol-treated rats display an exacerbation of the harmaline-associated vermal and paravermal Purkinje and granule cell loss, and more IO neuronal loss.120 Conceivably alcohol's antitremor efficacy may lure ET patients to alcohol-exacerbated cerebellar damage, to which they may be more vulnerable.
Antiepileptic drugs
De Ryck et al.130 found antitremor effects for primidone, clonazepam, gabapentin, and carbamazepine. Whereas levetiracetam had minimal effect on tremor, the derivative brivaracetam was effective. Similarly, Paterson et al.118 found that primidone, gabapentin, and carbamazepine suppress harmaline tremor in mice, but also reported that valproate does as well. Zonisamide suppresses harmaline tremor in mice, with 50 mg/kg more effective than 5 mg/kg.25 Zonisamide has shown efficacy for ET.131 Lacosemide, 0.3–30 mg/kg, suppresses harmaline tremor in rats;132 however, a clinical trial did not show efficacy for ET.133 Similarly, carisbamate suppressed harmaline tremor in preclinical testing, but demonstrated no antitremor efficacy in an ET trial.134
Gap junction blockers
Contrary to expectation, Cx36-null mice or mice with IO transduction of inactive mutant Cx36 mount a vigorous tremor response to harmaline.51, 52 On the other hand, harmaline tremor is suppressed by the broad-spectrum gap junction blocker carbenoxolone and by more specific mefloquine.135 It may be conjectured that other gap junctions also play a role in harmaline tremor, such as connexin 57.136 More research on this topic is warranted.
T-type calcium channels
Isomers of octanol suppress harmaline tremor in the rat.137 Because octanol blocks T-type calcium channels it was predicted that antagonists of these channels could be effective for tremor. However, octanol exerts multiple actions. We showed that each of five drugs that block T-type calcium channels suppress tremor in the harmaline model and in the GABAA α1-null genetic mouse tremor model.138 The best agent appeared to be NNC55-0396.139, 140
Of the three subtypes of T-type calcium channels, Cav3.1 is expressed in IO, Purkinje cells and some DCN neurons. Park et al.64 reported that Cav3.1-null mice generated in the laboratory of HS Shin are impaired in manifesting harmaline tremor. This was demonstrated with a low harmaline dose that in wild-type mice induced tremor for only 15 minutes. Tremor was greatly reduced in Cav3.1-null mice, but not abolished.64 Although these authors postulate Cav3.1 is the “pacemaker” for tremor, they do not explain how harmaline is able to induce any tremor in Cav3.1-null mice. Our laboratory found that a routine dose of harmaline that results in longer tremor produces as much tremor in Cav3.1-null mice generated by K Sakimura as in wild-type mice. Moreover, NNC55-0396 suppresses tremor in the Cav3.1-null mice.138 As NNC55-0396 may act on Cav3.2 and/or Cav3.3 channels as well as Cav3.1, it is possible harmaline has an effect on these subtypes, although such a role in tremor remains unexplored.
Adenosine
Harmaline tremor is enhanced by the adenosine receptor antagonist caffeine, 50–150 mg/kg, in rats,141 and by the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthiine (DPCPX), 4 mg/kg, in mice.80 Intrathalamic infusion of adenosine or the A1 receptor agonist 2-chloro-N6-cyclopentyladenosine reduces harmaline tremor. Infusion of DPCPX or the use of A1 receptor null mice reduces the threshold for DBS-induced involuntary movements (related to glutamate release), so that tremor cannot be suppressed by DBS. Thalamic DBS is suggested to cause local adenosine release that limits glutamate effects, enabling tremor to be suppressed at current levels below those associated with involuntary movements.80
Other drugs
Anticholinergics do not reduce harmine tremor in rodents.111 Harmaline tremor is suppressed by systemic lidocaine (12.5–50 mg/kg);142 dantrolene, 10 mg/kg;124 lithium, 2 mEq,118 in mice; and 16-methyl-prostaglandin E2 (PGE2), 25–50 μg/kg, in cats.143 Conversely, harmine tremor is enhanced by cyclosporin, 25–50 mg/kg, in mice.144 MK-0249, a histamine-3 receptor inverse agonist, suppresses harmaline tremor, but does not suppress ET tremor.145
Summary
Knowledge of the physiology and the basic circuit of harmaline tremor (Figure 3) suggests that drugs affecting specific ion conductances, such as the T-type calcium channel, gap junctions, glutamate, and GABA receptors should affect tremor; notions that have received support. In addition, the tremorigenic circuit is susceptible to influence by various neurotransmitter systems, including norepinephrine, 5-HT and DA. Further research is likely to reveal more neuromodulators of tremor.
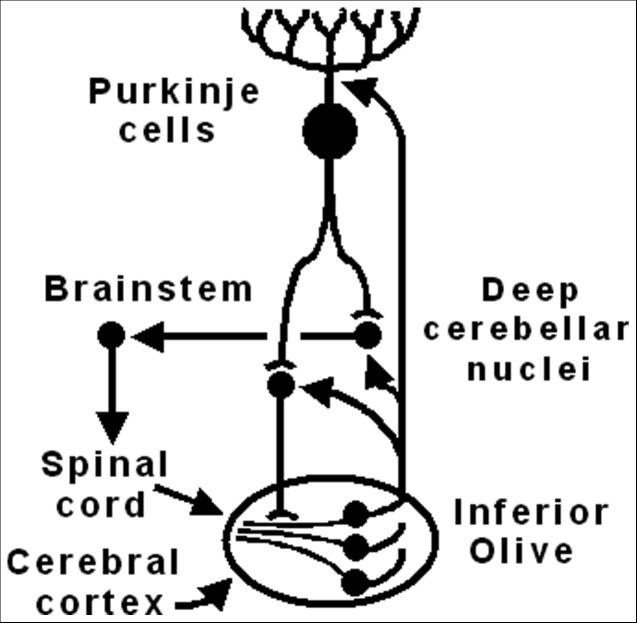
Figure 3. The Olivocerebellar Circuit Implicated in the Generation and Expression of Harmaline Tremor.
The Olivocerebellar Circuit Implicated in the Generation and Expression of Harmaline Tremor. Synaptic bar endings connote inhibition, while arrowhead endings connote excitation. Harmaline elicits rhythmic bursting in clusters of inferior olivary neurons, synchronized via dendritic glomeruli. Climbing fibers excite complex spikes in Purkinje cells, which project GABA to both GABAergic and excitatory output deep cerebellar nucleus cells. These cells also receive collaterals from climbing fibers. The deep cerebellar nuclei project GABA to inferior olivary glomeruli, opposing synchrony. Glutamate receptor activation in the inferior olive promotes synchrony. The deep cerebellar nuclei and inferior olive are required for harmaline tremor expression; the absence of Purkinje cells reduces but does not abolish tremor. The deep cerebellar outflow to the brainstem and spinal cord is sufficient for harmaline tremor expression without the need for supratentorial structures.
Harmaline tremor as a preclinical model of ET
Comparison with ET
Harmaline-induced tremor has been suggested as useful for preclinical screening of potential ET therapies. ET and the harmaline animal model differ in a number of respects, however, and the harmaline model possesses limitations.
Harmaline tremor is an acute state induced pharmacologically, following which animals are resistant to further doses. In contrast, ET develops gradually, and is chronic, without remission. Based on associations of lower risk for ET with smoking and a Mediterranean diet,13, 146 and findings of Purkinje cell loss with cerebellar cortical gliosis or locus ceruleus depletion and/or Lewy bodies, it appears likely that at least in some cases ET is a neurodegenerative disorder.147 Harmaline acts on the IO to produce tremor. In ET, the role of IO is less certain. In ET subjects, eye-blink conditioning, which depends on the olivocerebellar pathway, is impaired.148 One imaging study found increased glucose utilization in the IO region in ET,149 but another study found no change in IO blood flow.150 Cerebellar cortical hypermetabolism, known to depend in the harmaline model on climbing fiber activation, also occurs in ET.149–151 Interestingly, alcohol administration suppresses cerebellar hypermetabolism in ET subjects, and increases IO blood flow, which does not happen in controls, suggesting that IO physiology differs in ET.151
Each pharmacotherapy suppresses tremor in only a fraction of ET patients. Given ET's heterogeneity, it is uncertain to what extent harmaline or any other animal model can offer predictive success. Another caveat in comparing drug efficacy for harmaline vs. ET is that, based on published reports, it is difficult to assess to what extent suppression of harmaline tremor is a non-specific effect of sedation or reductions in motor activity. Potential approaches are to employ tremor measures that are insensitive to locomotor activity levels, and to select doses shown in independent behavioral tests not to affect motor activity.
How accurate is the harmaline model in predicting efficacy for ET?
An initial prediction was encouraging. Sinton et al.137 reported in 1989 that isomers of octanol suppress harmaline tremor in the rat, subsequently replicated in mice.135 Early-stage clinical trials later demonstrated that 1-octanol reduces tremor in ET.152 In humans, 1-octanol is rapidly converted to octanoic acid, which may be the active antitremor agent.153
Citalopram, imipramine and caffeine worsen both harmaline107, 143 and ET tremor (Table 1). We do not know of any agent that fails to suppress harmaline tremor yet is effective for ET. Phenoxybenzamine, levetiracetam, and anticholinergics do not suppress harmaline tremor, and do not usually suppress tremor in ET.
Table 1. Comparison of Harmaline Rodent Tremor Model and Essential Tremor.
| Feature | Harmaline Tremor | Essential Tremor |
| Clinical | ||
| Action tremor | Yes | Yes |
| Time course | Acute | Chronic |
| Inducing agent | Pharmacologic | Probably neurodegenerative |
| Role of inferior olive | Definite | Uncertain |
| Cerebellar hypermetabolism | Yes | Yes |
| Response to drugs | ||
| Caffeine | Worsens | Worsens in some |
| Citalopram, imipramine | Worsens | Worsens in some |
| Phenoxybenzamine | Does not suppress | Does not suppress |
| Anticholinergics | Do not suppress | Do not suppress |
| Levetiracetam | Does not suppress | Does not suppress |
| Primidone | Suppresses | Suppresses in some |
| Clonazepam, diazepam | Suppresses | Suppresses in some |
| Gabapentin | Suppresses | Suppresses in some |
| Carbamazepine | Suppresses | Does not suppress |
| Valproate | Suppresses | Does not suppress/worsens |
| Zonisamide | Suppresses | Suppresses in some |
| Lacosamide | Suppresses | Does not suppress |
| Carisbamate | Suppresses | Does not suppress |
| Propranolol | Suppresses | Suppresses in some |
| l-dopa, DA agonists | Suppresses | Do not suppress |
| Ethanol | Suppresses | Suppresses in some |
| Gamma-hydroxybutyrate | Suppresses | Suppresses in some |
| Lithium | Suppresses | Does not suppress/worsens |
| 1-Octanol | Suppresses | Suppresses in some |
| Memantine | Weakly suppreses | Weak or no suppression |
| MK-0249 | Suppresses | Does not suppress |
Of 16 agents reported to suppress harmaline tremor, including weakly effective memantine, seven fail to suppress ET tremor, including several anti-epileptic drugs,133, 134, 154 levodopa and dopamine agonists,103 lithium,118 and MK-0249. Matches between positive results in the harmaline model and efficacy in ET trials occurred in 9 out of 16 agents or a 56% concordance rate, and include propranolol, several anti-epileptic drugs, alcohol, memantine, and gamma-hydroxybutyrate.118, 155 Because some of these agents were tested in the model after having been found effective in ET, the true predictive success rate for the model may be lower than 56%.
Summary
As is often the case with neurological disease models, the harmaline model is prone to false positives. Because the harmaline state is poorly compatible with sensitive tests of drug intoxication, independent tests of behavioral tolerability in non-harmaline control subjects may be appropriate.
Conclusion
Like ET, harmaline induces an action tremor that involves cerebellar circuitry, and responds to drugs that suppress clinical tremor. Unlike ET, harmaline tremor is acute and temporary. Harmaline converts IO STOs to rhythmic burst firing that is propagated through the cerebellum, then ultimately activates spinal motoneurons to express tremor. The minimum cerebellar circuit required includes the IO, climbing fibers and DCN. Pharmacologic studies indicate that harmaline tremor severity is influenced by several glutamate receptors, GABA A and B receptors, serotonin receptors, norepinephrine receptors, dopamine receptors, gap junctions, T-type calcium channels, alcohol, and antiepileptic drugs. Of drugs that suppress harmaline tremor, approximately half suppress ET tremor.
Acknowledgments
We are grateful to Elan Louis, MD, for constructive comments and to Hovsep Kosoyan, PhD, for assistance with figures.
Footnotes
Funding: The author was supported by Veterans Affairs, the International Essential Tremor Foundation, and the Ralph M. Parsons Foundation.
Conflict of Interest: The author reports no conflict of interest.
References
- 1.Guan Y, Louis ED, Zheng W. Toxicokinetics of tremorogenic natural products, harmane and harmine, in male Sprague-Dawley rats. J Toxicol Environ Health A. 2001;64:645–660. doi: 10.1080/152873901753246241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callaway JC, Airaksinen MM, Salmela KS, Salaspuro M. Formation of tetrahydroharman (1-methyl-1,2,3,4-tetrahydro-beta-carboline) by Helicobacter pylori in the presence of ethanol and tryptamine. Life Sci. 1996;58:1817–1821. doi: 10.1016/0024-3205(96)00165-8. [DOI] [PubMed] [Google Scholar]
- 3.Fekkes D, Bernard BF, Cappendijk SL. Norharman and alcohol-dependency in male Wistar rats. Eur Neuropsychopharmacol. 2004;14:361–366. doi: 10.1016/j.euroneuro.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Bosin TR, Borg S, Faull KF. Harman in rat brain, lung and human CSF: effect of alcohol consumption. Alcohol. 1988;5:505–511. doi: 10.1016/0741-8329(88)90090-0. [DOI] [PubMed] [Google Scholar]
- 5.Spijkerman R, van den Eijnden R, van de Mheen D, Bongers I, Fekkes D. The impact of smoking and drinking on plasma levels of norharman. Eur Neuropsychopharmacol. 2002;12:61–71. doi: 10.1016/S0924-977X(01)00143-2. [DOI] [PubMed] [Google Scholar]
- 6.Yu AM, Idle JR, Krausz KW, Küpfer A, Gonzalez FJ. Contribution of individual cytochrome P450 isozymes to the O-demethylation of the psychotropic β-carboline alkaloids harmaline and harmine. J Pharmacol Exp Ther. 2003;305:315–322. doi: 10.1124/jpet.102.047050. [DOI] [PubMed] [Google Scholar]
- 7.Miksys S, Rao Y, Hoffmann E, Mash DC, Tyndale RF. Regional and cellular expression of CYP2D6 in human brain: higher levels in alcoholics. J Neurochem. 2002;82:1376–1387. doi: 10.1046/j.1471-4159.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- 8.Miksys S, Tyndale RF. Nicotine induces brain CYP enzymes: relevance to Parkinson's disease. J Neural Transm Suppl. 2006;70:177–180. doi: 10.1007/978-3-211-45295-0_28. [DOI] [PubMed] [Google Scholar]
- 9.Gearhart DA, Neafsey EJ, Collins MA. Phenylethanolamine N-methyltransferase has β-carboline 2N-methyltransferase activity: hypothetical relevance to Parkinson's disease. Neurochem Int. 2002;40:611–620. doi: 10.1016/S0197-0186(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 10.Herraiz T, Guillén H, Galisteo J. N-methyltetrahydro-β-carboline analogs of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin are oxidized to neurotoxic β-carbolinium cations by heme peroxidases. Biochem Biophys Res Commun. 2007;356:118–123. doi: 10.1016/j.bbrc.2007.02.089. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara K, Gonda T, Sawada H, et al. Endogenously occurring β-carboline induces parkinsonism in nonprimate animals: a possible causative protoxin in idiopathic Parkinson's disease. J Neurochem. 1998;70:727–735. doi: 10.1046/j.1471-4159.1998.70020727.x. [DOI] [PubMed] [Google Scholar]
- 12.Herraiz T, Guillén H, Arán VJ, Idle JR, Gonzalez FJ. Comparative aromatic hydroxylation and N-demethylation of MPTP neurotoxin and its analogs, N-methylated β-carboline and isoquinoline alkaloids, by human cytochrome P450 2D6. Toxicol Appl Pharmacol. 2006;216:387–398. doi: 10.1016/j.taap.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Louis ED, Benito-León J, Bermejo-Pareja F Neurological Disorders in Central Spain (NEDICES) Study Group. Population-based prospective study of cigarette smoking and risk of incident essential tremor. Neurology. 2008;70:1682–1687. doi: 10.1212/01.wnl.0000311271.42596.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCann SJ, Pond SM, James KM, Le Couteur DG. The association between polymorphisms in the cytochrome P-450 2D6 gene and Parkinson's disease: a case-control study and meta-analysis. J Neurol Sci. 1997;153:50–53. doi: 10.1016/S0022-510X(97)00179-2. [DOI] [PubMed] [Google Scholar]
- 15.Louis ED, Jiang W, Pellegrino KM, et al. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in essential tremor. Neurotoxicology. 2008;29:294–300. doi: 10.1016/j.neuro.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–396. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zetler G, Singbartl G, Schlosser L. Cerebral pharmacokinetics of tremor-producing harmala and iboga alkaloids. Pharmacology. 1972;7:237–248. doi: 10.1159/000136294. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Zheng W, Mao X, Shungu DC. Blood harmane is correlated with cerebellar metabolism in essential tremor: a pilot study. Neurology. 2007;69:515–520. doi: 10.1212/01.wnl.0000266663.27398.9f. [DOI] [PubMed] [Google Scholar]
- 19.Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther. 2003;306:73–83. doi: 10.1124/jpet.103.049882. [DOI] [PubMed] [Google Scholar]
- 20.Pennes HH, Hoch PH. Psychotomimetics, clinical and theoretical considerations: harmine, Win-2299 and nalline. Am J Psychiatry. 1957;113:887–892. doi: 10.1176/ajp.113.10.887. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed A, Taylor NR. The analysis of drug-induced tremor in mice. Br J Pharmacol Chemother. 1959;14:350–354. doi: 10.1111/j.1476-5381.1959.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Montigny C, Lamarre Y. Rhythmic activity induced by harmaline in the olivo-cerebello-bulbar system of the cat. Brain Res. 1973;53:81–95. doi: 10.1016/0006-8993(73)90768-3. [DOI] [PubMed] [Google Scholar]
- 23.Poirier LJ, Sourkes TL, Bouvier G, Boucher R, Carabin S. Striatal amines, experimental tremor and the effect of harmaline in the monkey. Brain. 1966;89:37–52. doi: 10.1093/brain/89.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Miwa H. Rodent models of tremor. Cerebellum. 2007;6:66–72. doi: 10.1080/14734220601016080. [DOI] [PubMed] [Google Scholar]
- 25.Miwa H, Hama K, Kajimoto Y, Kondo T. Effects of zonisamide on experimental tremors in rats. Parkinsonism Relat Disord. 2008;14:33–36. doi: 10.1016/j.parkreldis.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Fowler SC. Concurrent quantification of tremor and depression of locomotor activity induced in rats by harmaline and physostigmine. Psychopharmacology (Berl) 2001;158:273–280. doi: 10.1007/s002130100882. [DOI] [PubMed] [Google Scholar]
- 27.de Souza da Fonseca A, Pereira FR, Santos R. Validation of a new computerized system for recording and analysing drug-induced tremor in rats. J Pharmacol Toxicol Methods. 2001;46:137–143. doi: 10.1016/S1056-8719(02)00169-7. [DOI] [PubMed] [Google Scholar]
- 28.Martin FC, Thu Le A, Handforth A. Harmaline-induced tremor as a potential preclinical screening method for essential tremor medications. Mov Disord. 2005;20:298–305. doi: 10.1002/mds.20331. [DOI] [PubMed] [Google Scholar]
- 29.Stanford JA, Fowler SC. At low doses, harmaline increases forelimb tremor in the rat. Neurosci Lett. 1998;241:41–44. doi: 10.1016/S0304-3940(97)00974-9. [DOI] [PubMed] [Google Scholar]
- 30.Lutes J, Lorden JF, Beales M, Oltmans GA. Tolerance to the tremorogenic effects of harmaline: evidence for altered olivo-cerebellar function. Neuropharmacology. 1988;27:849–855. doi: 10.1016/0028-3908(88)90102-5. [DOI] [PubMed] [Google Scholar]
- 31.Lorden JF, Stratton SE, Mays LE, Oltmans GA. Purkinje cell activity in rats following chronic treatment with harmaline. Neuroscience. 1988;27:465–472. doi: 10.1016/0306-4522(88)90281-3. [DOI] [PubMed] [Google Scholar]
- 32.Stratton SE, Lorden JF. Effect of harmaline on cells of the inferior olive in the absence of tremor: differential response of genetically dystonic and harmaline-tolerant rats. Neuroscience. 1991;41:543–549. doi: 10.1016/0306-4522(91)90347-Q. [DOI] [PubMed] [Google Scholar]
- 33.O'Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55:303–310. doi: 10.1016/0306-4522(93)90500-F. [DOI] [PubMed] [Google Scholar]
- 34.Miwa H, Kubo T, Suzuki A, Kihira T, Kondo T. A species-specific difference in the effects of harmaline on the rodent olivocerebellar system. Brain Res. 2006;1068:94–101. doi: 10.1016/j.brainres.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 35.Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llinás R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang EJ, Sugihara I, Llinás R. Differential roles of apamin- and charybdotoxin-sensitive K+ conductances in the generation of inferior olive rhythmicity in vivo. J Neurosci. 1997;17:2825–2838. doi: 10.1523/JNEUROSCI.17-08-02825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Placantonakis DG, Schwarz C, Welsh JP. Serotonin suppresses subthreshold and suprathreshold oscillatory activity of rat inferior olivary neurones in vitro. J Physiol. 2000;524:833–851. doi: 10.1111/j.1469-7793.2000.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Placantonakis D, Welsh J. Two distinct oscillatory states determined by the NMDA receptor in rat inferior olive. J Physiol. 2001;534:123–140. doi: 10.1111/j.1469-7793.2001.t01-1-00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Degen J, Meier C, Van Der Giessen RS, et al. Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol. 2004;473:511–525. doi: 10.1002/cne.20085. [DOI] [PubMed] [Google Scholar]
- 42.Leznik E, Makarenko V, Llinás R. Electrotonically mediated oscillatory patterns in neuronal ensembles: an in vitro voltage-dependent dye-imaging study in the inferior olive. J Neurosci. 2002;22:2804–2815. doi: 10.1523/JNEUROSCI.22-07-02804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sotelo C, Llinás R, Baker R. Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol. 1974;37:541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- 44.Leznik E, Llinás R. Role of gap junctions in synchronized neuronal oscillations in the inferior olive. J Neurophysiol. 2005;94:2447–2456. doi: 10.1152/jn.00353.2005. [DOI] [PubMed] [Google Scholar]
- 45.De Zeeuw CI, Van Alphen AM, Hawkins RK, Ruigrok TJ. Climbing fibre collaterals contact neurons in the cerebellar nuclei that provide a GABAergic feedback to the inferior olive. Neuroscience. 1997;80:981–986. doi: 10.1016/S0306-4522(97)00249-2. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki K, Bower JM, Llinás R. Multiple purkinje cell recording in rodent cerebellar cortex. Eur J Neurosci. 1989;1:572–586. doi: 10.1111/j.1460-9568.1989.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 47.Lang EJ, Sugihara I, Llinás R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–275. doi: 10.1152/jn.1996.76.1.255. [DOI] [PubMed] [Google Scholar]
- 48.Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87:1993–2008. doi: 10.1152/jn.00477.2001. [DOI] [PubMed] [Google Scholar]
- 49.Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Giessen RS, Maxeiner S, French PJ, Willecke K, De Zeeuw CI. Spatiotemporal distribution of Connexin45 in the olivocerebellar system. J Comp Neurol. 2006;495:173–184. doi: 10.1002/cne.20873. [DOI] [PubMed] [Google Scholar]
- 51.Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Placantonakis DG, Bukovsky AA, Zeng XH, Kiem HP, Welsh JP. Fundamental role of inferior olive connexin 36 in muscle coherence during tremor. Proc Natl Acad Sci USA. 2004;101:7164–7169. doi: 10.1073/pnas.0400322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Placantonakis DG, Bukovsky AA, Aicher SA, Kiem HP, Welsh JP. Continuous electrical oscillations emerge from a coupled network: a study of the inferior olive using lentiviral knockdown of connexin36. J Neurosci. 2006;26:5008–5016. doi: 10.1523/JNEUROSCI.0146-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshall SP, van der Giessen RS, de Zeeuw CI, Lang EJ. Altered olivocerebellar activity patterns in the connexin36 knockout mouse. Cerebellum. 2007;28:1–13. doi: 10.1080/14734220601100801. [DOI] [PubMed] [Google Scholar]
- 55.Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26:1739–1748. doi: 10.1523/JNEUROSCI.3677-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teune TM, van der Burg J, de Zeeuw CI, Voogd J, Ruigrok TJ. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(SICI)1096-9861(19980309)392:2<164::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 57.Molineux ML, McRory JE, McKay BE, et al. Specific T-type calcium channel isoforms are associated with distinct burst phenotypes in deep cerebellar nuclear neurons. Proc Natl Acad Sci USA. 2006;103:5555–5560. doi: 10.1073/pnas.0601261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97:901–911. doi: 10.1152/jn.00974.2006. [DOI] [PubMed] [Google Scholar]
- 59.De Montigny C, Lamarre Y. Effects produced by local applications of harmaline in the inferior olive. Can J Physiol Pharmacol. 1975;53:845–849. doi: 10.1139/y75-116. [DOI] [PubMed] [Google Scholar]
- 60.Batini C, Bernard JF, Buisseret-Delmas C, Conrath-Verrier M, Horcholle-Bossavit G. Harmaline-induced tremor. II. Unit activity correlation in the interposito-rubral and oculomotor systems of cat. Exp Brain Res. 1981;42:383–391. doi: 10.1007/BF00237503. [DOI] [PubMed] [Google Scholar]
- 61.Llinás R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- 62.Batini C, Buisseret-Delmas C, Conrath-Verrier M. Harmaline-induced tremor. I. Regional metabolic activity as revealed by [14C]2-deoxyglucose in cat. Exp Brain Res. 1981;42:371–382. doi: 10.1007/BF00237502. [DOI] [PubMed] [Google Scholar]
- 63.Beitz AJ, Saxon D. Harmaline-induced climbing fiber activation causes amino acid and peptide release in the rodent cerebellar cortex and a unique temporal pattern of Fos expression in the olivo-cerebellar pathway. J Neurocytol. 2004;33:49–74. doi: 10.1023/B:NEUR.0000029648.81071.20. [DOI] [PubMed] [Google Scholar]
- 64.Park YG, Park HY, Lee CJ, et al. CaV3.1 is a tremor rhythm pacemaker in the inferior olive. Proc Natl Acad Sci USA. 2010;107:10731–10736. doi: 10.1073/pnas.1002995107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bernard JF, Buisseret-Delmas C, Compoint C, Laplante S. Harmaline induced tremor. III. A combined simple units, horseradish peroxidase, and 2-deoxyglucose study of the olivocerebellar system in the rat. Exp Brain Res. 1984;57:128–137. doi: 10.1007/BF00231139. [DOI] [PubMed] [Google Scholar]
- 66.Buisseret-Delmas C. Sagittal organization of the olivocerebellonuclear pathway in the rat. II. Connections with the nucleus interpositus. Neurosci Res. 1988;5:494–512. doi: 10.1016/0168-0102(88)90039-9. [DOI] [PubMed] [Google Scholar]
- 67.Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol (Berl) 1991;184:225–243. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- 68.Simantov R, Snyder SH, Oster-Granite ML. Harmaline-induced tremor in the rat: abolition by 3-acetylpyridine destruction of cerebellar climbing fibers. Brain Res. 1976;114:144–151. doi: 10.1016/0006-8993(76)91016-7. [DOI] [PubMed] [Google Scholar]
- 69.LeDoux MS, Lorden JF. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp Brain Res. 2002;145:457–467. doi: 10.1007/s00221-002-1127-4. [DOI] [PubMed] [Google Scholar]
- 70.Lorden JF, Oltmans GA, McKeon TW, Lutes J, Beales M. Decreased cerebellar 3',5'-cyclic guanosine monophosphate levels and insensitivity to harmaline in the genetically dystonic rat (dt). J Neurosci. 1985;5:2618–2625. doi: 10.1523/JNEUROSCI.05-10-02618.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milner TE, Cadoret G, Lessard L, Smith AM. EMG analysis of harmaline-induced tremor in normal and three strains of mutant mice with Purkinje cell degeneration and the role of the inferior olive. J Neurophysiol. 1995;73:2568–2577. doi: 10.1152/jn.1995.73.6.2568. [DOI] [PubMed] [Google Scholar]
- 72.Krauss JK, Wakhloo AK, Nobbe F, Tränkle R, Mundinger F, Seeger W. Lesion of dentatothalamic pathways in severe post-traumatic tremor. Neurol Res. 1995;17:409–416. [PubMed] [Google Scholar]
- 73.Elble RJ, Schieber MH, Thach WT., Jr Activity of muscle spindles, motor cortex and cerebellar nuclei during action tremor. Brain Res. 1984;323:330–334. doi: 10.1016/0006-8993(84)90308-1. [DOI] [PubMed] [Google Scholar]
- 74.Monzée J, Drew T, Smith AM. Effects of muscimol inactivation of the cerebellar nuclei on precision grip. J Neurophysiol. 2004;91:1240–1249. doi: 10.1152/jn.01124.2002. [DOI] [PubMed] [Google Scholar]
- 75.Gemba H, Sasaki K, Yoneda Y, Hashimoto S, Mizuno N. Tremor in the monkey with a cerebellar lesion. Exp Neurol. 1980;69:173–182. doi: 10.1016/0014-4886(80)90152-1. [DOI] [PubMed] [Google Scholar]
- 76.Llinás R, Mühlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamarre Y, Joffroy AJ, Dumont M, De Montigny C, Grou F, Lund JP. Central mechanisms of tremor in some feline and primate models. Can J Neurol Sci. 1975;2:227–233. doi: 10.1017/s0317167100020321. [DOI] [PubMed] [Google Scholar]
- 78.Battista AF, Nakatani S, Goldstein M, Anagnoste B. Effect of harmaline in monkeys with central nervous system lesions. Exp Neurol. 1970;28:513–524. doi: 10.1016/0014-4886(70)90189-5. [DOI] [PubMed] [Google Scholar]
- 79.Villablanca J, Riobó F. Electroencephalographic and behavioral effects of harmaline in intact cats and in cats with chronic mesencephalic transection. Psychopharmacologia. 1970;17:302–313. doi: 10.1007/BF00404235. [DOI] [PubMed] [Google Scholar]
- 80.Bekar L, Libionka W, Tian GF, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 2008;14:75–80. doi: 10.1038/nm1693. [DOI] [PubMed] [Google Scholar]
- 81.Deecher DC, Teitler M, Soderlund DM, Bornmann WG, Kuehne ME, Glick SD. Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies. Brain Res. 1992;571:242–247. doi: 10.1016/0006-8993(92)90661-R. [DOI] [PubMed] [Google Scholar]
- 82.Helsley S, Fiorella D, Rabin RA, Winter JC. Behavioral and biochemical evidence for a nonessential 5-HT2A component of the ibogaine-induced discriminative stimulus. Pharmacol Biochem Behav. 1998;59:419–425. doi: 10.1016/S0091-3057(97)00451-6. [DOI] [PubMed] [Google Scholar]
- 83.Grella B, Dukat M, Young R, et al. Investigation of hallucinogenic and related β-carbolines. Drug Alcohol Depend. 1998;50:99–107. doi: 10.1016/S0376-8716(97)00163-4. [DOI] [PubMed] [Google Scholar]
- 84.Robertson HA. Harmaline-induced tremor: the benzodiazepine receptor as a site of action. Eur J Pharmacol. 1980;67:129–132. doi: 10.1016/0014-2999(80)90020-5. [DOI] [PubMed] [Google Scholar]
- 85.Sugihara I, Lang EJ, Llinás R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–534. doi: 10.1111/j.1460-9568.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 86.Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res. 1997;770:26–29. doi: 10.1016/S0006-8993(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 87.Mousah H, Jacqmin P, Lesne M. Interaction of carbolines and some GABA receptor ligands with the GABA and the benzodiazepine receptors. J Pharmacol. 1986;17:686–691. [PubMed] [Google Scholar]
- 88.Schweri M, Cain M, Cook J, Paul S, Skolnick P. Blockade of 3-carbomethoxy-β-carboline induced seizures by diazepam and the benzodiazepine antagonists, Ro 15-1788 and CGS8216. Pharmacol Biochem Behav. 1982;17:457–460. doi: 10.1016/0091-3057(82)90304-5. [DOI] [PubMed] [Google Scholar]
- 89.Frostholm A, Evans JE, Cummings SL, Rotter A. Harmaline-induced changes in gamma aminobutyric acidA receptor subunit mRNA expression in murine olivocerebellar nuclei. Brain Res Mol Brain Res. 2000;85:200–208. doi: 10.1016/S0169-328X(00)00259-X. [DOI] [PubMed] [Google Scholar]
- 90.Ishida H, Sasa M, Takaori S, Ishida H. Effects of harmaline on membrane excitability and ATPase activity of the crayfish giant axon. Jpn J Pharmacol. 1981;31:801–807. doi: 10.1254/jjp.31.801. [DOI] [PubMed] [Google Scholar]
- 91.Splettstoesser F, Bonnet U, Wiemann M, Bingmann D, Büsselberg D. Modulation of voltage-gated channel currents by harmaline and harmane. Br J Pharmacol. 2005;144:52–58. doi: 10.1038/sj.bjp.0706024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smart L. Competitive inhibition of sodium-dependent high affinity choline uptake by harmala alkaloids. Eur J Pharmacol. 1981;75:265–269. doi: 10.1016/0014-2999(81)90553-7. [DOI] [PubMed] [Google Scholar]
- 93.McCormick SJ, Tunnicliff G. Inhibitors of synaptosomal gamma-hydroxybutyrate transport. Pharmacology. 1998;57:124–131. doi: 10.1159/000028233. [DOI] [PubMed] [Google Scholar]
- 94.Schwarz MJ, Houghton PJ, Rose S, Jenner P, Lees AD. Activities of extract and constituents of Banisteriopsis caapi relevant to parkinsonism. Pharmacol Biochem Behav. 2003;75:627–633. doi: 10.1016/S0091-3057(03)00129-1. [DOI] [PubMed] [Google Scholar]
- 95.Roberts E, Wong E, Svenneby G, Degener P. Sodium-dependent binding of GABA to mouse brain particles. Brain Res. 1978;152:614–619. doi: 10.1016/0006-8993(78)91119-8. [DOI] [PubMed] [Google Scholar]
- 96.Reid MS, Hsu K, Jr, Souza KH, Broderick PA, Berger SP. Neuropharmacological characterization of local ibogaine effects on dopamine release. J Neural Transm. 1996;103:967–985. doi: 10.1007/BF01291787. [DOI] [PubMed] [Google Scholar]
- 97.Pimpinella G, Palmery M. Interaction of β-carbolines with central dopaminergic transmission in mice: structure-activity relationships. Neurosci Lett. 1995;189:121–124. doi: 10.1016/0304-3940(95)11469-D. [DOI] [PubMed] [Google Scholar]
- 98.Cumming P, Vincent SR. Inhibition of histamine-N-methyltransferase (HNMT) by fragments of 9-amino-1,2,3,4-tetrahydroacridine (tacrine) and by β-carbolines. Biochem Pharmacol. 1992;44:989–992. doi: 10.1016/0006-2952(92)90133-4. [DOI] [PubMed] [Google Scholar]
- 99.Airaksinen MM, Lecklin A, Saano V, Tuomisto L, Gynther J. Tremorigenic effect and inhibition of tryptamine and serotonin receptor binding by β-carbolines. Pharmacol Toxicol. 1987;60:5–8. doi: 10.1111/j.1600-0773.1987.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 100.Miralles A, Esteban S, Sastre-Coll A, Moranta D, Asensio VJ, Garcia-Sevilla JA. High--affinity binding of β-carbolines to imidazoline I2B receptors and MAO-A in rat tissues: norharman blocks the effect of morphine withdrawal on DOPA/noradrenaline synthesis in the brain. Eur J Pharmacol. 2005;518:234–242. doi: 10.1016/j.ejphar.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 101.Wiklund L, Sjölund B, Björklund A. Morphological and functional studies on the serotoninergic innervation of the inferior olive. J Physiol (Paris) 1981;77:183–186. [PubMed] [Google Scholar]
- 102.Sjölund B, Björklund A, Wiklund L. The indolaminergic innervation of the inferior olive. 2. Relation to harmaline induced tremor. Brain Res. 1977;131:23–37. doi: 10.1016/0006-8993(77)90026-9. [DOI] [PubMed] [Google Scholar]
- 103.Costall B, Kelly DM, Naylor RJ. The importance of 5-hydroxytryptamine for the induction of harmine tremor and its antagonism by dopaminergic agonists assessed by lesions of the midbrain raphe nuclei. Eur J Pharmacol. 1976;35:109–119. doi: 10.1016/0014-2999(76)90305-8. [DOI] [PubMed] [Google Scholar]
- 104.Welsh JP, Chang B, Menaker ME, Aicher SA. Removal of the inferior olive abolishes myoclonic seizures associated with a loss of olivary serotonin. Neuroscience. 1998;82:879–897. doi: 10.1016/S0306-4522(97)00297-2. [DOI] [PubMed] [Google Scholar]
- 105.Mehta H, Saravanan KS, Mohanakumar KP. Serotonin synthesis inhibition in olivo-cerebellar system attenuates harmaline-induced tremor in Swiss albino mice. Behav Brain Res. 2003;145:31–36. doi: 10.1016/S0166-4328(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 106.Arshaduddin M, Kadasah S, Al Deeb S, Al Moutaery K, Tariq M. Exacerbation of harmaline-induced tremor by imipramine. Pharmacol Biochem Behav. 2005;81:9–14. doi: 10.1016/j.pbb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 107.Arshaduddin M, Al Kadasah S, Biary N, Al Deeb S, Al Moutaery K, Tariq M. Citalopram, a selective serotonin reuptake inhibitor augments harmaline-induced tremor in rats. Behav Brain Res. 2004;153:15–20. doi: 10.1016/j.bbr.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 108.Yamazaki M, Ikeda Y, Ishikawa M, Inagaki C, Tanaka C. Inhibition of harmaline induced tremor by L-threo-3, 4-dihydroxyphenylserine, an L-norepinephrine precursor. Nippon Yakurigaku Zasshi. 1976;72:363–369. doi: 10.1254/fpj.72.363. [DOI] [PubMed] [Google Scholar]
- 109.Yamazaki M, Tanaka C, Takaori S. Significance of central noradrenergic system on harmaline induced tremor. Pharmacol Biochem Behav. 1979;10:421–427. doi: 10.1016/0091-3057(79)90207-7. [DOI] [PubMed] [Google Scholar]
- 110.Kolasiewicz W, Kuter K, Nowak P, Pastuszka A, Ossowska K. Lesion of the cerebellar noradrenergic innervation enhances the harmaline-induced tremor in rats. Cerebellum. 2011;10:267–280. doi: 10.1007/s12311-011-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cox B, Potkonjak D. An investigation of the tremorgenic actions of harmine in the rat. Eur J Pharmacol. 1971;16:39–45. doi: 10.1016/0014-2999(71)90054-9. [DOI] [PubMed] [Google Scholar]
- 112.Kulkarni SK, Kaul PN. Modification by levo-propranolol of tremors induced by harmine in mice. Experientia. 1979;35:1627–1628. doi: 10.1007/BF01953232. [DOI] [PubMed] [Google Scholar]
- 113.Paul V. Involvement of β2-adrenoceptor blockade and 5-hydroxytryptamine mechanism in inhibition of harmaline-induced tremors in rats. Eur J Pharmacol. 1986;122:111–115. doi: 10.1016/0014-2999(86)90165-2. [DOI] [PubMed] [Google Scholar]
- 114.Niespodziany I, Klitgaard H, Margineanu D. Effects of brivaracetam (UCB 34714) on harmaline-induced electrophysiological changes in rat inferior olivary neurons. Soc Neurosci Abst. 2005;971:1. [Google Scholar]
- 115.Wood PL, Richard JW, Pilapil C, Nair NP. Antagonists of excitatory amino acids and cyclic guanosine monophosphate in cerebellum. Neuropharmacology. 1982;21:1235–1238. doi: 10.1016/0028-3908(82)90126-5. [DOI] [PubMed] [Google Scholar]
- 116.Koller WC. Ineffectiveness of phenoxybenzamine in essential tremor. J Neurol Neurosurg Psychiatry. 1986;49:222. doi: 10.1136/jnnp.49.2.222-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eblen F, Löschmann PA, Wüllner U, Turski L, Klockgether T. Effects of 7-nitroindazole, NG-nitro-L-arginine, and D-CPPene on harmaline-induced postural tremor, N-methyl-D-aspartate-induced seizures, and lisuride-induced rotations in rats with nigral 6-hydroxydopamine lesions. Eur J Pharmacol. 1996;299:9–16. doi: 10.1016/0014-2999(95)00795-4. [DOI] [PubMed] [Google Scholar]
- 118.Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur J Pharmacol. 2009;616:73–80. doi: 10.1016/j.ejphar.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 119.Du W, Harvey JA. Harmaline-induced tremor and impairment of learning are both blocked by dizocilpine in the rabbit. Brain Res. 1997;745:183–188. doi: 10.1016/S0006-8993(96)01148-1. [DOI] [PubMed] [Google Scholar]
- 120.Iseri PK, Karson A, Gullu KM, et al. The effect of memantine in harmaline-induced tremor and neurodegeneration. Neuropharmacology. 2011;61:715–723. doi: 10.1016/j.neuropharm.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 121.Handforth A, Bordelon Y, Frucht SJ, Quesada A. A pilot efficacy and tolerability trial of memantine for essential tremor. Clin Neuropharmacol. 2010;33:223–226. doi: 10.1097/WNF.0b013e3181ebd109. [DOI] [PubMed] [Google Scholar]
- 122.Mignani S, Bohme GA, Birraux G, et al. 9-Carboxymethyl-5H,10H-imidazo[1,2-a]indeno[1,2-e]pyrazin-4-one-2-carbocylic acid (RPR117824): selective anticonvulsive and neuroprotective AMPA antagonist. Bioorg Med Chem. 2002;10:1627–1637. doi: 10.1016/S0968-0896(01)00431-X. [DOI] [PubMed] [Google Scholar]
- 123.Kolasiewicz W, Kuter K, Wardas J, Ossowska K. Role of the metabotropic glutamate receptor subtype 1 in the harmaline-induced tremor in rats. J Neural Transm. 2009;116:1059–1063. doi: 10.1007/s00702-009-0254-5. [DOI] [PubMed] [Google Scholar]
- 124.Shinozaki H, Hirate K, Ishida M. Further studies on quantification of drug-induced tremor in mice: effects of antitremorgenic agents on tremor frequency. Exp Neurol. 1985;88:303–315. doi: 10.1016/0014-4886(85)90193-1. [DOI] [PubMed] [Google Scholar]
- 125.Tariq M, Arshaduddin M, Biary N, Al Moutaery K, Al Deeb S. Baclofen attenuates harmaline induced tremors in rats. Neurosci Lett. 2001;312:79–82. doi: 10.1016/S0304-3940(01)02166-8. [DOI] [PubMed] [Google Scholar]
- 126.Meert TF. Pharmacological evaluation of alcohol withdrawal-induced inhibition of exploratory behaviour and supersensitivity to harmine-induced tremor. Alcohol Alcohol. 1994;29:91–102. [PubMed] [Google Scholar]
- 127.Rappaport MS, Gentry RT, Schneider DR, Dole VP. Ethanol effects on harmaline--induced tremor and increase of cerebellar cyclic GMP. Life Sci. 1984;34:49–56. doi: 10.1016/0024-3205(84)90329-1. [DOI] [PubMed] [Google Scholar]
- 128.Rogers J, Madamba SG, Staunton DA, Siggins GR. Ethanol increases single unit activity in the inferior olivary nucleus. Brain Res 1986 22. 385:253–262. doi: 10.1016/0006-8993(86)91071-1. [DOI] [PubMed] [Google Scholar]
- 129.Garbourg Y, Welsh JP. Systemic ethanol induces inferior olive synchrony and oscillation in vivo. Soc Neurosci Abst. 2000;743:16. [Google Scholar]
- 130.De Ryck M, Matagne A, Kenda B, Michel P, Klitgaard H. Contrasting effects of UCB 34714 and drugs for essential tremor on harmaline-induced elicited versus spontaneous tremor and sedation in rats. Mov Disord. 2004;19:S443. [Google Scholar]
- 131.Handforth A, Martin FC, Kang GA, Vanek Z. Zonisamide for essential tremor: an evaluator-blinded study. Mov Disord. 2009;24:437–440. doi: 10.1002/mds.22418. [DOI] [PubMed] [Google Scholar]
- 132.Stöhr T, Lekieffre D, Freitag J. Lacosamide, the new anticonvulsant, effectively reduces harmaline-induced tremors in rats. Eur J Pharmacol. 2008;589:114–116. doi: 10.1016/j.ejphar.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 133.Gironell A, Pagonabarraga J, Pascual-Sedano B, Kulisevsky J. Lacosamide, another therapeutic failure in essential tremor: an open-label trial. Mov Disord. 2011;26:183–184. doi: 10.1002/mds.23296. [DOI] [PubMed] [Google Scholar]
- 134.Elble RJ, Biondi DM, Ascher S, Wiegand F, Hulihan J. Carisbamate in essential tremor: brief report of a proof of concept study. Mov Disord. 2010;25:634–638. doi: 10.1002/mds.22872. [DOI] [PubMed] [Google Scholar]
- 135.Martin FC, Handforth A. Carbenoxolone and mefloquine suppress tremor in the harmaline mouse model of essential tremor. Mov Disord. 2006;21:1641–1649. doi: 10.1002/mds.20940. [DOI] [PubMed] [Google Scholar]
- 136.Zappalà A, Parenti R, La Delia F, Cicirata V, Cicirata F. Expression of connexin57 in mouse development and in harmaline-tremor model. Neuroscience. 2010;171:1–11. doi: 10.1016/j.neuroscience.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 137.Sinton CM, Krosser BI, Walton KD, Llinás RR. The effectiveness of different isomers of octanol as blockers of harmaline-induced tremor. Pflugers Arch. 1989;414:31–36. doi: 10.1007/BF00585623. [DOI] [PubMed] [Google Scholar]
- 138.Handforth A, Homanics GE, Covey DF, et al. T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor. Neuropharmacology. 2010;59:380–387. doi: 10.1016/j.neuropharm.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quesada A, Bui PH, Homanics GE, Hankinson O, Handforth A. Comparison of mibefradil and derivative NNC 55-0396 effects on behavior, cytochrome P450 activity, and tremor in mouse models of essential tremor. Eur J Pharmacol. 2011;659:30–36. doi: 10.1016/j.ejphar.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bui PH, Quesada A, Handforth A, Hankinson O. The mibefradil derivative NNC55-0396, a specific T-type calcium channel antagonist, exhibits less CYP3A4 inhibition than mibefradil. Drug Metab Dispos. 2008;36:1291–1299. doi: 10.1124/dmd.107.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Al-Deeb S, Al-Moutaery K, Arshaduddin M, Biary N, Tariq M. Effect of acute caffeine on severity of harmaline induced tremor in rats. Neurosci Lett. 2002;325:216–218. doi: 10.1016/S0304-3940(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 142.Biary N, Arshaduddin M, Al Deeb S, Al Moutaery K, Tariq M. Effect of lidocaine on harmaline-induced tremors in the rat. Pharmacol Biochem Behav. 2000;65:117–121. doi: 10.1016/S0091-3057(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 143.Berti F, Fano M, Folco GC, Longiave D, Omini C. Inhibition of harmaline induced tremors by 16 (S)-16-methyl PGE2 in different mammalian species: a correlation with central cyclic nucleotides and prostaglandins. Prostaglandins. 1978;15:867–874. doi: 10.1016/0090-6980(78)90153-3. [DOI] [PubMed] [Google Scholar]
- 144.Shuto H, Kataoka Y, Kanaya A, Matsunaga K, Sueyasu M, Oishi R. Enhancement of serotonergic neural activity contributes to cyclosporine-induced tremors in mice. Eur J Pharmacol. 1998;341:33–37. doi: 10.1016/S0014-2999(97)01441-6. [DOI] [PubMed] [Google Scholar]
- 145.Zoethout RW, Iannone R, Bloem BR, et al. The effects of a novel histamine-3 receptor inverse agonist on essential tremor in comparison to stable levels of alcohol. J Psychopharmacol. 2012;26:292–302. doi: 10.1177/0269881111398685. [DOI] [PubMed] [Google Scholar]
- 146.Scarmeas N, Louis ED. Mediterranean diet and essential tremor. A case-control study. Neuroepidemiology. 2007;29:170–177. doi: 10.1159/000111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Louis ED, Faust PL, Vonsattel JP. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 148.Kronenbuerger M, Gerwig M, Brol B, Block F, Timmann D. Eyeblink conditioning is impaired in subjects with essential tremor. Brain. 2007;130:1538–1551. doi: 10.1093/brain/awm081. [DOI] [PubMed] [Google Scholar]
- 149.Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci. 1993;114:45–48. doi: 10.1016/0022-510X(93)90047-3. [DOI] [PubMed] [Google Scholar]
- 150.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. 1994;36:636–642. doi: 10.1002/ana.410360413. [DOI] [PubMed] [Google Scholar]
- 151.Boecker H, Wills AJ, Ceballos-Baumann A, et al. The effect of ethanol on alcohol-responsive essential tremor: a positron emission tomography study. Ann Neurol. 1996;39:650–658. doi: 10.1002/ana.410390515. [DOI] [PubMed] [Google Scholar]
- 152.Shill HA, Bushara KO, Mari Z, Reich M, Hallett M. Open-label dose-escalation study of oral 1-octanol in patients with essential tremor. Neurology. 2004;62:2320–2322. doi: 10.1212/WNL.62.12.2320. [DOI] [PubMed] [Google Scholar]
- 153.Nahab FB, Wittevrongel L, Ippolito D, et al. An open-label, single-dose, crossover study of the pharmacokinetics and metabolism of two oral formulations of 1-octanol in patients with essential tremor. Neurotherapeutics. 2011;8:753–762. doi: 10.1007/s13311-011-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Handforth A, Martin FC. Pilot efficacy and tolerability: a randomized, placebo-controlled trial of levetiracetam for essential tremor. Mov Disord. 2004;19:1215–1221. doi: 10.1002/mds.20147. [DOI] [PubMed] [Google Scholar]
- 155.Frucht SJ, Houghton WC, Bordelon Y, Greene PE, Louis ED. A single-blind, open-label trial of sodium oxybate for myoclonus and essential tremor. Neurology. 2005;65:1967–1969. doi: 10.1212/01.wnl.0000188670.38576.bd. [DOI] [PubMed] [Google Scholar]