Abstract
Background
The transcription factor Oct-4, is an important marker of undifferentiating level and a key regulating factor for maintenance of pluripotency in cells. Establishment of an Oct-4 promoter-based reporter system is an appropriate tool for monitoring the differentiation of embryonic stem cells both in vivo and in vitro.
Methods
In the present study, we report construction of a recombinant vector, pDB2 Oct4 promoter/EGFP, in which expression of Enhanced Green Fluorescent Protein (EGFP) was controlled by the mouse Oct-4 promoter.
Results
In transfected mouse embryonic stem cells with this vector, EGFP was predicted to be specifically expressed in pluripotency state. After transfection, high-level expression of EGFP under the control of Oct-4 promoter was observed in manipulated embryonic stem cells.
Conclusion
Thus, our new cellular reporter showed that both the properties of embryonic cells and expression the EGFP could be of great help in studying the differentiating and reprogramming mechanisms of mESCs.
Keywords: Embryonic stem cells, Enhanced green fluorescent protein, Mice, Transcription factors
Introduction
A new potential research area has been developed in the field of stem cell research, since the creation of inducible stem cells in 2006 (1), which enables identifying critical genes and factors responsible for stem cell reprogramming. Moreover, a large number of methods and reports have been described to study the pluripotency sate in the stem cells (2). The pluripotency, which is a fascinating feature of stem cells, is controlled through gene expression regulation by several transcription factors including Oct-4 (POU transcription factor), Sox2, Klf4, and c-Myc (1, 3).
Among these transcription factors, Oct-4 is one of the well-characterized transacting elements, and is sufficient to generate pluripotent stem cells independently (4). In fact, the Oct-4 is a protein of Pit-Oct-Unc family (5) which is characterized by exclusive expression in blastomeres, in the inner cell mass of the blastocyst, and in the germ cell lineage (6, 7). Although Oct-4 is expressed initially in all blastomeres, its expression is decreased during gastrulation, when cells differentiate into the three germ layers, and there is no apparent expression in somatic lineages. Interestingly, Oct4 is dispensable for both self-renewal and maintenance of somatic stem cells in the adult mammal cells (8). Moreover, the expression level of Oct-4 shows the dual role of Oct4 in cell differentiation, and Oct4 not only acts on the control of pluripotency, but also regulates differentiation programs in embryonic cells, according to its level of expression (9). Therefore, Oct-4 expression must be closely regulated, as any little change in its expression triggers differentiation state of the cells.
Thus, the regulation of Oct-4 expression using different (bio)-chemical agents and its effects on downstream cellular events remains as an interesting research field in manipulation of cellular fates (10, 11). Furthermore, understanding the molecular events of Oct-4 expression under the influence of different drugs would be required for drug screening (12, 13), in the term of medicinal experiments. The expression of Oct-4 is regulated by Oct-4 gene promoter whose structure has been studied extensively (14–16). It was reported that the Oct-4 gene regulatory region consists of at least three upstream elements. Moreover, in mouse, it was proposed that there are several important elements in the Oct-4 promoter including putative Sp1/Sp3 binding site, overlapping hormone responsive element, and a repeated motif (CCCA/TCCC) may be involved in Oct-4 expression regulation (14). Currently, Oct-4 expression analysis under the control of respective promoter is considered as the most valid marker for epigenetic reprogramming and pluripotency state analyses (17). Furthermore, the expression of Oct4 usually is studied by several methods, including:
PCR-based technologies: The analysis of Oct-4 expression was extensively carried out using different PCR strategies such as semi-quantitative RT-PCR (18) and real time PCR (19),
Blotting techniques: Both southern and western blot analyses were used for the expression studies of OcT-4 at RNA and protein levels, respectively (20, 21),
Cell imaging: The expression of Oct-4 in intact cells was shown using different reports like luciferase (22) and GFP (23) under the control of Oct-4 promoter.
Although the analyses of expression by PCR or blotting methods are effective techniques, especially when an unknown system is being studied, these methods are time-consuming and usually need the destruction of cell or tissue integrity. While, imaging technology is an interesting method that provides a fast and real-time protocol for studying the cell differentiation-promoting agents. Using the same approach, a cell line of the mouse embryonic stem cells (mESCs)-Royan B1- was-stably transfected with the EGFP under the control of the Oct4 promoter, to produce a whole-(mESCs) cell bio-reporter.
Materials and Methods
Genomic PCR and T/A cloning
Genomic DNA was extracted from mouse mesenchymal cells using the genomic DNA extraction kit (Bioneer, Korea) according to the protocol.
In order to amplify mouse Oct-4 promoter, three set of primers were designated based on the sequence data for Oct-4 promoter (NCBI data for mouse chromosome 17, region: 21834554-21836862) (Table 1). Oligonucleotide primers were ordered from Metabion Company (Germany). The genomic DNA was used as template for the Polymerase Chain Reaction (PCR)(24). Utilizing Ex taq DNA polymerase (TaKaRa, Japan) two sets of PCR were carried out primary amplifications of the two DNA fragments with the size of 1221 and 1292 bp using F1 and R1 primer set and F2 and R2 primer set, respectively. Both fragments were utilized as templates in the next step for SOE-PCR under the following conditions: initial denaturation at 94°C for 5 min, 25 cycles of 94°C for 1 min, 65°C for 1 min and 72°C for 2 min, and a final extension for 5 min at 72°C. Then, the primary PCR pro-ducts were extracted using gel-extraction kit (Qiagen, Germany) to remove excess primers and templates, and the resulting fragments from the primary PCRs were mixed in a 1:1 molar ratio. Thus, the amount of DNA added to the second PCR (SOE-PCR) was approxi-mately 100 ng. SOE-PCR conditions were similar with those of the primary PCRs, ex-cept that F1 and R2 primers were added to amplify a 2309 bp fragment of DNA. Finally, a nested PCR was performed using F3 and R3 primers with 2 µl the SOE-PCR product as a template.
Table 1.
List of primers used in this study
| Name | Sequence (5’- 3’) |
|---|---|
| F1 | ATGTCTCTTGTCCTGGCCAGTGAGTCACC |
| R1 | AATCCCCTCACACAAGACTTCCCCAGC |
| F2 | TAGGGAAGTTCAGGGTAGGCTCTCTGC |
| R2 | AAGGCGAAGTCTGAAGCCAGGTGTC |
| F3 | ATTAATGAATACAGACAGGACTGCTGGGCTGC VspI |
| R3 | AGCTAGCTGGAAAGACGGCTCACCTAGGGACG NheI |
F and R, are referred as forward and reverse primers, respectively
In order to amplify mouse Oct-4 promoter, three set of primers were designated based on the sequence data for Oct-4 promoter (NCBI data for mouse chromosome 17, region: 21834554-21836862) (Table 1). Oligonucleotide primers were ordered from Metabion Company (Germany). The genomic DNA was used as template for the Polymerase Chain Reaction (PCR)(24). Utilizing Ex taq DNA polymerase (TaKaRa, Japan) two sets of PCR were carried out primary amplifications of the two DNA fragments with the size of 1221 and 1292 bp using F1 and R1 primer set and F2 and R2 primer set, respectively. Both frag-ments were utilized as templates in the next step for SOE-PCR under the following con-ditions: initial denaturation at 94°C for 5 min, 25 cycles of 94°C for 1 min, 65°C for 1 min and 72°C for 2 min, and a final extension for 5 min at 72°C. Then, the primary PCR products were extracted using gel-extraction kit (Qiagen, Germany) to remove excess primers and templates, and the resulting fragments from the primary PCRs were mixed in a 1:1 molar ratio. Thus, the amount of DNA added to the second PCR (SOE-PCR) was approximately 100 ng. SOE-PCR conditions were similar with those of the primary PCRs, except that F1 and R2 primers were added to amplify a 2309 bp fragment of DNA. Finally, a nested PCR was performed using F3 and R3 primers with 2 µl the SOE-PCR product as a template.
Thermal program was carried out based on the following conditions: 1 cycle of 5 min at 95°C as denaturation, 35 cycles of 1 min at 94°C, 1 min at 60°C and 2 min at 72°C and 1 final cycle of 15 min. at 72°C. Amplicon with expected size (2177 bp) was purified by gel extraction kit (Qiagen, Germany). The purified product was then cloned in a T/A cloning vector using T/A cloning kit and pTZ57/R cloning vector (Fermentas) according to the manual of the manufacturer. DH5α competent cells were transformed by the product of the T/A cloning reaction and colony selection was performed by white and blue screening method on LB plates containing ampicillin (100 µg/ml), IPTG and X-gal. This vector was named pTZ57/Oct-4 promoter plasmid.
Construction of Oct-4 promoter/EGFP and ▵ promoter/EGFP cassettes
A mammalian expression vehicle (25) including pCMV-Int and pDB2, encoding PhiC31 integrase and EGFP coding region flanked by the cytomegalovirus promoter respectively, was kindly provided by Prof. Calos (Stanford University, USA).
In order to construct a recombinant pDB2 plasmid expressing EGFP under the control of Oct4 promoter, a fragment encompassing the sequences of CMV promoter in pDB2 was removed by double digestion with NheI and VspI (Fermentas, Lithuania). Subsequently, the recombinant pTZ57/Oct-4 promoter plasmid was double digested with the same restricted enzymes. To obtain Oct-4 promoter/EGFP cassette, the ligation was performed with digested pDB2 and the fragment containing Oct4 promoter using DNA Ligation Kit (Takara, Japan). Similarly, a plasmid without the CMV promoter (▵ promoter/EGFP) was constructed using digested pDB2 plasmid which its ends were blunted by klenow and ligation reactions. This construct was used as a negative control.
Again, DH5α competent cells were transformed by the ligation mixture and positive colonies were screened on LB plates containing kanamycin (30 µg/ml). Colony PCR was performed using F1 and R1 primers to select bacterial colonies with the recombinant plasmids. Selected positive colonies were cultured overnight and recombinant plasmids were purified by Miniprep Kit (Qiagen) and sequenced (Metabion company, Germany) to ensure the accurate cloning of Oct4 promoter without any mutation.
Mouse embryonic stem cells (mESCs) culture and transfection
Royan B1, mESCs derived from the C57BL/ 6 strain of mouse (26) were grown on a feeder layer of the primary Mouse Embryonic Fibroblasts (MEF) in tissue culture flasks. Cells were maintained in ESCs medium that consisted of DMEM (Gibco) containing 15% fetal bovine serum (Gibco), 0.1 mM β-mercap-toethanol (Sigma), 2 mM glutamine (Gibco), 0.1 mM non-essential amino acids (Sigma) and 1000 IU/ml leukemia inhibitory factor (Chemicon).
Transient transfection of mESCs was performed by electroporation. Approximately 3.5×106 of mESCs were re-suspended in PBS buffer (250 µl) and mixed with the recombinant plasmids, including Oct-4 promoter/ EGFP (10 µg) and negative control. Moreover, pCMVInt (10 µg) was added to each tube to perform co-transfection. Each cell/DNAs mixture was transferred into an electroporation cuvette (4 mm gap) and pulsed in the gene pulser using the following conditions: U=280 V, C=500 µF.
After electroporation, mESCs were allowed to spread on pre-coated gelatin-plastic flasks containing mitomycin C-inactivated feeder layer of primary cultures of mouse embryonic fibroblasts (MEF) in ESC medium containing G418 (Sigma, USA). Cell culture incubation was performed for 10 days in a humidified atmosphere of 5% CO2 at 37°C. Finally, the preliminary screening was carried out for pDB2/Oct-4 promoter by PCR.
Transient and stable cell line
Two independent G418-resistant colonies were picked up, cultured and routinely passaged every 2 days in ESCs medium containing 100 µM G418 (Invitrogen). After 4 passages, G418-resistant ESCs were obtained and grown on a feeder layer of primary mouse embryonic fibroblasts. Genomic DNA of the stable cell lines were extracted using genomic DNA extraction kit and the presence of the EGFP was shown in both Oct4 promotor/ EGFP and ▵ prompter/EGFP by PCR.
Fluorescence microscopy and flow cytometry analysis
The percentage of GFP expressing cells and fluorescence intensity was assessed by flow cytometry. Control cells, ▵ promoter/ EGFP cells, were used to determine the average number of GFP-positive cells. For the flow cytometry analysis, the ESCs were isolated from MEFs by treatment of the cells with trypsin-ethylenediamine tetraacetic acid (EDT A) solution (Gibco). The supernatant containing cells was then collected and centrifuged (6 min, 1,200 rpm). The isolated cells were resuspended (5×105 cells/ml in phosphate buffered saline (PBS) and assessed by a flow cytometer (Becton Dickinson) at 488 nm. Acquired data were then analyzed using Win MDI software.
Results
In this study, a new whole-cell bio-reporter based on mouse embryonic stem cell, Royan B1, and EGFP under the control of Oct-4 promoter was developed, and its initial functionality was shown using the fluorescence microscopy and flow cytometery. The new bio-reporter may be used for screening the effect of cell differentiating agents as well as the effect of drugs on Oct-4 expression.
Isolating the Oct-4 promoter and cloning
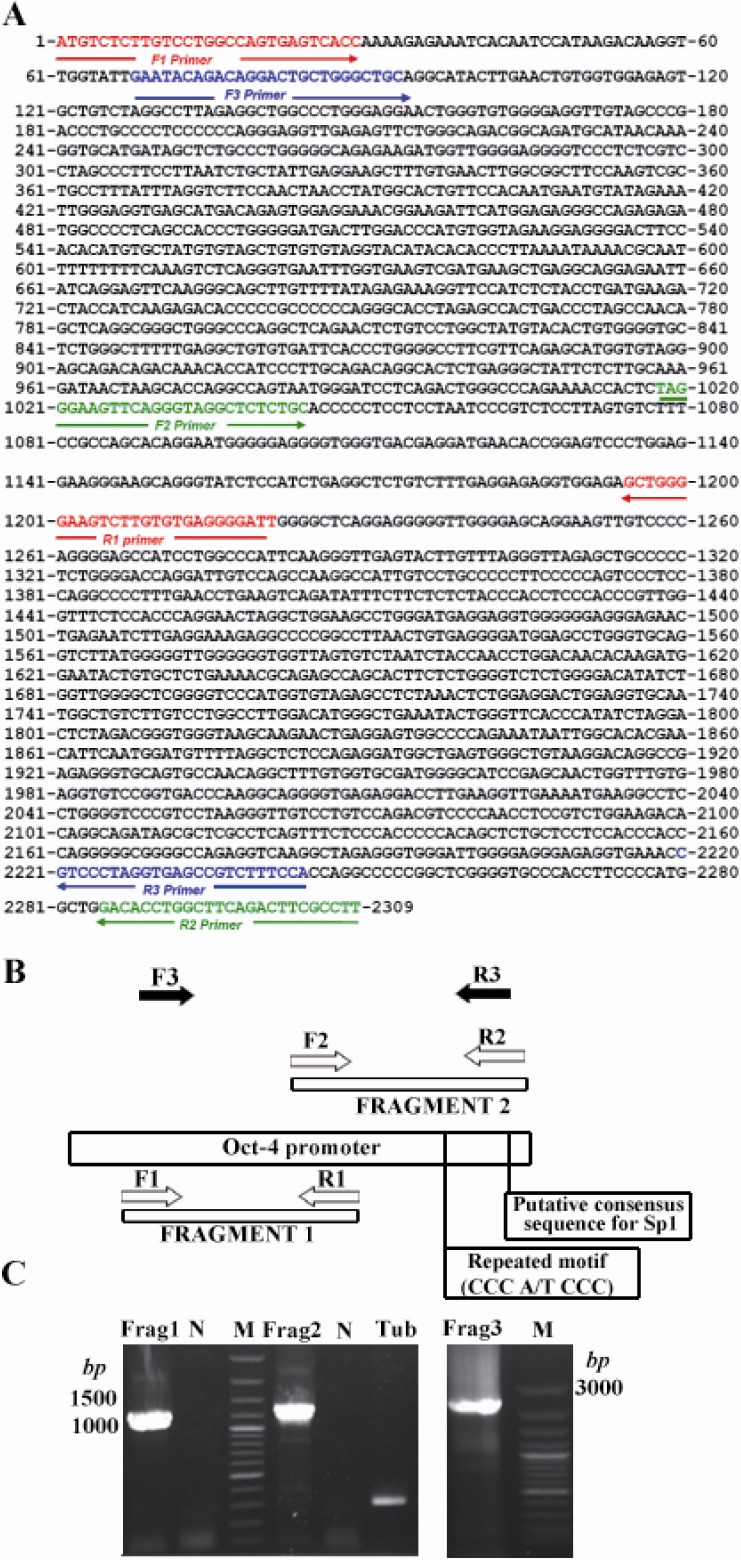
To obtain the sequence of the basic elements of Oct-4 promoter, several sets of PCR on the basis of the DNA sequence of mouse mesenchymal cells were carried out using designated primer pairs (Figures 1A and B). Several fragments were obtained by SOE-PCR. Fragment 1 consists of 1221 bp (Figure 1C, left hand panel, Frag 1) which corresponds to the upstream part of the Oct-4 promoter. Fragment 2 contains 1292 bp, which includes both repeated motif (CCC A/T CCC) and putative consensus sequence for Sp1 (Figure 1C, left hand panel, Frag 2). Finally, the full-length of Oct-4 promoter was amplified by nested PCR using cloning primers. Amplicon was approximately 2.17 kb and it was extracted from agarose gel. Then it was inserted into the T/A vector and was sequenced (Figure 1C, right hand panel, Frag 3). The sequence has shown 100% identity to Oct-4 promoter sequence (GenBank accession no. NT_039649.7). Finally, cloning of the amplicon was achieved by insertion into pDB2 vector in place of CMV promoter. The recombinant vector was named pDB2-Oct4 (Figure 2A).
Figure 1.
Position of the fragments and PCR products during the constructing of Oct-4 promoter. A) The orientation of the primers used for the amplification of each fragment is indicated by arrows; B) Schematic representation for the PCRs for cloning various parts of Oct-4 promoter; C) Product band for each fragment after electrophoresis is shown. Frag 1 (1221 bp) and 2 (1292 bp) are the products of first and second rounds of PCR which were used as templates for the SOE-PCR to amplify the Frag 3 as the respective product with the length of 2309 bp. M is 100 bp ladder (Fermentas)
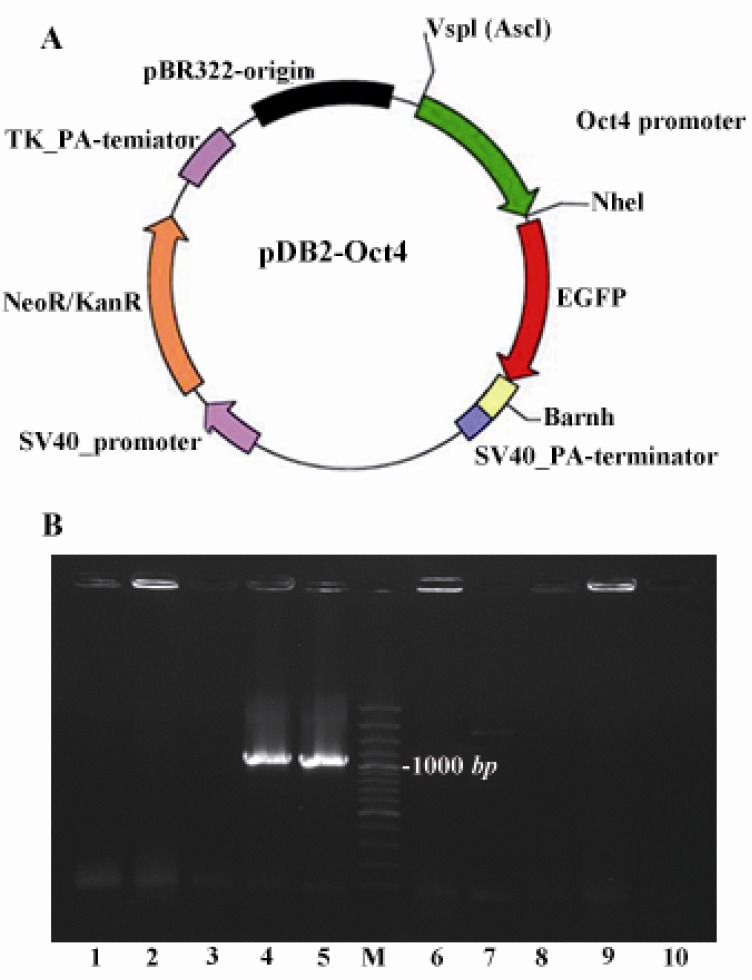
Figure 2.
A) Schematic map of the Oct-4 promoter/EGFP cassette in pDB2 vector; B) Bacterial colony insert check on transformed colonies was evident that two independent colonies out of ten colonies contained recombinant vector as described in the text. M is 100 bp ladder (Fermentas)
Bacterial colony insert check on the transformed colonies was evident that two independent colonies out of ten colonies contained the recombinant vector, pDB2-Oct4 (Figure 2B). This recombinant expression vector encoding the EGFP reporter gene in conjunction with Oct-4 transcriptional response element was a reporter cassette user extracted and used for the next experiment.
Development of stable cell line
In order to develop an Oct-4 based cellular reporter line as a pluripotency marker, mESCs, Royan/B1cells were transfected with aforementioned reporter cassette using a non-viral strategy, which a reporter embryonic cell line was created. Transfection was carried out using both user pCMVInt and the reporter construct, and the transient cells were obtained with high efficiency (data not shown). Cells were screened by PCR and RT-PCR to confirm the presence of GFP (data not shown). Positive colonies were passaged four times in the presence of G418 to generate stably transfected cell line. The results showed that reporter cassette was able to express both EGFP and G418 resistance coding sequences for selection in mouse ES cells.
Live cell imaging
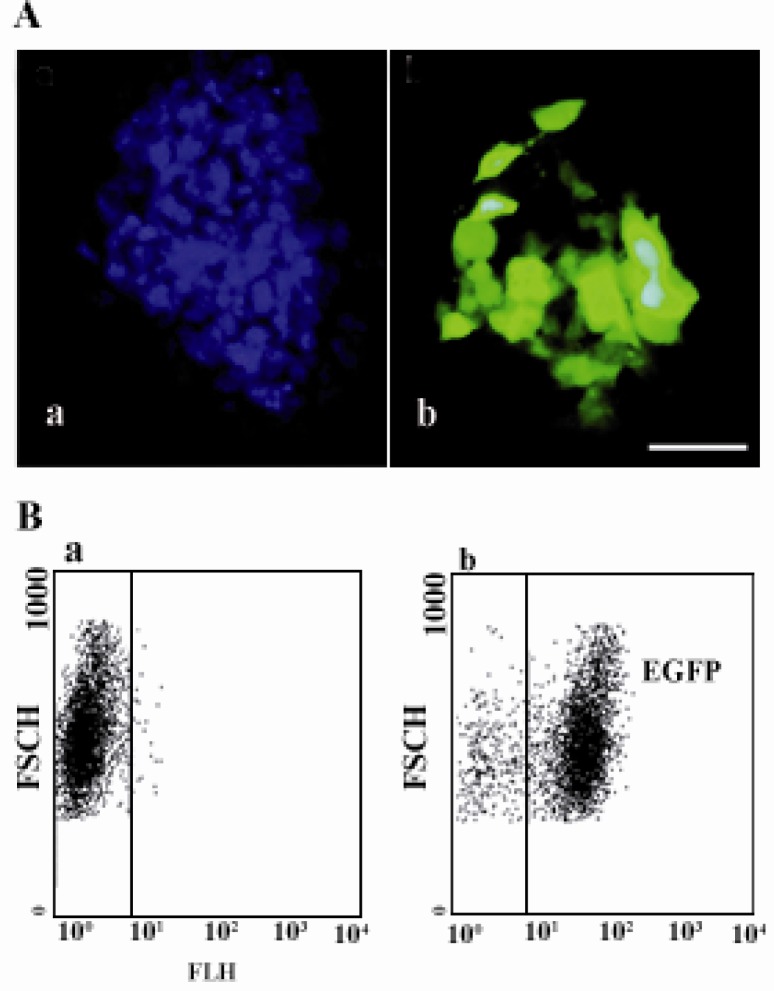
To confirm that the new reporter from mESCs is functional, live cell imaging was performed. Both the stable mESCs and control cell lines were grown in pre-coated gelatin-plastic flasks containing feeder layer of neomycin resistance MEFs in ESC medium which were utilized at 80-90% of confluency. The ability of the new cell reporter to produce detectable signal was assayed using the fluorescence microscopy. Interestingly, large number of cellular reporter expressed EGFP under the control of Oct4, and were imaged under fluorescence microscopy as shown in Figure 3A. The results indicated that the screened Oct-4 promoter/EGFP cell line was the clonal population that exhibits the strongest expression in comparison to the control and interestingly retains the embryonic features of the parental Royan /B1 cells.
Figure 3.
A) Fluorescence microscopic imaging of EGFP reporter plasmid expression in mESCs (b) was merged with DAPI (a) Bar is 100 µM; B) Flow cytometric assay of manipulated mESCs. Two-dimensional dot plot of green fluorescence protein in Oct4-GFP negative cells is shown in left (a) and RB1 Oct4-GFP positive cells is shown in right (b)
Flow cytometry analysis
The quantitative studies of the new Oct-4 based gene reporter was carried out using the flow cytometry. Both the new cell reporter and control cell [(stably transformed with pDB2 (▵ promoter/EGFP) plasmid)] lines were treated by trypsin and assayed using a FACS with excitation at 488 nm for EGFP. The results were collected at 530/30 nm and the expression efficiency of EGFP in Royan/ B1 cell was analyzed using WinMDI 2.9 software. As shown in Figure 3B, the stably cellular reporter line that constitutively expressed EGFP could be detected with strong signal (up to 95%) by flow cytometry (Figure 3B). The results were consistent with the results obtained from fluorescence microscopy which serves as a qualitative index of Oct4 activity, and it seems that the new reporter system-Royan B1 Oct4/EGFP-allows analyzing the effect of differentiation-prompting agents with high efficiency.
Discussion
The whole-cell reporters for studying components such as differentiation promoting agents, apoptosis inducer factors and small metabolites are potentially useful tools for the real-time monitoring of cellular events (27, 28). In this study, we created a new cell reporter from a mESC for cell imaging. The specific nature of this new reporter is a simple alternative to in vitro methods like real-time PCR and blotting, which are relatively time-consuming and need cell disruption. The new cellular reporter consists of user promoter from Oct-4 promoter fused to EGFP.
It was reported that Oct-4 is critically involved in the self-renewal of undifferentiated embryonic stem cells, and expression of this transcription factor is regarded as the most valid and common marker for epigenetic reprogramming and pluripotency, both in vivo and in vitro (9, 29). Moreover, it was proposed that the full-length Oct-4 promoter has two critical domains which are important for Oct-4 regulation (14). In order to obtain an efficient reporter for pluripotency, a 2.1 kb fragment containing key response elements of Oct-4 was ligated by the flank of EGFP (Figure 2). Therefore, the new reporter cassette may provide a powerful tool to study cell differentiation-promoting agents.
Moreover, considering EGFP as a reporter has several advantages comparing to other bioluminescent reporters. In fact, the protein accumulation of EGFP as a reporter of gene expression could be directly observable in living cells. Especially, it lacks the requirement for an exogenous cofactor or substrate (30). Therefore, the assay of Royan B1 Oct4/ EGFP as a cellular reporter could simplify the required strategies.
Non-viral transfection strategy based on φC31 integrase system was adopted for the integration of the reporter cassette into the genome of Royan /B1 cells. In comparison to other conventional methods such as lentiviral vectors or non-integrating vectors, this system is fast, efficient and non invasive (25). More-over, compared to other non-specific methods previously reported for integration, the obtained cell line shows a high level expression of EGFP (Figure 3A). It was reported that the integration of gene cassette using φ C31 integrase is specific and probably carried out in exonic locations (25). These areas are probably more suitable for the expression of the interested sequences and may describe the high efficiency of EGFP expression in Royan/ B1 cells.
Moreover, flow cytometric studies showed that the cellular reporter ex-pressed EGFP under the control of Oct-4 promoter up to 95% (Figure 3B). Therefore, the new cellular reporter showed the expression of EGFP with high efficiency. The cell reporter may be a useful tool for subsequent studies such as screening for drugs or agents which induce differentiation.
Conclusion
One of the major properties of stem cells is self-renewal property which exerts continuous tissue and cell regeneration for the persistence of tissue homeostasis and repair. Oct-4 has important role for this feature of stem cells. Therefore, Oct-4 promoter-based reporter system provides an opportunity for studying the differentiating levels of embryonic stem cells, and for considering the effect of biochemical agents on differentiation. mESCs Oct4/EGFP is a whole-cell reporter for assay Oct-4 promoter activity. This new reporter system is functional and can be studied by both fluorescence microscopy and flow cytometry. The reporter is unique in respect to high level expression of EGFP and provides a high-sensitivity tool for further analysis and it seems it may have a potential application for high-throughput screening of drugs.
Acknowledgement
This study was funded by a grant in aid of research from Royan Institute in support of Reza Ghorbani for obtaining his M.Sc. degree from the University of Isfahan.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24(20):2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith K, Dalton S. Myc transcription factors: key regulators behind establishment and maintenance of pluripotency. Regen Med. 2010;5(6):947–959. doi: 10.2217/rme.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JB, Sebastiano V, Wu G, Araúzo-Bravo M J, Sasse P, Gentile L, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Latchman DS. POU family transcription factors in the nervous system. J Cell Physiol. 1999;179(2):126–133. doi: 10.1002/(SICI)1097-4652(199905)179:2<126::AID-JCP2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby P, et al. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 7.Rappolee D, Basilico C, Patel Y, Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development. 1994;120(8):2259–2269. doi: 10.1242/dev.120.8.2259. [DOI] [PubMed] [Google Scholar]
- 8.Berg JS, Goodell MA. An argument against a role for Oct4 in somatic stem cells. Cell Stem Cell. 2007;1(4):359–360. doi: 10.1016/j.stem.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24(4):372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 10.Lee KL, Keat Lim SK, Orlov YL, Yit LY, Yang H, Ang LT, et al. Graded nodal/activin signaling titrates conversion of quantitative phospho-smad2 levels into qualitative embryonic stem cell fate decisions. PLoS Genetics. 2011;7(6):e1002130. doi: 10.1371/journal.pgen.1002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blandino G, Deppert W, Hainaut P, Levine A, Lozano G, Olivier M, et al. Mutant p53 protein, master regulator of human malignancies: a report on the fifth mutant p53 workshop. Cell Death Differ. 2012;19(1):180–183. doi: 10.1038/cdd.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaccarino FM, Urban AE, Stevens HE, Szekely A, Abyzov A, Grigorenko EL, et al. Annual research review: The promise of stem cell research for neuropsychiatric disorders. J Child Psychol Psychiatry. 2011;52(4):504–516. doi: 10.1111/j.1469-7610.2010.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soto-Gutierrez A, Tafaleng E, Kelly V, Roy-Chowdhury J, Fox IJ. Modeling and therapy of human liver diseases using induced pluripotent stem cells: How far have we come? Hepatology. 2011;53(2):708–711. doi: 10.1002/hep.24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordhoff V, Hübner K, Bauer A, Orlova I, Malapetsa A, Schöler HR. Comparative analysis of human, bovine, and murine Oct-4 upstream promoter sequences. Mamm Genome. 2001;12(4):309–317. doi: 10.1007/s003350010279. [DOI] [PubMed] [Google Scholar]
- 15.Yang HM, Do HJ, Oh JH, Kim JH, Choi SY, Cha KY, et al. Characterization of putative cis-regulatory elements that control the transcriptional activity of the human Oct4 promoter. J Cell Biochem. 2005;96(4):821–830. doi: 10.1002/jcb.20588. [DOI] [PubMed] [Google Scholar]
- 16.Simonsson S, Gurdon J. DNA demethylation is necessary for the epigenetic reprogramming of somatic cell nuclei. Nat Cell Biol. 2004;6(10):984–990. doi: 10.1038/ncb1176. [DOI] [PubMed] [Google Scholar]
- 17.Pesce M, Schöler HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19(4):271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 18.Ono M, Kajitani T, Uchida H, Arase T, Oda H, Nishikawa-Uchida S, et al. OCT4 expression in human uterine myometrial stem/progenitor cells. Hum Reprod. 2010;25(8):2059–2067. doi: 10.1093/humrep/deq163. [DOI] [PubMed] [Google Scholar]
- 19.He W, Li K, Wang F, Qin YR, Fan QX. Expression of Oct-4 in human esophageal squamous cell carcinoma is significantly associated with poorer prognosis. World J Gastroenterol. 2012;18(7):712–719. doi: 10.3748/wjg.v18.i7.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linn DE, Yang X, Sun F, Xie Y, Chen H, Jiang R, et al. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer. 2010;1(9):908–916. doi: 10.1177/1947601910388271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, et al. Oct4-Induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Saxe JP, Tomilin A, Schöler HR, Plath K, Huang J. Post-translational regulation of Oct4 transcriptional activity. PLoS ONE. 2009;4(2):e4467. doi: 10.1371/journal.pone.0004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, et al. E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells. 2010;28(8):1315–1325. doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- 24.Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 25.Keravala A, Portlock J, Nash JA, Vitrant DG, Robbins PD, Calos MP. PhiC31 integrase mediates integration in cultured synovial cells and enhances gene expression in rabbit joints. J Gene Med. 2006;8(8):1008–1017. doi: 10.1002/jgm.928. [DOI] [PubMed] [Google Scholar]
- 26.Baharvand H, Matthaei KI. Culture condition difference for establishment of new embryonic stem cell lines from the C57BL/6 and BALB/c mouse strains. In Vitro Cell Dev Biol Anim. 2004;40(3-4):76–81. doi: 10.1290/1543-706x(2004)040<0076:ccdfeo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Balaguer P, Boussioux AM, Demirpence E, Nicolas JC. Reporter cell lines are useful tools for monitoring biological activity of nuclear receptor ligands. Luminescence. 2001;16(2):153–158. doi: 10.1002/bio.630. [DOI] [PubMed] [Google Scholar]
- 28.Motoike T, Loughna S, Perens E, Roman B, Liao W, Chau TC, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28(2):75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Habermann FA, Wuensch A, Sinowatz F, Wolf E. Reporter genes for embryogenesis research in livestock species. Theriogenology. 2007;68(Suppl 1):S116–S124. doi: 10.1016/j.theriogenology.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 30.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]