Abstract
Cancer cell resistance to paclitaxel continues to be a major clinical problem. In this study, we utilized miRNA arrays to screen for differentially expressed miRNAs in paclitaxel-resistant cell lines established in vitro. We observed concordant upregulation of miR-135a in paclitaxel-resistant cell lines representing three human malignancies. Subsequently, the role of miRNA-135a was evaluated in an in vivo model of paclitaxel resistance. In this model, mice were inoculated subcutaneously with a non-small cell lung carcinoma cell line and treated with paclitaxel for a prolonged period. In paclitaxel-resistant cell lines, established either in vitro or in vivo, blockage of miR-135a sensitized resistant cell lines to paclitaxel-induced cell death. We further demonstrated a correlation between paclitaxel response and miR-135a expression in paclitaxel-resistant subclones that were established in vivo. The paclitaxel-resistant phenotype of these subclones was maintained upon retransplantation in new mice as shown by decreased tumor response upon paclitaxel treatment compared to controls. Upregulation of miR-135a was associated with reduced expression of the adenomatous polyposis coli gene (APC). APC knockdown increased paclitaxel resistance in parental cell lines. Our results indicate that paclitaxel resistance is associated with upregulation of miR-135a both in vitro and in vivo, and is in part mediated by miR-135a-mediated downregulation of APC.
Keywords: paclitaxel resistance, miRNA-135a, APC, carcinoma
Introduction
Taxanes, such as paclitaxel and docetaxel, exert their cytotoxic effect via interaction with tubulin subunits, the building blocks of microtubules. Microtubules, formed by polymerization of heterodimeric α- and β-tubulin subunits, play fundamental roles in a wide range of cellular processes, such as maintenance of cell shape, cell signaling and cell division (Gelfand and Bershadsky, 1991). By stabilizing microtubules and inhibiting their disassembly to tubulin, taxanes interfere with proper formation of the mitotic spindle, which leads to activation of the mitotic spindle check point and mitotic arrest (Schiff et al., 1979). Drug-treated cells eventually escape mitotic arrest without assembling a normal mitotic spindle. Depending on the cell type and concentration of taxanes used, these cells will undergo apoptosis during mitotic arrest or as a result of the abnormal mitosis (Shi et al., 2008). The mechanisms of taxane-induced apoptosis are poorly understood, but involve both phosphorylation of Bcl-2 and activation of caspases-3 and -9 (Haldar et al., 1996; Perkins et al., 1998).
Since the original US Food and Drug Administration (FDA) approval of paclitaxel for clinical use in advanced ovarian cancer in 1992, significant activity has been demonstrated against a broad spectrum of solid malignancies. At present, taxanes, used either as single-agent or in combination with multiple other anti-cancer agents, are routinely used in the adjuvant, neoadjuvant and metastatic setting for a wide range of solid malignancies, including those of the breast, prostate, ovary, lung, and head and neck (Chu et al., 2005; Dombernowsky et al., 1996; Mackler and Pienta, 2005; Wakelee et al., 2005). Despite their widespread use, the clinical effectiveness of taxanes is limited by the emergence of taxane-resistant cancer cells, which ultimately leads to relapse and poor prognosis. Various mechanisms have been implicated in acquired or secondary taxane resistance. The best-studied mechanism is the upregulation of P-glycoprotein and related drug efflux pumps (Greenberger et al., 1988; Oguri et al., 2008). Other mechanisms may involve inadequate interaction with spindle microtubules due to post-translational modification or altered expression of tubulin and microtubule-associated proteins (McGrogan et al., 2008; Mozzetti et al., 2005; Villeneuve et al., 2006) or functional aberrations in molecular pathways, such as cell cycle control, the spindle assembly checkpoint and apoptosis (Anand et al., 2003; McGrogan et al., 2008; Sudo et al., 2004, Patel et al., 2010). The contribution of each of these mechanisms to clinical resistance remains uncertain (Patel et al., 2010).
MicroRNAs (miRNAs) are a class of small non-protein coding RNAs that negatively modulate expression of cognate mRNAs (He and Hannon, 2004). They act by targeting the RNA-induced silencing complex (RISC) to complementary sites within the 3′ untranslated region (UTR) of their target mRNAs. Depending on the degree of base pairing between the miRNA and the 3′ UTR, either degradation or translational repression of the targeted mRNA will occur. Although they account for less than 1% of all human genes, miRNAs have been estimated to regulate up to 30% of all protein-encoding genes (Xie et al., 2005). Altered miRNA expression has been observed in various human malignancies (Esquela-Kerscher and Slack, 2006; Iorio et al., 2005). Surprisingly, miRNA expression profiles can predict tumor type and stage in human cancers with a better accuracy than the classical mRNA expression profiles (Lu et al., 2005). The significant correlation between microRNA expression patterns and compound potency in the NCI-60 panel of cell lines suggested that microRNAs have a role in chemoresistance (Blower et al., 2007). Indeed, particular miRNAs have been shown to be involved in tumor responsiveness to chemotherapies, including paclitaxel (Cochrane et al., 2009; Fujita et al., 2010; Kovalchuk et al., 2008; Sorrentino et al., 2008; Xia et al., 2008; Zhou et al., 2010). All studies to date have been performed in in vitro established paclitaxel-resistant cell lines. Although these studies have provided valuable insight into the mechanisms of paclitaxel resistance, the clinical implication is uncertain.
In the present study, we have utilized miRNA arrays to identify microRNAs associated with the development of paclitaxel resistance in a panel of cell lines representing various human solid malignancies, which were made paclitaxel-resistant in vitro. Subsequently, the in vivo significance of the most discriminating miRNA was evaluated in a mouse model of paclitaxel resistance.
Results
miRNA-135a is upregulated in various paclitaxel-resistant cell lines
A screen to identify miRNAs involved in paclitaxel resistance was performed in four cell lines that were made paclitaxel-resistant by continuous exposure to paclitaxel in vitro. We identified 18 miRNAs to be dysregulated at least 2-fold in resistant compared to paclitaxel-sensitive parental cells (Supplementary Figure S1). Supervised hierarchical clustering using the 18 differentially expressed miRNAs clustered cell lines according to cancer type rather than paclitaxel response (Figure 1). Since miR-135a was most highly and concordantly upregulated in more than one paclitaxel-resistant cell line, i.e. 6.4-fold in PC-14TXT cells and 9.1-fold in MES-SADX5 cells, we further investigated the role of miR-135a in paclitaxel resistance. Quantitative real-time-polymerase chain reaction (qRT-PCR) examination of miR-135a levels revealed a 1.5-fold upregulation in PC-14TXT cells (P=0.029) and a 28-fold upregulation in MES-SADX5 cells (P<0.001), confirming our miRNA array results (Figure 2). In addition, we observed upregulation of miR-135a in two other paclitaxel-resistant cell lines, i.e. 2-fold in A549TR (P=0.016) cells and 9.2-fold in SKOVTR cells (P=0.029), indicating that the association between miR-135a and paclitaxel resistance is not cell line-specific (Figure 2).
Figure 1. miRNA array of 4 paclitaxel-resistant cell lines.

Supervised hierarchical clustering of cell lines based on expression of miRNAs with ΔLMR≥2 in at least one cell line. Each column represents a cell line and each row a probe set. The heat maps indicate high (red) or low (blue) level of expression relative to the mean as per the scale shown.
Figure 2. Upregulation of miR-135a in a panel of paclitaxel-resistant cancer cell lines.

Real-time PCR analysis of miR-135a expression in parental MES-SA, SKOV, A549 and PC-14 cells (gray bars) compared to the expression of their paclitaxel-resistant subclones (black bars). Bars represent mean and % s.e.m. from triplicate experiments. *: P<0.05, **: P<0.001 as determined by Wilcoxon’s rank sum test.
Sensitivity to paclitaxel is modulated by changes in miR-135a expression in vitro
If paclitaxel resistance is causally related to miR-135a upregulation, then altering miR-135a expression levels should modulate paclitaxel sensitivity. We found this to be true when paclitaxel-resistant MES-SADX5 cells were transfected with a miR-135a inhibitor (antagomir). In this experiment, cell survival was 102.2% in cells transfected with a scrambled, non-targeting miRNA. In contrast, cell survival was reduced to 62.7% when the same cells were transfected with a miR-135a inhibitor and then treated with paclitaxel (Figure 3A, P=0.003). A similar result was found with A549TR cells (Figure 3A) where transfection with miR-135 inhibitor resulted in a trend toward increased cell sensitivity (P=0.054). To complement these studies, we elevated miR-135a levels in parental, paclitaxel-sensitive cells with the expectation that this treatment would result in acquisition of the resistant phenotype. In this experiment (Figure 3B), only 21.8% cell survival was observed in the MES-SA cells after treatment with 100 nM paclitaxel for 72 hours. In contrast, 43.6% of the cells survived paclitaxel treatment after transfection with a miR-135a mimic. Again, as shown in Figure 3B, a similar trend was observed using the A549 parental cells (p=0.051) in the presence and absence of the miR-135a mimic. Complete dose response curves are provided in Supplementary Figure S2. Consistent with the above results, when paclitaxel-induced apoptosis was determined by measurement of Annexin-positive cells, miR-135a inhibition increased apoptosis in the paclitaxel resistant A549TR cells (Figure 3C, P<0.001) and transfection with the miR-135a mimic decreased paclitaxel-induced apoptosis in the parental A549 cells (Figure 3D, P<0.001).
Figure 3. miR-135a is functionally involved in the paclitaxel response of cancer cell lines.
The paclitaxel-resistant cell lines MES-SADX5 and A549TR were transfected with a scrambled non-targeting miRNA (control) and a miR-135a inhibitor (inhibitor) and the paclitaxel-sensitive parental MES-SA and A549 cells with a scrambled non-targeting miRNA (control) and a miR-135a mimic (mimic). Cells were subsequently treated with 100 nM paclitaxel and cell viability was assessed using the MTT assay (A–B). The % of apoptotic cells was assessed by Annexin V-staining and FACS analysis (C–D). Values are presented as % cell survival in paclitaxel-treated cells relative to untreated cells. *: P<0.05, **: P<0.001 as determined by Wilcoxon’s rank sum test.
Overexpression of miR-135a leads to APC downregulation
It has been demonstrated that the tumor suppressor adenomatous polyposis coli gene (APC) is regulated by miR-135a (Nagel et al., 2008). We confirmed that miR-135a targeted the 3′ untranslated region of APC in both A549 (Figure 4A) and MES-SA cells (Figure 4B, P=0.029). To further explore the role of miR-135a upregulation in paclitaxel resistance, we examined APC expression in paclitaxel-sensitive and -resistant cell lines. Analysis of APC mRNA expression levels revealed a 2.6-fold downregulation in A549TR cells (P<0.001) and a 7-fold downregulation in MES-SADX5 cells (Figure 4C, P<0.001). A concordant downregulation of APC was also observed at the protein level (Figure 4C). Transfection with the anti-miR-135a inhibitor restored APC expression both at the mRNA (Figure 4D) and protein level (Figure 4E).
Figure 4. miR-135a modulates APC expression in paclitaxel-resistant cancer cells.
MES-SA (A) or A549 (B) cells were transfected with a luciferase reporter construct fused to APC 3 ′UTR or a control luciferase reporter vector with a random 3 ′UTR (random UTR). Subsequently, cells were cotransfected with either a miR-135a mimic or a non-targeting miRNA control (control miR). Values are shown as the percent of luciferase expression compared to the control. (C) Endogenous APC mRNA expression was quantified by real-time PCR analysis in A549, A549TR, MES-SA and MES-SADX5 cells. Bars represent mean and s.e.m. from duplicate experiments. NS: not significant, **: P<0.001 as determined by Wilcoxon’s rank sum test. In addition, APC protein expression was examined by western blotting (bottom panel). (D) A549TR cells were transfected with scrambled non-targeting miRNA (control) or miR-135a inhibitor. At the indicated time points, APC mRNA expression was examined by real-time quantitative PCR. (E) A549TR cells were mock-transfected, transfected with a scrambled miRNA (control inhibitor) or with a miR-135a inhibitor. Cells were fixed with paraformaldehyde, permeabilized and stained with APC-specific polyclonal antibodies, followed by fluorophore-conjugated secondary antibodies; nuclei were stained with DAPI. In addition, cells were lysed and APC protein levels were examined by western blot (bottom panel).
miR-135a-induced paclitaxel resistance is partly mediated by APC downregulation
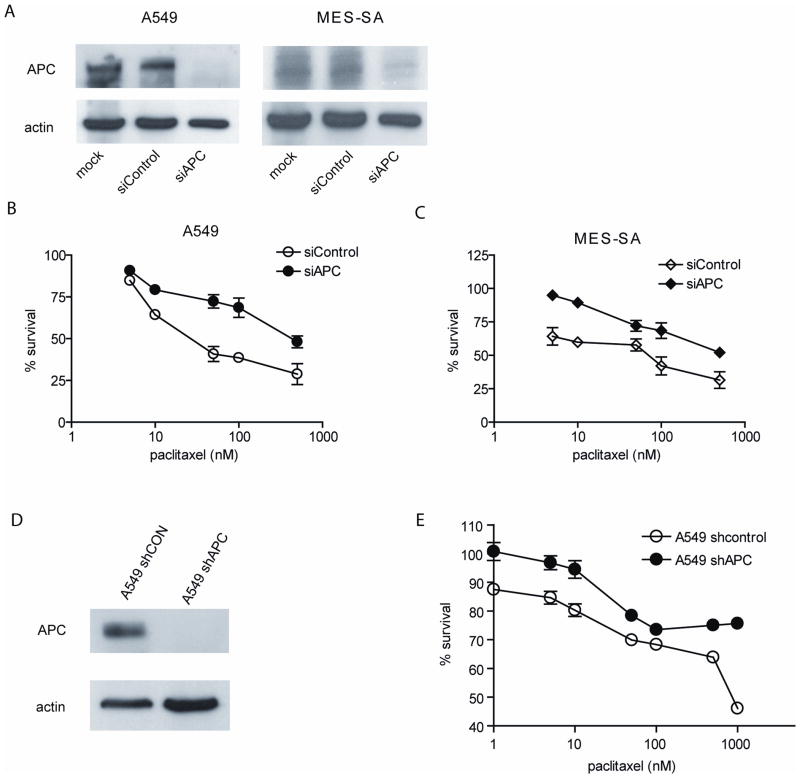
To test the role of APC in the cellular response to paclitaxel, APC expression was suppressed in parental A549 and MES-SA cells. Transfection with siRNA against APC completely suppressed APC expression in both cell lines (Figure 5A) and led to a decrease in paclitaxel-induced cytotoxicity in both cell lines (Figures 5B and 5C). Similar results were obtained in A549 cells stably expressing shRNA against APC (Figures 5D and 5E). These results suggest that downregulation of APC may be partly responsible for the effects of miR-135a on paclitaxel sensitivity in these cell lines.
Figure 5. miR-135a-mediated APC suppression contributes to paclitaxel resistance.
MES-SA and A549 cells were mock-transfected (mock), transfected with a scrambled siRNA (siControl) or with siRNA directed against APC (siAPC). APC expression was examined by immunoblotting (A). Transfected MES-SA (B) and A549 (C) cells were treated with paclitaxel and viability was assessed using the MTT assay. Values are presented as percentage of cell survival in paclitaxel-treated cells relative to untreated cells. A549 cells stably expressing either non-targeting shRNA (shCON) or shRNA against APC (shAPC) were generated. (D) Western blot analysis shows the expression level of APC in both cells. (E) Both A549-shCON and -shAPC cells were treated with paclitaxel and cell viability was measured using the MTT assay. Values are presented as percentage of cell survival in paclitaxel-treated cells relative to untreated cells.
The generation of paclitaxel-resistant cell lines in vivo
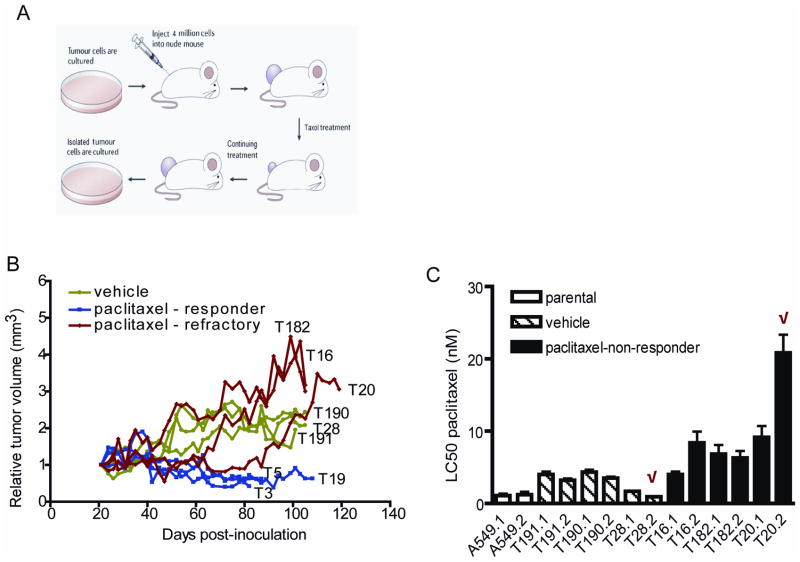
To further establish the role of miR-135a in paclitaxel resistance, we adapted the A549 xenograft mouse model previously utilized in our laboratory (Patel et al., 2010). Briefly, nude mice bearing subcutaneous A549 xenografts were injected intraperitoneally with either vehicle or 15 mg/kg paclitaxel until tumors that responded initially started to regrow (Figure 6A). Representative tumor growth curves are shown in Figure 6B. Vehicle-treated tumors show rapid progressive growth (green lines). Paclitaxel-treated tumors show two general types of response: 7 out of 10 mice showed prolonged response (blue lines) and 3 out of 10 mice became paclitaxel-refractory, as demonstrated by an increase in tumor volume after initial volume reduction during continued treatment (red lines). After 120 days of paclitaxel treatment, control and paclitaxel-refractory tumors were harvested. Within two passages in vitro, RNA was harvested for miRNA evaluation and determination of paclitaxel response. Flow cytometric analysis demonstrated that the harvested cells were composed solely of human A549 cells with no contaminating mouse cells (Supplementary Figure S3). Cell lines established from paclitaxel-refractory tumors (black bars) were more resistant to paclitaxel in vitro than either parental A549 cells (white bars) or vehicle-treated tumors (striped bars, Figure 6C). Treatment of xenotransplanted mice with 10 and 12.5 mg/kg paclitaxel did not generate any paclitaxel-refractory tumors (Supplementary Figure S4).
Figure 6. Establishment of A549 paclitaxel-resistant cells in vivo.
(A) A suspension of A549 cells was injected subcutaneously into the flank of a nude mouse. When the average tumor volume was at least 120 mm3, vehicle or paclitaxel (15 mg/kg) was administered i.p. every other day. Treatment was continued until tumors reached approximately four times their initial volume. Paclitaxel-refractory as well as vehicle-treated tumors were digested with collagenase and cultured in vitro. (B) Tumor growth was determined as the tumor volume on the day of treatment relative to the tumor volume at the start of treatment and presented as a ratio. Tumors treated with vehicle are shown in green, tumors responsive to paclitaxel in blue and tumors with continuous growth in the presence of paclitaxel in red. Each line represents the growth of an individual tumor (T) in an individual mouse (indicated by a number). Curves for representative tumors per group are shown. (C) Vehicle-treated tumors (hatched bars) or paclitaxel-refractory tumors (black bars) were harvested from mice and used to generate two cell lines per tumor. The indicated cell lines were cultured in vitro and cell viability was assessed 72 h after paclitaxel addition using the MTT assay. Bars represent average LC50 ± s.e.m. from triplicate experiments. The most resistant and sensitive cell lines in vitro, indicated with a red checkmark, were selected for reinjection.
Cells selected for paclitaxel resistance remain resistant upon retransplantation
Tumor clone 20.2 (T20.2), the most paclitaxel-resistant tumor in the series, was transplanted into 20 syngeneic mice. Half of these animals were treated with vehicle and half were subjected to another round of i.p. treatment with 15 mg/kg paclitaxel. Tumor clone 28.2 served as a sensitive control in this experiment because: (1) it has an LC50 value for paclitaxel that is similar to the parental A549 cells; (2) it is paclitaxel-naïve; and (3) it is similar to T20.2, as both were previously grown in mice. As shown in Figure 7A, xenografts established from T20.2 showed only a slight delay in tumor growth when tested with paclitaxel, whereas xenografts established from the paclitaxel-sensitive control T28.2 shrank substantially upon paclitaxel treatment. At the end of the experiment, the relative tumor burden was reduced 4.5-fold in paclitaxel-sensitive control cells (P=0.003), but only 1.4-fold in mice bearing T20.2 xenografts (Figure 7B). The decreased paclitaxel response of T20.2 compared to T28.2 in mice was associated with a decreased paclitaxel response in vitro (Figure 7C, P=0.01). The maintenance of paclitaxel resistance upon retransplantation suggests that paclitaxel resistance is associated with stable changes in the tumor rather than in transient changes induced by exposure to the host environment.
Figure 7. Establishment of an A549 in vivo paclitaxel resistance model: retransplantation.
(A) Tumor cell lines established after an initial round of inoculation and treatment with either vehicle (blue lines, T28.2) or 15 mg/kg paclitaxel (red lines, T20.2) were harvested during log-phase growth and reinjected subcutaneously into the flanks of nude mice. When the average tumor volume was ~120 mm3, vehicle (open circles or squares) or 15 mg/kg paclitaxel (closed circles or squares) was administered i.p. every other day. Treatment was continued until tumors reached approximately four times their initial volume. Each curve represents the average tumor growth ± s.e.m. for 10 mice per group. (B) Relative tumor burden at the end of the experiment. Each bar represents the average tumor growth ± s.e.m. for 10 mice per group. NS: not significant, *: P<0.05 as determined by Wilcoxon’s rank sum test. (C) Tumors were harvested, cultured in vitro and cell viability was assessed using the MTT assay. Values are presented as percentage of cell survival in paclitaxel-treated cells relative to untreated cells. White bars represent LC50s from tumor cell lines established from T28.2 tumors treated with vehicle. Black bars represent LC50s from cell lines established from T20.2 tumors treated with paclitaxel. Shown are the mean ± s.e.m. of two independent experiments, each performed in triplicate. *:P<0.05 as determined by Wilcoxon’s rank sum test.
miR-135a is upregulated in in vivo paclitaxel resistance
In our in vitro experiments we established a role for miR-135a in the cellular response to paclitaxel. To explore the role of miR-135a expression in vivo, we examined miR-135a expression in three cell lines representing various degrees of paclitaxel resistance. Paclitaxel resistance increased gradually from a median LC50 value of 1.14 nM in the parental A549 cells to 13.27 nM after one in vivo passage (P=0.030) and to 126.6 nM after re-injection (P=0.008, Figure 8A). As shown in Figure 8B, decreased paclitaxel response is significantly associated with increased miR-135a expression in tumors selected for paclitaxel resistance in vivo. Cells have a median 1.9-fold higher expression after the initial in vivo passage (round 1) and a 4.1-fold higher expression of miR-135a compared to the parental A549 cells (P=0.004). The correlation between miR-135a expression and paclitaxel response was observed in all cell lines derived from these tumors (Figure 8C, P<0.001). Transfection with a miR-135a inhibitor modestly increased paclitaxel-induced cytotoxicity in paclitaxel-resistant cells (Figure 8D). Furthermore, transfection of a miR-135a mimic suppressed paclitaxel-induced cytotoxicity in paclitaxel-sensitive cells (Figure 8E). Together, these results show that miR-135a is involved in the paclitaxel sensitivity of the cell lines established after long-term exposure to paclitaxel in vivo.
Figure 8. miR-135a plays a role in in vivo paclitaxel resistance.
(A). The parental A549 cells, a cell line that became refractory during in vivo treatment with paclitaxel (T20.2 round 1) and the same cell line established after yet another round of in vivo paclitaxel treatment (T20.2 reinjection) were treated with paclitaxel and cell viability was assessed using the MTT assay. Represented are average LC50 ± s.e.m. determined by MTT assay for parental A549 cells (white boxes), **:P<0.001, *: P<0.05 as determined by Wilcoxon’s rank sum test for T20.2 round 1 cells (hatched boxes) and for T20.2 re-injection cells (filled boxes). (B) The expression of miR-135a was examined in these cells by real-time quantitative PCR. *: P<0.05 as determined by Wilcoxon’s rank sum test. (C) The expression of miR-135a was examined in the cell lines established in vivo. The correlation between paclitaxel response and miR-135a expression was calculated using the Spearman’s rank test. (D–E) The paclitaxel-resistant cell line T800.1 (D) or the paclitaxel-sensitive T824.1 cell line (E) established after two rounds of treatment with vehicle were transfected with a scrambled non-targeting miRNA (control), a miR-135a inhibitor (inhibitor) in the resistant cells or a miR-135a mimic (mimic) in the sensitive cells. Subsequently, cells were treated with the indicated concentrations of paclitaxel and cell viability was assessed using the MTT assay. Values are presented as percentage of cell survival in paclitaxel-treated cells relative to untreated cells. Shown are the mean and s.e.m. of two independent experiments, each performed in triplicate.
miR-135a modulates tumor response to paclitaxel in vivo
We subsequently investigated the role of miR-135a in paclitaxel sensitivity in vivo. To this aim, A549 cells stably expressing miR-135a were generated, which showed expression of miR-135a approximating that of A549TR cells (P<0.01) (Figure 9A). As expected, miR-135a knockdown attenuated paclitaxel-induced cell death (Figure 9B) and APC protein expression (Figure 9C) relative to control transfected A549 cells in vitro. If overexpression of miR-135a is causally involved in paclitaxel-resistance in vivo, tumors artificially overexpressing miR-135a should show decreased paclitaxel sensitivity. To investigate this hypothesis, A549 control cells and A549 cells stably overexpressing a miR-135a mimic were inoculated subcutaneously into the flanks of nude mice. Paclitaxel treatment was given three times a week for three weeks and tumor size in each group was monitored during the course of treatment. At the end of the experiment, the relative tumor burden was reduced 5-fold in A549 control cells (P<0.001). A549 cells overexpressing miR-135a showed increased cell growth in vivo relative to control untreated cells. In vivo growth of miR-135a overexpression was not reduced by paclitaxel exposure (Figure 9D). This result demonstrates that miR-135a overexpression alone induces taxane resistance that is maintained during tumor growth in vivo.
Figure 9. Overexpression of miR-135a confers paclitaxel resistance in A549 cells in vivo.
A549 cells stably expressing either non-targeting premiR (A549-control) or premiR-135a (A549-miR-135a) were generated. (A) miR-135a expression level in A549-control and -miR-135a was determined and compared with A549 and A549TR cells using quantitative real time PCR analysis. *: P<0.01. (B) Paclitaxel response of A549-control and A549-premiR-135a cells was determined using the MTT assay. (C) APC protein expression was examined using Western blot analysis in A549-control and -premiR-135a cells. (D) A549-control and -premiR-135a cells were inoculated subcutaneously into the flanks of nude mice. Paclitaxel treatment was given three times a week for three weeks. The tumor size in each group was measured during the course of treatment and was calculated relative to the size prior to treatment initiation. Each curve represents the average tumor growth ± s.e.m. for 10 mice per group. *: P<0.05, **: P<0.001 as determined by Wilcoxon’s rank sum test.
Discussion
Despite their widespread use, the clinical effectiveness of taxanes is limited by the emergence of taxane-resistant cancer cells. Our current knowledge of the mechanisms underlying paclitaxel resistance is largely derived from in vitro models where resistance is induced by repeated or prolonged exposure of cultured cells to gradually increasing drug concentrations. Although this methodology has imparted important knowledge, it has potential disadvantages. First, cells in solid tumors in vivo tend to be more drug-resistant than the same cells grown in a monolayer in vitro (Hoffman, 1991; Kobayashi et al., 1993). This can be explained by various factors including decreased drug penetration, quiescence of cells in deeper layers due to cell contact inhibition and hypoxia, or resistance arising from an adaptive, reciprocal signaling between tumor cells and the surrounding microenvironment (Meads et al., 2009; Pollard, 2004). Second, treatment in culture bears little resemblance to in vivo pharmacokinetics, and does not allow the formation of active metabolites that may be produced by metabolic activity. Third, the in vitro situation does not address the role of the tumor microenvironment in the generation of drug resistance. To confirm the role of miRNA-135a in paclitaxel resistance, we established a new in vivo mouse model of paclitaxel resistance.
One way to circumvent this problem is to use mice that develop spontaneous tumors as a consequence of conditional tissue-specific mutations in proto-oncogenes and tumor suppressor genes. Using this approach, Rottenberg et al. generated docetaxel-resistant Brca1−/−;p53−/− mammary tumors (Rottenberg et al., 2007). In that study, tumors that responded to paclitaxel were allowed to grow back to their original size before treatment was resumed. We employed a different approach to the in vivo selection of paclitaxel-resistant tumors. In our model, mice were inoculated with paclitaxel-sensitive tumor cells and then treated with paclitaxel 3x weekly, consistent with current clinical practice.
Similar approaches have been employed by others to establish drug-resistant cell lines (Okugawa et al., 2004; Starling et al., 1990; Teicher et al., 1990). Interestingly, contrary to our model, resistance was not maintained when the established cell lines were exposed to paclitaxel in vitro in these models. The authors suggested that drug resistance in in vivo established cell lines may be dependent on an interaction between the tumor and the host stromal tissues rather than changes at the cellular level. The maintenance of drug resistance in vitro in our model could be explained by our selection method. Both Okugawa and Teicher et al retransplanted cells in a new recipient mouse after a single drug dose. This procedure was repeated six and ten times in a 6-month period, respectively. Starling’s mice were treated 7 times in 8 weeks with a treatment-free interval of 3 weeks between the first and second rounds of treatment. In contrast, our 3x weekly schedule for 42 weeks is associated with a prolonged and continuous selective pressure. It has been proposed that acquired drug resistance arises in a step-wise fashion as a consequence of random mutations in tumor subclones (Goldie and Coldman, 1979). Some of these mutations will, by chance, result in an increased survival in the presence of the drug being used, causing this subclone to be selected during therapy. Resistance at the cellular level, without the necessity of signals from the microenvironment, may occur at a later stage in the development of resistance, explaining the maintenance of paclitaxel resistance ex vivo in our model. Since clinical paclitaxel resistance usually takes several months to develop, our model may represent a promising new paradigm elucidate mechanisms of paclitaxel resistance.
miR-135a is a member of the miR-135 subfamily, which is comprised of miR-135a and miR-135b. Our current work demonstrates that upregulation of miR-135a correlates with the acquisition of taxane resistance in cells selected for resistance either in vitro or in vivo. Manipulation of miR-135a levels has a functional effect on taxane sensitivity in these lines. Our results suggest that suppression of miR-135a may be a reasonable approach to improving or prolonging drug sensitivity.
A recent report showed that the tumor suppressor gene APC is targeted by the miR-135a family (Nagel et al., 2008). In the present paper we confirm that APC is a miR-135a target and suggest miR-135a-mediated APC-downregulation as one possible mechanism for miR-135a-induced taxane resistance. The best-known function of the APC protein is the regulation of the Wnt signaling cascade through down-regulation of β-catenin (Giles et al., 2003). Loss of APC expression leads to nuclear accumulation of β-catenin and inappropriate activation of its target genes (Giles et al., 2003), including the growth-promoting genes c-myc and cyclin-D1 and the anti-apoptotic gene survivin (Zhang et al., 2001). Collectively, these changes can lead to reduced paclitaxel-induced apoptosis (Dikovskaya et al., 2007). In addition, defects in the mitotic check point function have been associated with reduced paclitaxel-induced apoptosis (Anand et al., 2003; Sudo et al., 2004). APC regulates the mitotic checkpoint by binding to microtubules during mitosis (Fodde et al., 2001) and interacting with kinetochore-associated proteins (Aoki and Taketo, 2007; Kaplan et al., 2001). Consequently, knockdown of APC by miR-135a may contribute to paclitaxel resistance by decreasing apoptosis directly or indirectly by interference with the mitotic spindle checkpoint.
Additional mechanisms for miR-135a induction of paclitaxel resistance are possible and merit future testing. A recent report showed that treatment with the angiogenesis inhibitor endostatin significantly improved the anti-tumor efficacy of paclitaxel in Lewis lung cell carcinoma (Huang and Chen, 2010). This implicates increased angiogenesis in paclitaxel resistance. Hypoxia-inducible factor 1 (HIF-1) is the key regulator of oxygen homeostasis that controls angiogenesis, erythropoiesis, and glycolysis via transcriptional activation of target genes under hypoxic conditions (Fong, 2008). Hypoxia-inducible factor 1-alpha inhibitor (HIF1AN) is a protein that binds to HIF-1α and inhibits its transcriptional activity (Mahon et al., 2001). HIF1AN is a potential miR-135a target listed in both the TargetScan and PicTar databases. Efforts to identify deregulation of other miR-135 targets in our model are currently being pursued in our laboratory using a combination of genomic approaches and in silico analysis of previously published datasets.
The role in paclitaxel resistance that we propose here for miR-135a offers the intriguing possibility that clinical taxane resistance could be reversed by inhibiting miR-135a and/or its deregulated downstream pathways. The use of anti-miRs as a therapeutic tool was demonstrated in a recent study where intratumoral injection of anti-miR-221/222 led to a reduction in tumor growth in a subcutaneous prostate cancer xenograft tumor model (Mercatelli et al., 2008). Unfortunately, intratumoral injection is not feasible for the majority of cancers and the success of systemic gene-based therapy has been limited to date by fundamental barriers to RNA delivery. While new approaches to direct miRNA delivery are being widely investigated, non-gene-based approaches may be feasible as well. For example, several small-molecule antagonists capable of disrupting APC have been identified (Lepourcelet et al., 2004) and represent potential alternatives to genetic manipulation.
In conclusion, we demonstrate that miR-135a is involved in paclitaxel resistance both in vitro and in vivo. In addition, we show that miR-135a-mediated paclitaxel resistance is in part mediated by downregulation of APC. Although the present paper solely focused on miR-135a, this miRNA represents only one of the many possible miRNAs that are likely to be involved in paclitaxel resistance, as we found additional miRNAs to be upregulated in the initial screening of our cell lines. Our in vivo model represents a promising new paradigm to elucidate mechanisms of paclitaxel resistance. Future genomic or proteomic profiling of the paclitaxel-resistant cell clones generated in this model is likely to generate important new insights into the mechanisms of paclitaxel resistance.
Materials and methods
Cell lines
The human NSCLC line A549 and its paclitaxel-resistant derivative cell lines were described previously (Patel et al., 2010). The human non-small cell lung cancer cell line (NSCLC) PC-14 and its docetaxel-resistant cell line PC-14/TXT were provided by Dr. Nagahiro Saijo (National Cancer Center Research Institute, Tokyo, Japan), the human breast cancer cell line MCF-7 and the multidrug-resistant derivative MCF-7TAX by Dr. Amadeo Parissenti (Laurentian University, Sudbury, ON, Canada) (Villeneuve et al., 2006), the human prostate cancer cell line PC-3 and the paclitaxel resistant variant PC-3TXR by Dr. Atsushi Mizokami (University of Kanazawa, Japan) (Takeda et al., 2007) and the human ovarian cancer cell line SKOV-3 and the paclitaxel resistant variant SKOV-3TR by Dr. Zhenfeng Duan (Massachusetts General Hospital, Boston, MA). Multi-drug resistant human uterine sarcoma cells MES-SADX5 were purchased from ATCC (Manassas, VA). All cells were cultured at 37°C in a humidified atmosphere with 5% CO2 in media supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Life Technologies, USA). Media and paclitaxel (Cytoskeleton, USA) concentrations used are specified in Supplementary Figure S5.
RNA purification, labeling and hybridization
Cells were harvested in log-phase growth and total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol with an additional extraction with phenol:chloroform:isoamyl alcohol 25:24:1. RNA integrity was assessed with an Agilent Bioanalyser 2100 (Agilent, Palo Alto, CA). Two μg of total RNA was labeled using the miRCURY™ Hy3™/Hy5™ labeling kit and hybridized on the miRCURY™ LNA Array v.11.0 (Exiqon, Vedbaek, Denmark) according to the manufacturer’s protocol. A common reference pool was constructed using RNA from all samples. Hybridization of the Hy5-labeled pool to each array facilitates comparison of ratios across datasets (Yang and Speed, 2002).
Processing of microarray data
Arrays were scanned using a laser confocal scanner (Agilent) and Hy3 (cell line) and Hy5 (common reference pool) signal intensities were calculated. Arrays were repeated if control oligonucleotides did not produce signals within the expected range. In successful arrays, signal intensities were background corrected using the normexp method (Ritchie et al., 2007) with offset value k=10 and normalized using the global Lowess (locally weighted scatterplot smoothing) regression algorithm (Cleveland, 1979). The LMR value was calculated per miRNA probe set by log2-transformation of the mean Hy3/Hy5 ratio (MR). The ΔLMR value, i.e. the difference between the LMR values in the parental cells and their paclitaxel-resistant subclone, was subsequently used to calculate the fold change per miRNA using the following formula: 2ΔLMR. For subsequent analysis, we used miRNAs which passed the filtering criteria on variation across samples, i.e. LMR ≥ 2.0.
Establishment of in vivo paclitaxel-resistant cell lines
Approximately 4×106 human A549 NSCLC cells were resuspended in 50% Matrigel (BD Biosciences, Bedford, MA) and injected subcutaneously into the hind flanks of 8-wk old male athymic (nu/nu) nude mice (Taconic Farms, Inc., Hudson, NY). When the average tumor volume reached ~150 mm3, mice (N=10 per group) were randomly assigned to the treatment or control group (Day 0). Mice were treated with vehicle (control) or 15 mg/kg paclitaxel (Bristol-Myers Squibb Co, Princeton, NJ) intraperitoneally 3 times per week (on Mondays, Wednesdays, and Fridays). This concentration of paclitaxel had minimal effects on mouse morbidity as measured by mouse weight (Supplementary Figure S6). Tumor size was determined by digital caliper measurements (length and width in mm), and tumor volume (mm3) was estimated using the formula (length x width2)/2. Effects on tumor growth rate were assessed per mouse by determining the tumor volume on the day of treatment relative to the tumor volume on Day 0. Animals were sacrificed once morbidity became evident or their tumor size exceeded 1000 mm3. Following the indicated treatments, A549 tumors were removed, minced, and expanded in vitro without further exposure to paclitaxel and stored in liquid nitrogen. All animal studies were conducted in accordance with the guidelines established by the internal Institutional Animal Care and Use Committee.
Cytotoxicity assays
Cell growth inhibition was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) bromide assay. Briefly, ~2.5×104 cells were plated in a 96-well plate and paclitaxel was added in appropriate concentrations 24 hours later. An equivalent amount of diluent (DMSO) was added to culture medium as a negative control. After 72 hours of drug incubation, 20 μl of MTT (Sigma Chemicals, St. Louis, MO; 20 mg/ml) was added to each well. After incubation for an additional 4 hours, 200 μl of isopropanol-HCl solution was added to each well to dissolve the cell pellets. Absorbance was determined using a 96-well SpectraMax plate reader (Molecular Devices, Sunnyvale, CA) at 560 nm and 650 nm (background).
Quantitative real time PCR (qRT-PCR)
Total cellular RNA was extracted using Trizol reagent (Invitrogen). In order to quantify APC mRNA expression levels, cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Richmond, VA, USA) and qRT-PCR were carried out using iQ SYBR Green master mix (Bio-Rad). Primer sequences used are: APC, 5′-GGAAGCAGAGAGAAAGTACTGGA-3′ (sense) and 5′-CTGAAGTTGAGCGT AATACCAG-3′ (antisense), GAPDH, 5′-GTATTGGGCGCCTGGTCACC-3′ (sense) and 5′-CGGGAAGATG GTGATGG-3′ (antisense). Expression of miR-135a and 5S sRNA as a reference was analyzed by the mirVana™ qRT-PCR miRNA Detection Kit (Ambion/Applied Biosystems, Austin, TX) in conjunction with real-time PCR with SYBR Green I (Bio-Rad). All reactions were conducted in triplicate in a LightCycler® 480 Real-Time PCR System (Roche, Indianapolis, IN). Gene expression was calculated relative to GAPDH (for APC) or 5S rRNA (for miR-135a) using the comparative cycle time (Ct) method.
Transfections and luciferase assay
Double-stranded RNA oligos representing a mature sequence that mimics endogenous miR-135a, anti-miR-135a designed to inhibit endogenous miR-135a, and anti-miR negative control were all obtained from Ambion and were transfected into cell lines at ~50% confluence at 30 nM concentration with siLentFect (Bio-Rad). For luciferase activity analysis, cells were seeded in 96-well plates and 100 ng Luc-APC-3′UTR reporter vector (SwitchGear Genomics, Menlo Park, CA) and 30 nM miR-135a mimic or 30 nM non-targeting control were cotransfected with Lipofectamine 2000 (Invitrogen). An empty vector, containing random genomic fragments served as a negative control. The next day, luciferase activity was measured using the Dual-Glo® Luciferase assay system (Promega, Madison, WI). For siRNA-mediated APC knockdown, a siRNA against human APC and a scrambled non-targeting siRNA (ON-Target PLUS Smartpool, Dharmacon) were transfected with Dharmacon Transfection reagent I into A549 cells before analyzing for protein expression at 48 hr post-transfection. For the paclitaxel dose response, cells were re-seeded 24h after transfection and treated with paclitaxel the next day and harvested 48 hr post-treatment using MTT assay. In generating A549 cells stably expressing miR-135a, expression plasmids for human miRNA-135a and its non-targeting control (Origene, Rockville, MD) were transfected using Lipofectamine 2000, before selection with 750 μg/ml Neomycin.
Annexin V assay
Annexin V/7-amino-actinomycin D labeling was performed according to the manufacturer’s instructions (BD Pharmingen, 559763) and samples were analyzed by flow cytometry. Briefly, cells were treated for 48–72 hours with either vehicle or 100 nM paclitaxel. Cells were trypsinized and washed with PBS before resuspending in assay binding buffer. Annexin V and 7-amino-actinomycin labeling was performed at room temperature for 15 minutes before analysis by flow cytometry (BD FACScan).
Western blot and immunocytochemical studies
Protein was extracted and the protein content was determined using the Bio-Rad Protein Assay Kit (Bio-Rad) with bovine serum albumin as the standard. Protein samples (50 μg) were fractionated by SDS–PAGE (7% polyacrylamide gels) and transferred to PVDF membrane (Millipore, Bedford, MA). The samples were incubated overnight at 4°C with a primary antibody directed against the C-terminus of APC (Santa Cruz Biotechnology, Santa Cruz, CA). Bound secondary HRP-conjugated antibodies were detected using the ECL detection system (Amersham/GE Healthcare, Pittsburgh, PA). Immunofluorescence for APC was carried out on cells seeded on round glass coverslips coated with 10 μg/ml fibronectin (BD Biosciences) in 24-well plates. Prior to staining, cells were treated with 30 nM of miRNA inhibitor. At the end of treatment, cells were washed with ice-cold PBS containing 1 mM MgCl2 and 1 mM CaCl2. Cells were fixed with 3.7% formaldehyde (Sigma) for 15 minutes at room temperature and stained for APC (1:50) with the primary antibody for 1 hr followed by extensive washing and incubation with secondary antibody (Alexa 488-conjugated donkey anti-goat IgG, 1:1000) in the dark for 30 minutes.
Statistical analysis
Statistical evaluation for data analysis was determined by using the unpaired Student’s t-test or Wilcoxon’s rank sum test. All data were shown as the mean ± standard error (SE). A statistical difference of P < 0.05 was considered significant.
Supplementary Material
All differentially expressed miRNAs have a ΔLMR≥2, i.e. fold change ≥4 (in red) or ≤0.25 (in blue), in at least one cell line pair.
MESSADX5 (A) and A549TR (B) cells were transfected with a non-targeting control miRNA or a miR-135a inhibitor and MESSA (C) or A549 (D) cells with either a control or a miR-135a mimic. Post-transfection, cell viability was assessed after exposure to paclitaxel. Shown are the mean and s.e.m. of two independent experiments.
28.2CON (A) and 20.2TR cells (B) were stained with anti-human HLA-ABC and anti-mouse H-2kb-PE/H-2Kd-PE to identify proportions of human and mouse cells, respectively. Pooled cells isolated from the bone marrow of 5 mice were used as a positive control for the anti-mouse and a negative control for the anti-human antibody (C).
Nude mice were inoculated with A549 cells. At an average tumor volume of ~120 mm3, vehicle-control and 10 mg/kg (A) or 12.5 mg/kg paclitaxel (B) was administered i.p. every other day. Each curve represents the tumor volume on the day of treatment relative to day 0 per mouse.
Tumor-bearing nude mice were treated i.p with 15 mg/kg paclitaxel every other day. Animals were weighed before each injection on a digital scale. Each curve represents the mean weight ± s.e.m. of 10 mice per day of treatment.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (B.R.Z), Kendle (A.H.), KWF Cancer Foundation (A.H.) Stichting Catharine van Tussenbroek (A.H.) and a U.S. Department of Defense PCRP fellowship (I.C.).
We sincerely thank Dr. John Heymach of M.D. Anderson Cancer Institute, Houston, TX and Jeremy Force, Children’s Hospital Boston, for kindly providing us with the A549TR cells. This work was supported in part by grant CA37393 from the National Institutes of Health (B.R.Z), Kendle (A.H.), KWF Cancer Foundation (A.H.), the Stichting Catharine van Tussenbroek (A.H.), and a U.S. Department of Defense PCRP fellowship (I.C.).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary information is available at the Oncogene website (http://www.nature.com/onc)
References
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–35. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- Blower PE, Verducci JS, Lin S, Zhou J, Chung JH, Dai Z, et al. MicroRNA expression profiles for the NCI-60 cancer cell panel. Mol Cancer Ther. 2007;6:1483–91. doi: 10.1158/1535-7163.MCT-07-0009. [DOI] [PubMed] [Google Scholar]
- Chu Q, Vincent M, Logan D, Mackay JA, Evans WK. Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer. 2005;50:355–74. doi: 10.1016/j.lungcan.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatter plots. J Amer Statist Assoc. 1979;74:829–836. [Google Scholar]
- Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovskaya D, Schiffmann D, Newton IP, Oakley A, Kroboth K, Sansom O, et al. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J Cell Biol. 2007;176:183–95. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombernowsky P, Gehl J, Boesgaard M, Paaske T, Jensen BV. Doxorubicin and paclitaxel, a highly active combination in the treatment of metastatic breast cancer. Semin Oncol. 1996;23:23–7. [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–8. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- Fong GH. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11:121–40. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Kojima K, Ohhashi R, Hamada N, Nozawa Y, Kitamoto A, et al. MiR-148a attenuates paclitaxel-resistance of hormone-refractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem. 2010;25:19076–84. doi: 10.1074/jbc.M109.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand VI, Bershadsky AD. Microtubule dynamics: mechanism, regulation, and function. Annu Rev Cell Biol. 1991;7:93–116. doi: 10.1146/annurev.cb.07.110191.000521. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979;63:1727–33. [PubMed] [Google Scholar]
- Greenberger LM, Lothstein L, Williams SS, Horwitz SB. Distinct P-glycoprotein precursors are overproduced in independently isolated drug-resistant cell lines. Proc Natl Acad Sci U S A. 1988;85:3762–6. doi: 10.1073/pnas.85.11.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996;56:1253–5. [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hoffman RM. Three-dimensional histoculture: origins and applications in cancer research. Cancer Cells. 1991;3:86–92. [PubMed] [Google Scholar]
- Huang G, Chen L. Recombinant human endostatin improves anti-tumor efficacy of paclitaxel by normalizing tumor vasculature in Lewis lung carcinoma. J Cancer Res Clin Oncol. 2010;136:1201–11. doi: 10.1007/s00432-010-0770-6. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–32. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Man S, Graham CH, Kapitain SJ, Teicher BA, Kerbel RS. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc Natl Acad Sci U S A. 1993;90:3294–8. doi: 10.1073/pnas.90.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–9. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Mackler NJ, Pienta KJ. Drug insight: Use of docetaxel in prostate and urothelial cancers. Nat Clin Pract Urol. 2005;2:92–100. doi: 10.1038/ncpuro0099. [DOI] [PubMed] [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–86. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–74. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Mercatelli N, Coppola V, Bonci D, Miele F, Costantini A, Guadagnoli M, et al. The inhibition of the highly expressed miR-221 and miR-222 impairs the growth of prostate carcinoma xenografts in mice. PLoS ONE. 2008;3:e4029. doi: 10.1371/journal.pone.0004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- Nagel R, le Sage C, Diosdado B, van der Waal M, Oude Vrielink JA, Bolijn A, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008;68:5795–802. doi: 10.1158/0008-5472.CAN-08-0951. [DOI] [PubMed] [Google Scholar]
- Oguri T, Ozasa H, Uemura T, Bessho Y, Miyazaki M, Maeno K, et al. MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non-small cell lung cancer. Mol Cancer Ther. 2008;7:1150–5. doi: 10.1158/1535-7163.MCT-07-2088. [DOI] [PubMed] [Google Scholar]
- Okugawa K, Kobayashi H, Hirakawa T, Sonoda T, Ogura T, Nakano H. In vivo establishment and characterization of a paclitaxel-resistant human ovarian cancer cell line showing enhanced growth properties and drug-resistance only in vivo. J Cancer Res Clin Oncol. 2004;130:178–86. doi: 10.1007/s00432-003-0516-9. [DOI] [PubMed] [Google Scholar]
- Patel N, Chatterjee SK, Vrbanac V, Chung I, Mu CJ, Olsen RR, et al. Rescue of paclitaxel sensitivity by repression of Prohibitin1 in drug-resistant cancer cells. Proc Natl Acad Sci U S A. 2010;107:2503–8. doi: 10.1073/pnas.0910649107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins C, Kim CN, Fang G, Bhalla KN. Overexpression of Apaf-1 promotes apoptosis of untreated and paclitaxel- or etoposide-treated HL-60 cells. Cancer Res. 1998;58:4561–6. [PubMed] [Google Scholar]
- Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- Ritchie ME, Silver J, Oshlack A, Holmes M, Diyagama D, Holloway A, et al. A comparison of background correction methods for two-colour microarrays. Bioinformatics. 2007;23:2700–7. doi: 10.1093/bioinformatics/btm412. [DOI] [PubMed] [Google Scholar]
- Rottenberg S, Nygren AO, Pajic M, van Leeuwen FW, van der Heijden I, van de Wetering K, et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci U S A. 2007;104:12117–22. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008;68:3269–76. doi: 10.1158/0008-5472.CAN-07-6699. [DOI] [PubMed] [Google Scholar]
- Sorrentino A, Liu CG, Addario A, Peschle C, Scambia G, Ferlini C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol Oncol. 2008;111:478–86. doi: 10.1016/j.ygyno.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Starling JJ, Maciak RS, Hinson NA, Hoskins J, Laguzza BC, Gadski RA, et al. In vivo selection of human tumor cells resistant to monoclonal antibody-Vinca alkaloid immunoconjugates. Cancer Res. 1990;50:7634–40. [PubMed] [Google Scholar]
- Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–8. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- Takeda M, Mizokami A, Mamiya K, Li YQ, Zhang J, Keller ET, et al. The establishment of two paclitaxel-resistant prostate cancer cell lines and the mechanisms of paclitaxel resistance with two cell lines. Prostate. 2007;67:955–67. doi: 10.1002/pros.20581. [DOI] [PubMed] [Google Scholar]
- Teicher BA, Herman TS, Holden SA, Wang YY, Pfeffer MR, Crawford JW, et al. Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science. 1990;247:1457–61. doi: 10.1126/science.247.4949.1457. [DOI] [PubMed] [Google Scholar]
- Villeneuve DJ, Hembruff SL, Veitch Z, Cecchetto M, Dew WA, Parissenti AM. cDNA microarray analysis of isogenic paclitaxel- and doxorubicin-resistant breast tumor cell lines reveals distinct drug-specific genetic signatures of resistance. Breast Cancer Res Treat. 2006;96:17–39. doi: 10.1007/s10549-005-9026-6. [DOI] [PubMed] [Google Scholar]
- Wakelee H, Ramalingam S, Belani CP. Docetaxel in advanced non-small cell lung cancer. Expert Rev Anticancer Ther. 2005;5:13–24. doi: 10.1586/14737140.5.1.13. [DOI] [PubMed] [Google Scholar]
- Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–9. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, et al. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–45. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Speed T. Design issues for cDNA microarray experiments. Nat Rev Genet. 2002;3:579–88. doi: 10.1038/nrg863. [DOI] [PubMed] [Google Scholar]
- Zhang T, Otevrel T, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–7. [PubMed] [Google Scholar]
- Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi Y, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) J Biol Chem. 2010;285:21496–507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All differentially expressed miRNAs have a ΔLMR≥2, i.e. fold change ≥4 (in red) or ≤0.25 (in blue), in at least one cell line pair.
MESSADX5 (A) and A549TR (B) cells were transfected with a non-targeting control miRNA or a miR-135a inhibitor and MESSA (C) or A549 (D) cells with either a control or a miR-135a mimic. Post-transfection, cell viability was assessed after exposure to paclitaxel. Shown are the mean and s.e.m. of two independent experiments.
28.2CON (A) and 20.2TR cells (B) were stained with anti-human HLA-ABC and anti-mouse H-2kb-PE/H-2Kd-PE to identify proportions of human and mouse cells, respectively. Pooled cells isolated from the bone marrow of 5 mice were used as a positive control for the anti-mouse and a negative control for the anti-human antibody (C).
Nude mice were inoculated with A549 cells. At an average tumor volume of ~120 mm3, vehicle-control and 10 mg/kg (A) or 12.5 mg/kg paclitaxel (B) was administered i.p. every other day. Each curve represents the tumor volume on the day of treatment relative to day 0 per mouse.
Tumor-bearing nude mice were treated i.p with 15 mg/kg paclitaxel every other day. Animals were weighed before each injection on a digital scale. Each curve represents the mean weight ± s.e.m. of 10 mice per day of treatment.