Abstract
Objective
To assess variation of total prostate-specific antigen (tPSA), free PSA (fPSA), percent fPSA, human glandular kallikrein 2 (hK2), and intact PSA measured three times within two weeks. Knowledge of the variation in an individual’s PSA level is important for clinical decision-making.
Patients and Methods
Study participants were 149 patients referred for prostate biopsy, of which 97 had benign disease and 52 had prostate cancer. Three blood samples were drawn with a median of four hours between first and second samples and 12 days between first and third samples. Variability was described by absolute differences, ratios and intra-individual coefficients of variation. Total PSA, fPSA, hK2, and intact PSA were measured in anti-coagulated blood plasma.
Results
At baseline, the median tPSA was 6.8 (IQR 4.5, 9.6) ng/mL. The intra-individual variation was low for all biomarkers, and lowest for tPSA. For 80% of participants, the ratio between first and second time points for tPSA was between 0.91 and 1.09 and the ratio for percent fPSA was between 0.89 and 1.15. Total coefficients of variation between time 1 and 2 for tPSA, fPSA, percent fPSA, hK2 and intact PSA were 4.0%, 6.6%, 6.0%, 9.2%, and 9.5%, respectively. The measurements taken several days apart varied more than those taken on the same day, but the variation between both time points were not large.
Conclusion
The intra-individual variation for all the kallikrein-like markers studied was relatively small, especially for samples drawn the same day. Few cases are reclassified between the time points. This indicates high short-term biological and technical reproducibility of the tests in clinical use.
Keywords: Free PSA, Prostate cancer, PSA, Screening, Variation
Introduction
Prostate-specific antigen (PSA) is an important and established clinical tool to assess prostate cancer risk and detect prostate cancer. Several other biomarkers have been proposed as a means to strengthen the specificity of PSA: the closely related kallikrein-related peptidase 2 (hK2) [1], free PSA (fPSA), percentage of fPSA [2–5], and intact PSA (iPSA) [6].
Clinical decisions are often based on laboratory tests associated with considerable intraindividual variability [7,8]. Recognizing variations is important to avoid unnecessary investigations. With PSA, changes over a long term may indicate disease progression [9], and therefore long-term changes are not a subject for this study. However, PSA changes over a very short period of time reflect the variation in the assay, which can be considered as the sum of analytical and biological variability. Analytical variation results from variation in factors such as sampling of blood, laboratory processing, and assay performance. Biological variation results from individual factors; for intra-individual variation in total PSA (tPSA), relevant factors include physical and sexual activity, urinary tract infection (UTI), digital rectal examination (DRE), prostate biopsy, and treatment with alpha-reductase inhibitors [8, 10–13]. Prior studies have shown that DRE does not importantly influence the clinical interpretation of PSA-levels [14], whereas prostate biopsy and UTI may cause pronounced increase of the PSA levels in serum. Short-term intraindividual variation in PSA has been examined in two meta-analyses [15,16]. In a meta-analysis of 27 studies [14], the typical biological variation of tPSA was close to 20% (range, 2.1–19.6%) over a period of days to weeks. A meta-analysis of 12 studies yielded a total intraindividual coefficient of variation (CV) for tPSA of about 13% [16]. Only a few studies have addressed variability of percent fPSA [10,17–19], and to our knowledge none have addressed hK2 or iPSA.
The critical question for the clinician is what PSA level should prompt further diagnostic tests. Therefore, knowledge is needed of the random variation of PSA in the blood that can be expected within a short time interval. We undertook a prospective study to analyze the variation in a standardized manner over short time periods—within hours and within two weeks—for tPSA, fPSA, percent fPSA, hK2, and iPSA.
Patients and Methods
Patients
Participants were recruited during 2001–2004 at the Department of Urology at Malmö University Hospital. The study was approved by the regional ethics committee at Lund University. Consecutive patients (n=219) who were scheduled for prostate biopsy were asked to participate. The patients included both referred outpatients and patients referred from other departments due to symptoms suspicious for prostatic disease. Reasons for referral were: increased PSA level, urinary tract symptoms, or a pathological DRE finding. All but three patients (n=216) agreed to participate. All patients had to produce a urine sample that was tested with a dip-stick. No UTIs were found. Immediately prior to the biopsy, 750 mg of ciprofloxacin was given as a prophylaxis. A first blood sample was drawn more than two weeks after the biopsy (Time 1), a second sample was drawn 4–7 hours later (Time 2), and a third sample (Time 3) approximately two weeks later. There was a time elapse of more than two weeks between prostate biopsy and the first blood draw with a median time elapse of 7 weeks (IQR 5, 9). Exclusion criteria were manipulation of the prostate, hormonal therapy (n=24), or other therapy (n=12) that would affect PSA levels. An additional 31 patients were excluded because of missing biomarker measurements, leaving 149 for the analysis. All participants provided written consent for participation and for retrieval of information from medical records
Sample handling and laboratory methods
Venous blood was collected into EDTA vials, centrifuged 3200x g for 10 min, then stored at −80°C within 3 hours of venipuncture.
Total PSA and fPSA were analyzed within two weeks of sample collection and within three hours of thawing using the dual-label DELFIA immunofluorometric assay (Prostatus™ PSA Free/Total PSA, Perkin-Elmer Life Sciences, Turku, Finland) [20] calibrated against the WHO 96/670 (PSA-WHO) and WHO 68/668 (free PSA-WHO) standards. The assay’s CV for tPSA is 5.0% at 2.32 ng/mL and for fPSA is 5.9% at 0.25 ng/mL. The assay for hK2 was as described [21]. The between-assay imprecision values (mean CVs) range from 5.7% to 11% for high and low hK2 controls, respectively. The iPSA assay is based on a europium-labeled detection antibody (4D4 or 5C3) that recognizes iPSA but not PSA cleaved at Lys145 or Lys146 (i.e. nicked PSA) [6, 22]. The CV of this assay is 8.9% [23].
Statistical methods
To describe the variability of the biomarkers between Time 1 and Time 2 and between Time 1 and Time 3, we summarized both the absolute and relative changes, and calculated the intra-individual CVs. These CVs were calculated as the ratio of the standard deviation to the mean within each individual and are expressed as percentages. All statistical analyses were conducted using Stata 10 (Stata Corp, College Station, TX).
Results
Overall, 149 patients provided biomarker measurements across three time points. Their median age was 65 years (IQR: 60, 70). The median interval between Time 1 and Time 2 was 4 hours (IQR 3, 7); the median interval between Time 1 and Time 3 was 12 days (IQR 6, 38). Median biomarker levels did not change importantly across the three time points (Table 1). Prostate biopsy revealed benign histology in 97 patients (65%) and prostate cancer in 52 (35%). Those with benign histology were significantly younger than those with prostate cancer (median age: 63 [IQR 59–68] versus 68 [IQR 61–72] years).
Table 1.
Biomarker levels at time 1, time 2 and time 3. All values are median (IQR).
| Time 1 | Time 2 | Time 3 | |
|---|---|---|---|
| tPSA (ng/mL) | 6.8 (4.5, 9.6) | 6.7 (4.6, 9.9) | 6.5 (4.3, 9.8) |
| fPSA (ng/mL) | 1.06 (0.67, 1.66) | 1.02 (0.70, 1.74) | 1.01 (0.66, 1.72) |
| Percent fPSA (%) | 16 (12, 20) | 16 (12, 21) | 16 (13, 21) |
| hK2 (pg/mL) | 75 (41, 125) | 71 (40, 120) | 73 (41, 127) |
| iPSA (ng/mL) | 0.58 (0.33, 0.89) | 0.56 (0.34, 0.89) | 0.56 (0.32, 0.85) |
Table 2 shows the absolute and relative change in each biomarker across time points. We found no important differences in mean biomarker levels, although percent fPSA differed by a very small but statistically significant amount between Times 1 and 2 (mean absolute difference: 0.60% [95% CI: 0.18, 1.02] and mean relative difference: 1.03 [95% CI: 1.01, 1.06]).
Table 2.
Absolute differences and ratio of biomarker levels between time 1 and time 2 and between time 1 and time 3.
| Biomarker | Mean Absolute Difference (95% CI) | Mean Ratio (95% CI) | ||
|---|---|---|---|---|
|
| ||||
| Time 1–Time 2 | Time 1–Time 3 | Time 1/Time 2 | Time 1/Time 3 | |
| tPSA (ng/mL) | −0.02 (−0.12, 0.09) | −0.03 (−0.34, 0.28) | 1.00 (0.98, 1.01) | 0.98 (0.95, 1.02) |
| fPSA (ng/mL) | 0.05 (0.01, 0.10) | 0.03 (−0.06, 0.12) | 1.03 (1.00, 1.06) | 1.01 (0.97, 1.06) |
| Percent fPSA (%) | 0.60 (0.18, 1.02) | 0.56 (−0.08, 1.19) | 1.03 (1.01, 1.06) | 1.04 (1.00, 1.08) |
| hK2 (pg/mL) | −1.70 (−6.71, 3.31) | 0.26 (−8.07, 8.60) | 0.98 (0.94, 1.02) | 1.02 (0.96, 1.08) |
| iPSA (ng/mL) | 0.01 (−0.01, 0.04) | 0.02 (−0.02, 0.06) | 1.02 (0.98, 1.05) | 1.02 (0.98, 1.07) |
To illustrate the range of changes in individual men, we summarized the proportion of patients with various absolute differences in each biomarker (Table 3). The absolute change in tPSA between the first and second time point was between 0 and 1 ng/ml for the 133 participants (89%). A greater proportion of participants exhibited larger changes between the first and third measurement (26 [17%] had a tPSA change of more than 2 ng/ml). Only one participant had an absolute change in tPSA that was greater than 5 ng/ml. Similar results were seen for the other biomarkers (Table 3). While a few patients showed large absolute differences in biomarker levels between time points, the relative change was usually less dramatic. For example, the one individual whose tPSA increased by more than 5 ng/ml had 56 ng/ml at baseline and 69 ng/mL at Time 3, a relative change of 23%.
Table 3.
Number and proportion of patients with various absolute differences in several biomarkers between measurements taken at baseline and those taken several hours (time 2) or several days (time 3) later.
| Time 2 | Time 3 | |
|---|---|---|
| tPSA | ||
| 0–1 ng/mL | 133 (89%) | 91 (61%) |
| 1–2 ng/mL | 14 (9%) | 32 (21%) |
| 2+ ng/mL | 2 (1%) | 26 (17%) |
| fPSA | ||
| 0.0–0.05 ng/mL | 114 (77%) | 80 (54%) |
| 0.05–0.2 ng/mL | 20 (13%) | 27 (18%) |
| 0.2+ ng/mL | 15 (10%) | 42 (28%) |
| Percent fPSA (%) | ||
| 0–2 | 114 (77%) | 82 (55%) |
| 2–4 | 22 (15%) | 40 (27%) |
| 4+ | 13 (9%) | 27 (18%) |
| hK2 | ||
| 0.00–0.01 ng/mL | 102 (68%) | 90 (60%) |
| 0.01–0.02 ng/mL | 31 (21%) | 39 (26%) |
| 0.02+ ng/mL | 16 (11%) | 20 (13%) |
| iPSA | ||
| 0.0–0.1 ng/mL | 108 (72%) | 89 (60%) |
| 0.1–0.2 ng/mL | 29 (19%) | 32 (21%) |
| 0.2+ ng/mL | 12 (8%) | 28 (19%) |
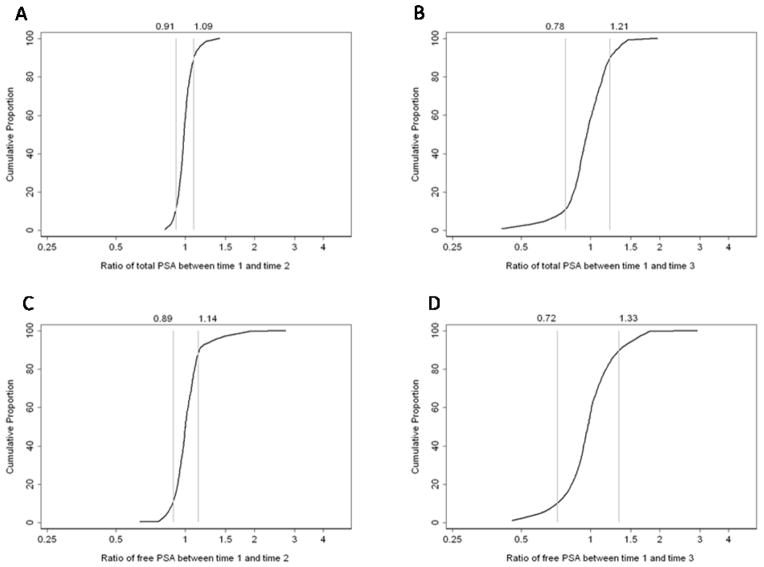
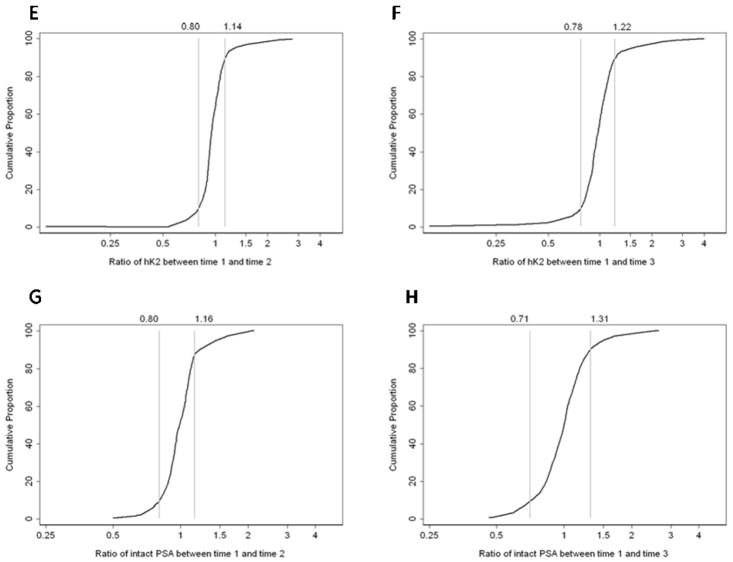
Like the absolute differences, the relative differences in biomarker measurements between time points were small for the majority of participants (Figure 1). For example, for 80% of patients the ratio of tPSA from Time 1 to Time 2 was 0.91–1.09; between Time 1 and Time 3 the ratio was wider (80% had a ratio of 0.78–1.21). Similar results were seen for fPSA and percent fPSA. The distributions of hK2 and iPSA had slightly longer tails (Figures 1E–H), indicating that a few patients exhibited larger relative changes; however, these changes were not particularly large in absolute terms. For example, the most extreme ratio for iPSA, 2.6, was for a change from 0.31 to 0.82 ng/ml.
Figure 1.
Cumulative proportion of the relative differences in the biomarkers. The vertical lines represent the 10th and 90th centiles of the relative difference. Left column, differences between Times 1 and 2; right column, differences between Times 1 and 3. AB, tPSA; C–D, percent fPSA; E–F, hK2; G–H, intact PSA.
The intra-individual CVs between the time points (Table 4) were low for all biomarkers. The variation for tPSA was 4% between Time 1 and 2, and 11.0% between Time 1 and 3. Variation between Time 1 and Time 2 was somewhat higher for the other biomarkers than for tPSA. For all biomarkers, variation was higher for the longer interval between Times 1 and 3 than between Times 1 and 2. Interestingly, however, CVs for the different biomarkers were more uniform between Times 1 and 3 than between Times 1 and 2.
Table 4.
Mean (SD) Intra-Individual Coefficients of Variation.
| CV between Time 1 and 2 (%) | CV between Time 1 and 3 (%) | |
|---|---|---|
| tPSA | 4.0 (3.7) | 11.0 (10.7) |
| fPSA | 6.6 (8.0) | 13.0 (12.7) |
| Percent fPSA | 6.0 (6.5) | 10.3 (10.0) |
| hK2 | 9.2 (12.6) | 11.8 (15.3) |
| iPSA | 9.5 (9.4) | 12.8 (11.9) |
We were particularly concerned about intra-individual changes that could have changed whether biopsy was recommended (ie, tPSA changing from above to below a biopsy threshold or vice versa). Sixty-four subjects had relatively large changes in tPSA (more than 1 ng/ml between either time point), but only four went from above to below the biopsy threshold of 3 ng/ml used at out institution. For the more traditional biopsy threshold of 4 ng/mL, ten men (7%) had at least one tPSA measurement above and one below the threshold.
As a sensitivity analysis, we repeated all statistical analyses separately for men who were diagnosed (n=52) and not diagnosed (n=97) with prostate cancer. Our results were not importantly changed. For example, the mean absolute difference in tPSA between Time 1 and Time 2 was 0.00 (95% CI: −0.22, 0.22) and −0.03 (95% CI: −0.14, 0.08) for men who were and were not diagnosed with prostate cancer, respectively. Similar results were seen for other biomarkers and for differences in the ratios.
Discussion
For the clinical use of serum PSA and other kallikrein-related peptidase measurements, knowledge about reproducibility and short-term variation is essential. In this study, we have assessed the total variation of PSA and of several other kallikrein markers (fPSA, percent fPSA, hK2 and iPSA) of potential clinical value in cancer-risk assessment [24]. We demonstrate a low variation for all biomarkers over the course of time points spanning less than two weeks. The different biomarkers showed similar degrees of variation. Measurements taken on the same day vary less than measurements taken two weeks apart, especially for tPSA and percent fPSA, although even the two-week differences are not large.
The short-term intra-individual variability we measured for PSA is much lower than in prior reports (summarized in Table 5). The variability was particularly high in early studies, which were less well standardized. Riehmann et al, who reported the highest CV (58%), used a Pros-Check™ assay with a rather high analytical CV, up to 13% [25]. In the latest studies, the reported tPSA variation has been lower: total CVs around 12–15%. In a meta-analysis [16], Yan derived a total CV of 13% from the 12 studies that met the required criteria. There are only a few prior studies on variation of fPSA, all of them reporting higher variation than the values we measured (Table 5). In general, the reported variation has been higher for fPSA than for tPSA.
Table 5.
Coefficients of variation reported for tPSA and fPSA.
| Study | Time Span, weeks | Prostate Conditions | No. Participants | Measure | tPSA | fPSA |
|---|---|---|---|---|---|---|
| Riehmann, 1993 [23] | 30 | BPH | 129 | CVt | ≤58% | na |
| Prestigiacomo, 1996 [29] | 3 | PCa, BPH | 169 | CVb | 23.5% | na |
| Nixon, 1997 [16] | 2 | PCa, BPH | 9 | CVt | 7.5% | 13.9% |
| Ornstein, 1997 [10] | 2 | BPH | 84 | CVt | 15% | 16.5% |
| Morote, 1999 [17] | 4–8 | PCa, BPH | 107 | CVt | 15% | 32% |
| Boddy, 2004 [30] | 4 | BPH | 58 | CVb | 9.5% | na |
| Kobayashi, 2005 [18] | 12 | PCa, BPH | 126 | CVt | 16.1% | 15.4% |
| This study | 2 | PCa, BPH | 149 | CVt | 11.0% | 13.0% |
| 0.02 | PCa, BPH | 149 | CVt | 4.0% | 6.6% |
BPH, benign prostatic hyperplasia; CVb, coefficient of biological variation; CVt, coefficient of total variation; PCa, prostate cancer; na, not available
The wide discrepancies in reported variation may be due to small sample sizes, short intervals, non-representative study cohorts, or non-standardized sampling. Another factor is the level of tPSA. Some investigators reported that intraindividual variations in serum PSA were greater among men with lower serum PSA values [18,25], and we found that the largest relative variation was in patients with the lowest biomarker levels. However, this was not shown by Ornstein or Kobayashi [10,19].
We did observe a few patients with moderately large absolute changes in biomarker measurements; one patient’s PSA increased 13 ng/ml between Time 1 and Time 3, 7 patients had changes that were larger than 4 ng/ml. However, these changes were typically in men with high values (eg, PSA varying between 20 and 25 ng/ml) and thus were not clinically relevant. Furthermore, for only a modest number of men (≤7%) would the tPSA variation have caused a reclassification with respect to biopsy thresholds of 3 or 4 ng/mL. In contrast, in a study by Eastham et al, annual PSA measurements in men without known prostate cancer showed that nearly half of the men with one abnormal PSA level subsequently had a normal level [26], suggesting that PSA level fluctuation may result in many false-positive elevations. Comparing our results with those of Eastham et al is troublesome because the variation between annual measurements is more likely to be affected by local disease processes such as infection and obstruction. Our results, however, show that the short-term variability is very low and results in reclassification of few cases.
The advantage of our prospective study is the well standardized sampling procedure. The short interval between the first and second samples is unique compared to previous studies. The use of plasma strengthens our results, as PSA, especially fPSA, is more stable in plasma than in serum [27]. However, we note several limitations. First, participants were sampled only three times. Additional measurements could have uncovered greater intra-individual variation; however, the intra-individual variation was small and relatively homogenous across participants, and thus it is unlikely that more blood measurements would importantly affect our results. Second, short-term variations may span an interval longer than 12 days. However, use of longer intervals would lead to uncertainty as to whether the variability was affected by progression of prostate cancer. We did not collect any information as to whether the men in our study cohort had ejaculated within the last 48 hours, but our data would suggest that influence from recent ejaculation would be small and not significantly change our results, which is consistent with at least some prior reports [28]. Dipstick-testing but not bacterial cultures were routinely performed on the urinary samples from the patients in our cohort. The limited sensitivity of dipstick-testing could have led us to include men with urinary tract infections, but all men received prophylactic treatment with ciprofloxacin immediately prior to biopsy, and none of these men developed post-biopsy septicemia. Lastly, our study participants were a mixed group, having either BPH or prostate cancer. On the other hand, this cohort is highly representative of patients referred to a urology clinic for PSA measurements and further diagnostic work-up. Furthermore, our results were not importantly changed when men with and without prostate cancer were analyzed separately.
Conclusions
Our study results clearly show a low intra-individual variation for tPSA, fPSA, percent fPSA, hK2, and iPSA over short periods: a few hours or two weeks. This low variation can be attained by using stable assays and sampling procedures as in good clinical practice. The low short-term variability reflects the stability of the analyte as a diagnostic marker and strengthens the precision, reliability, and clinical utility of these prostate cancer biomarkers.
Acknowledgments
This research was funded by a Fulbright Research Scholar Award; P50-CA92629 SPORE from the National Cancer Institute (Pilot Project 7), Swedish Cancer Society [08-0345], Swedish Research Council ([Medicine no. 20095; Biomedical Engineering for Better Health 2006-7600]; Fundación Federico SA, funds from David H. Koch provided through the Prostate Cancer Foundation, the Cancer Research Fund of University Hospital, Malmö, the Faculty of Medicine, Lund University, Region Skåne and The Sidney Kimmel Center for Prostate and Urologic Cancers.
We thank Janet Novak, PhD, for writing assistance, which was paid for by Memorial Sloan-Kettering Cancer Center. We also thank Ms Gun-Britt Eriksson and Ms Kerstin Håkansson for expert assistance with immunoassays.
Footnotes
Conflict of Interest Statement: Dr. Hans Lilja holds patents for free PSA, hK2, and intact PSA assays.
Contributor Information
Anders Christensson, Email: Anders.christensson@med.lu.se.
Laila Bruun, Email: Laila.Bruun@med.lu.se.
Thomas Björk, Email: Thomas.Bjork@skane.se.
Angel M. Cronin, Email: serioa@mskcc.org.
Andrew J. Vickers, Email: vickersa@mskcc.org.
Caroline J. Savage, Email: savagec@mskcc.org.
Hans Lilja, Email: liljah@mskcc.org.
References
- 1.Becker C, Piironen T, Pettersson K, Hugosson J, Lilja H. Clinical value of human glandular kallikrein 2 and free and total prostate-specific antigen in serum from a population of men with prostate-specific antigen levels 3.0 ng/mL or greater. Urology. 2000;55:694–9. doi: 10.1016/s0090-4295(99)00585-3. [DOI] [PubMed] [Google Scholar]
- 2.Morgan TO, McLeod DG, Leifer ES, Murphy GP, Moul JW. Prospective use of free prostate-specific antigen to avoid repeat prostate biopsies in men with elevated total prostate-specific antigen. Urology. 1996;48:76–80. doi: 10.1016/s0090-4295(96)00615-2. [DOI] [PubMed] [Google Scholar]
- 3.Lilja H, Christensson A, Dahlen U, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618–25. [PubMed] [Google Scholar]
- 4.Christensson A, Bjork T, Nilsson O, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–5. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 5.Woodrum DL, Brawer MK, Partin AW, Catalona WJ, Southwick PC. Interpretation of free prostate specific antigen clinical research studies for the detection of prostate cancer. J Urol. 1998;159:5–12. doi: 10.1016/s0022-5347(01)63996-x. [DOI] [PubMed] [Google Scholar]
- 6.Nurmikko P, Vaisanen V, Piironen T, Lindgren S, Lilja H, Pettersson K. Production and characterization of novel anti-prostate-specific antigen (PSA) monoclonal antibodies that do not detect internally cleaved Lys145-Lys146 inactive PSA. Clin Chem. 2000;46:1610–8. [PubMed] [Google Scholar]
- 7.Costongs GM, Janson PC, Bas BM, Hermans J, van Wersch JW, Brombacher PJ. Short-term and long-term intra-individual variations and critical differences of clinical chemical laboratory parameters. J Clin Chem Clin Biochem. 1985;23:7–16. [PubMed] [Google Scholar]
- 8.Roehrborn CG, Pickens GJ, Carmody T., 3rd Variability of repeated serum prostate-specific antigen (PSA) measurements within less than 90 days in a well-defined patient population. Urology. 1996;47:59–66. doi: 10.1016/s0090-4295(99)80383-5. [DOI] [PubMed] [Google Scholar]
- 9.Ulmert D, Serio AM, O’Brien MF, et al. Long-term prediction of prostate cancer: prostate-specific antigen (PSA) velocity is predictive but does not improve the predictive accuracy of a single PSA measurement 15 years or more before cancer diagnosis in a large, representative, unscreened population. J Clin Oncol. 2008;26:835–41. doi: 10.1200/JCO.2007.13.1490. [DOI] [PubMed] [Google Scholar]
- 10.Ornstein DK, Smith DS, Rao GS, Basler JW, Ratliff TL, Catalona WJ. Biological variation of total, free and percent free serum prostate specific antigen levels in screening volunteers. J Urol. 1997;157:2179–82. [PubMed] [Google Scholar]
- 11.Bruun L, Savage C, Cronin AM, Hugosson J, Lilja H, Christensson A. Increase in percent free prostate-specific antigen in men with chronic kidney disease. Nephrol Dial Transplant. 2009;24:1238–41. doi: 10.1093/ndt/gfn632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorente JA, Arango O, Bielsa O, Cortadellas R, Gelabert-Mas A. Effect of antibiotic treatment on serum PSA and percent free PSA levels in patients with biochemical criteria for prostate biopsy and previous lower urinary tract infections. Int J Biol Markers. 2002;17:84–9. doi: 10.5301/jbm.2008.1602. [DOI] [PubMed] [Google Scholar]
- 13.Thompson IM, Chi C, Ankerst DP, et al. Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst. 2006;98:1128–33. doi: 10.1093/jnci/djj307. [DOI] [PubMed] [Google Scholar]
- 14.Cevik I, Türkeri L, Ozveri H, Ilker Y, Akdas A. Short-term effect of digital rectal examination on serum prostate-specific antigen. A prospective study. Eur Urol. 1996;29:403–6. doi: 10.1159/000473787. [DOI] [PubMed] [Google Scholar]
- 15.Soletormos G, Semjonow A, Sibley PE, et al. Biological variation of total prostate-specific antigen: a survey of published estimates and consequences for clinical practice. Clin Chem. 2005;51:1342–51. doi: 10.1373/clinchem.2004.046086. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y. Intraindividual variation of prostate specific antigen measurement and implications for early detection of prostate carcinoma. Cancer. 2001;92:776–80. doi: 10.1002/1097-0142(20010815)92:4<776::aid-cncr1382>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Nixon RG, Lilly JD, Liedtke RJ, Batjer JD. Variation of free and total prostate-specific antigen levels: the effect on the percent free/total prostate-specific antigen. Arch Pathol Lab Med. 1997;121:385–91. [PubMed] [Google Scholar]
- 18.Morote J, Raventos CX, Lorente JA, Enbabo G, Lopez M, de Torres I. Intraindividual variations of total and percent free serum prostatic-specific antigen levels in patients with normal digital rectal examination. Eur Urol. 1999;36:111–5. doi: 10.1159/000067981. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi M, Kurokawa S, Tokue A. Intraindividual variation in total and percent free prostate-specific antigen levels in prostate cancer suspects. Urol Int. 2005;74:198–202. doi: 10.1159/000083548. [DOI] [PubMed] [Google Scholar]
- 20.Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 21.Väisänen V, Eriksson S, Ivaska KK, Lilja H, Nurmi M, Pettersson K. Development of sensitive immunoassays for free and total human glandular kallikrein 2. Clin Chem. 2004;50:1607–17. doi: 10.1373/clinchem.2004.035253. [DOI] [PubMed] [Google Scholar]
- 22.Nurmikko P, Pettersson K, Piironen T, Hugosson J, Lilja H. Discrimination of prostate cancer from benign disease by plasma measurement of intact, free prostate-specific antigen lacking an internal cleavage site at Lys145-Lys146. Clin Chem. 2001;47:1415–23. [PubMed] [Google Scholar]
- 23.Steuber T, Vickers A, Haese A, Kattan MW, Eastham JA, Scardino PT, Huland H, Lilja H. Free PSA isoforms and intact and cleaved forms of urokinase plasminogen activator receptor in serum improve selection of patients for prostate cancer biopsy. Int J Cancer. 2007;120:1499–504. doi: 10.1002/ijc.22427. [DOI] [PubMed] [Google Scholar]
- 24.Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Göteborg, Sweden. BMC Med. 2008;8:6–19. doi: 10.1186/1741-7015-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riehmann M, Rhodes PR, Cook TD, Grose GS, Bruskewitz RC. Analysis of variation in prostate-specific antigen values. Urology. 1993;42:390–7. doi: 10.1016/0090-4295(93)90364-g. [DOI] [PubMed] [Google Scholar]
- 26.Eastham J, Riedel E, Scardino P, et al. Variation of serum prostate-specific antigen levels. An evaluation of year-to-year fluctuations. JAMA. 2003;289:2695–2700. doi: 10.1001/jama.289.20.2695. [DOI] [PubMed] [Google Scholar]
- 27.Ulmert D, Becker C, Nilsson J-Å, Piironen T, Bjork T, Hugosson J, Berglund G, Lilja H. Reproducibility and Accuracy of Measurements of Free and Total Prostate-Specific Antigen in Serum vs Plasma after Long-Term Storage at −20 °C. Clin Chem. 2006;52:235–9. doi: 10.1373/clinchem.2005.050641. [DOI] [PubMed] [Google Scholar]
- 28.Tchetgen M, Song J, Strawderman M, Jacobsen S, Oesterling J. Ejaculation increases the serum prostate-specific antigen concentration. Urology. 1996;47:511–6. doi: 10.1016/S0090-4295(99)80486-5. [DOI] [PubMed] [Google Scholar]
- 29.Prestigiacomo AF, Stamey TA. Physiological variation of serum prostate specific antigen in the 4.0 to 10.0 ng./ml. range in male volunteers. J Urol. 1996;155:1977–80. [PubMed] [Google Scholar]
- 30.Boddy JL, Dev S, Pike DJ, Malone PR. Intra-individual variation of serum prostate specific antigen levels in men with benign prostate biopsies. BJU Int. 2004;93:735–8. doi: 10.1111/j.1464-410X.2003.04717.x. [DOI] [PubMed] [Google Scholar]